Abstract
A growing body of evidence indicates that freshwater bodies, particularly eutrophic systems, are significant sources of the greenhouse gases (GHGs) carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O). Unlike marine environments, freshwater systems are generally shallower and more directly influenced by terrestrial inputs, including nutrient enrichment, organic matter deposition, and steep redox gradients in both the water column and sediments. These conditions promote intense phytoplankton growth, including massive harmful cyanobacterial blooms (HCBs), and stimulate microbial processes that drive GHG production and release. This opinion article examines the biogeochemical mechanisms underlying these emissions and evaluates the potential of mitigation treatments to both enhance carbon sequestration and reduce CH4 and N2O emissions. We argue that effective control of HCBs, whether through nutrient load reduction or direct mitigation protocols, would not only provide communities with toxin-free water but also significantly lower GHG emissions from eutrophic waterbodies. As this is an opinion paper rather than a comprehensive review, we intentionally avoided citing widely accepted concepts, since doing full justice to the many excellent contributions across all relevant subfields would not be possible within the scope of this work.
1. Brief Introduction
The rising atmospheric CO2 concentrations from pre-industrial levels of about 280 ppm to over 420 ppm today, primarily due to fossil fuel combustion and deforestation, and that of other (methane and nitrous oxide) greenhouse gases (GHG) is widely recognized as a major driver of global warming. Along with other GHGs, elevated CO2 levels contribute to a range of adverse effects, including ocean acidification, extreme weather events, and loss of biodiversity, all of which have serious implications for both human and ecological systems. International agreements, such as the Paris Agreement (https://unfccc.int/files/meetings/paris_nov_2015/application/pdf/paris_agreement_english_.pdf?utm_source=chatgpt.com, accessed on 20 July 2025) aim to limit the global temperature increase to well below 2 °C above pre-industrial levels. Achieving this target requires not only substantial reductions in ongoing emissions but also active removal of existing GHG from the atmosphere. However, despite global efforts and commitments, the concentration of GHG continues to rise and the trend is accelerating highlighting the urgent need for more effective and immediate action.
Various frameworks have been developed to sequester atmospheric CO2, broadly classified into two main categories: engineered technologies and nature-based solutions. Engineered approaches such as direct air capture, carbon mineralization, and geological storage typically involve substantial energy requirements, complex infrastructure, and extended development timelines. In contrast, nature-based solutions leverage ecological processes like photosynthesis to remove atmospheric CO2, generally with lower energy demands and a range of ecological co-benefits. Beyond their role in climate mitigation, biologically driven strategies also support biodiversity conservation, water quality improvement, and ecosystem resilience [1]. Nevertheless, despite the progress achieved through existing initiatives, the total volume of CO2 sequestered to date remains far below the scale needed to significantly alter the atmospheric carbon trajectory, highlighting the magnitude of the challenge that lies ahead.
1.1. Intensification of Harmful Blooms
One of the consequences of global warming is the intensification of HCBs during the last few decades and their spreading to relatively cold regions where such bloom events were previously unheard of. The blooms are defined as a rapid rise in phytoplankton abundance reaching values higher than 2 × 104 cells/mL or 10 µg chlorophyll a/L. For harmful blooms, readers may refer to the World Health Organization guidelines for relevant toxin thresholds (https://www.who.int/publications/i/item/9789241549950, accessed on 20 July 2025). The blooms capitalize on the elevated dissolved CO2 concentrations, a rising nutrient supply due to leaching from agricultural fields or poorly treated wastewater and the warming lakes temperatures [2,3,4,5,6] altogether enhancing their photosynthetic activity and enabling them to produce massive, and frequently toxic blooms [4]. These blooms substantially alter the optical and biogeochemical characteristics of the waterbody and their biodiversity, severely affecting the water quality in thousands of waterbodies in both developed and underdeveloped countries and hence the wellbeing of people and wildlife. It is estimated that 4.4 billion people currently lack access to clean drinking water worldwide [7], intensification of the HCBs severely aggravate the problem by adding toxins to the infested water.
In my view (and that of many others often discussed in scientific meetings) it is time we begin to recognize the intensification and global spread of HCBs as a man-made pandemic, much like the way our colleagues in the health sector classify widespread threats to public well-being. As long as these blooms continue to be regarded and hence dismissed as “natural phenomena,” policymakers and the public will remain reluctant to allocate the resources urgently needed for their control or mitigation. The consequence is that communities will go on drinking water laced with cyanotoxins or turning to bottled water, thereby compounding the problem with plastic pollution. Reframing HCBs as a preventable, human-driven crisis is essential if we are to galvanize the political will and societal commitment necessary to restore water safety, protect ecosystems, and safeguard human health.
This raises a fundamental question: if we term HCBs “natural phenomena,” how do we define “natural processes”? This distinction is critical, as many policymakers, environmental agencies, and public service leaders remain reluctant to confront HCBs despite their severe global impacts. While HCBs do occur naturally, extensive laboratory and field evidence demonstrates that their proliferation and intensification, particularly over the past three decades, are largely driven by human activities, including nutrient enrichment and climate change. These pressures have enabled HCBs to expand into previously unaffected, cooler regions such as Scandinavia, Canada, southern Australia, and the southern parts of Argentina and Chile.
Given this context, it is difficult to view today’s intense blooms as entirely “natural”. With the development of effective, environmentally friendly mitigation protocols, it may be possible to partially “cure” affected lakes, restoring ecological stability, biodiversity, and toxin-free water supplies, much like returning them to a pre-bloom state. In doing so, we are not merely intervening, but actively healing these ecosystems, enabling them to regain ecological stability and biodiversity. A mitigation treatment, when implemented, should selectively target the dominant bloom-forming harmful species, particularly Microcystis spp. which are responsible for most ecological disruptions in eutrophic freshwater systems. As discussed below, the impact of the applied mitigation protocol on both the rate and magnitude of biomass sedimentation strongly influences the spatial and temporal patterns of GHGs emissions, primarily through its effects on oxygen availability and the redox potential along the water column. Without detailing specific protocols, one possible approach exploits the high sensitivity of cyanobacteria to oxidative stress. As the earliest oxygen-producing organisms, cyanobacteria are less equipped to cope with oxidative damage than eukaryotes in the phytoplankton assemblage [5]. Mild oxidative stress can trigger programmed cell death-like processes in cyanobacteria without significantly harming other phytoplankton groups [5].
As mentioned, one of the consequences of global warming and HCB proliferation is a dramatic decline in the biodiversity of waterbodies where toxic cyanobacteria may represent over 90% of the photosynthetic organisms. It is widely recognized that, in addition to nutrient availability and various abiotic conditions, both inter- and intra-species interactions, particularly allelopathy, play a significant role in shaping the phytoplankton community composition [8,9,10,11,12]. One example is growth experiments that demonstrated interspecies competition between the dinoflagellate Peridinium gatunense and the cyanobacterium Microcystis aeruginosa which strongly inhibit each other, with the final cell abundance and community composition determined by their initial inoculum ratios and the temperature [13,14]. Since, in most cases, the optimal temperature for dinoflagellate, green algae and diatoms growth is lower than that of toxic cyanobacteria strains, the warming winters plays a major role determining the seasonal succession in freshwater systems and in the intensification of HCBs [15]. This is further aggravated by the larger fraction of cyanobacterial cells that overwinter under warmer conditions [16,17,18] serving as an inoculum for the development of HCB populations in the spring. In our view, the competitive advantage provided by this larger overwintering inoculum is one of the principal drivers behind the intensification of HCBs and their spread into cooler regions over recent decades.
1.2. Shallow Lakes Are Hotspots for HCB
Shallow eutrophic lakes are more vulnerable to massive phytoplankton blooms compared to deeper lakes. Examples include the (many) lakes in the drainage basin of the Yangtze River in China, Lake Okeechobee in Florida, parts of Lake Victoria in Africa and many others. The main reasons being that the nutrients in the sediments are more accessible to the phytoplankton, closer to the photic zone compared with deeper lakes where they may sink to the hypolimnion and become unavailable for the rest of the summer, due to stratification, awaiting for the forthcoming water mixing in the winter. In shallow waterbodies, where the smaller volumes warm up more rapidly in the spring [17], cyanobacteria which thrive in warm conditions gain an early-season advantage, allowing them to establish dominance and outcompete other algae.
Given the strong link between rising atmospheric CO2 and the growth of HCBs, we examine how mitigation treatments that promote biomass sinking may enhance both organic- and inorganic C sequestration. We also explore strategies to reduce other GHGs emissions from freshwater bodies, focusing on key processes that govern GHG formation and exchange with the atmosphere. Rather than a comprehensive literature review, we highlight mechanisms affected by algal bloom mitigation, as illustrated in the Figure 1.
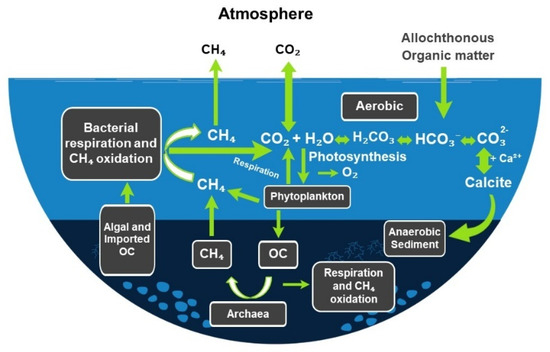
Figure 1.
Conceptual scheme of biogeochemical processes in a eutrophic freshwater body during a phytoplankton bloom. Photosynthesis removes CO2 and generates O2, supporting aerobic respiration and methane (CH4) oxidation in surface waters. Anaerobic sediment layers promote CH4 production by Archaea utilizing organic carbon (OC), while additional CH4 may be produced by phytoplankton in oxic zones. Allochthonous and algal-derived OC are subject to bacterial respiration, consumption by zooplankton and fish, or burial in sediments where it may eventually contribute to fossil fuel formation or undergo mineralization. Overwintering cells can serve as the inoculum for the following spring bloom. Dissolved CO2 equilibrates with HCO3− and CO32−, enabling CaCO3 precipitation (where sufficient Ca2+ is present) particularly under high pH, with calcite settling to and buried in the sediments. Fluxes of CH4 and CO2 to the atmosphere are indicated. Local environmental conditions control the magnitude and direction of these fluxes. Note: Nitrous oxide (N2O) fluxes are omitted for clarity; see text.
This approach is especially relevant for eutrophic waterbodies burdened by HCBs, offering a potential pathway to reduce their environmental impact while contributing to climate change mitigation.
2. Sequestration of Organic C
In aquatic systems, organic carbon (OC) is produced as part of the “biological CO2 pump” Figure 1 and [19,20,21], whereby atmospheric CO2, dissolved in the sunlit upper layers of the water column, is converted into organic matter through photosynthesis and associated cellular processes. In addition to this autochthonous production, allochthonous organic matter can also enter the waterbody from the surrounding terrestrial environment. A portion of the OC sinks to the sediments, contributing to long-term carbon sequestration, while another fraction undergoes microbial decomposition and respiration, releasing CO2 and methane (see below) and thereby completing the carbon cycle (Figure 1). One of the central challenges is to better understand the drivers of these fluxes—what determines the balance between them—and whether it is possible to increase the proportion of OC that remains buried over long timescales.
2.1. Are Lakes a Source or a Sink of CO2?
As mentioned earlier, photosynthetic CO2 fixation promotes the dissolution of atmospheric CO2 into waterbodies. Within aquatic environments, CO2 arises primarily from two processes: (1) the microbial decomposition of OC and (2) calcification, in which two bicarbonate ions combine to form carbonate, releasing CO2 in the process (Figure 1). Numerous studies have shown that lakes play a dual and complex role in the global carbon cycle, acting alternately as sources and sinks of CO2 depending on their geographical location, season, time of day, organic carbon inputs, and other environmental factors [3,22,23,24,25].
Eutrophic lakes generally emit much larger quantities of GHGs per unit surface area than oceans (oligotrophic systems), although likely less per cell because of self-shading effects on redox balance. Model-based estimates suggest that, globally, lakes may contribute up to 20% of fossil fuel–equivalent CO2 emissions [22]. However, because a portion of the photosynthetically produced OC becomes buried in sediments rather than fully mineralized, lakes should, in principle, act as sinks for atmospheric CO2. Consequently, the observation of a net CO2 efflux may appear to contradict the second law of thermodynamics, since part of the fixed carbon remains stored instead of being respired. This apparent paradox is resolved by recognizing that the balance between OC burial and its decomposition ultimately determines whether a lake functions as a net CO2 (and O2) source or sink. A key factor in this balance is the input of allochthonous OC—terrestrial carbon fixed on land and transported into lakes via runoff [26,27,28]. Once introduced, this external carbon is subject to microbial degradation, contributing additional CO2 (and methane) emissions. As a result, even systems showing strong in-lake CO2 drawdown through photosynthetic alkalization and elevated dissolved oxygen (DO) concentrations (see Figure 2) may, on a whole lake scale, exhibit net GHG release.
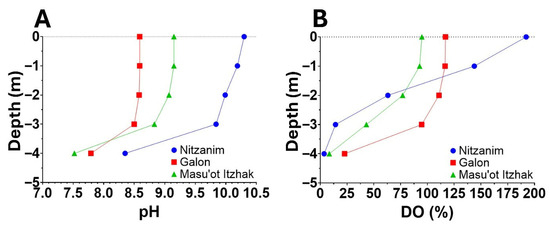
Figure 2.
Vertical profiles of pH (A) and dissolved oxygen (DO) (B) with water depth in three irrigation reservoirs. Nitzanim and Galon are filled with nutrient-rich, treated recycled water, while Masu’ot Itzhak receives floodwater collected from a nearby stream. Aerial images of the reservoirs (Figure 3A–C) show an extensive bloom in Nitzanim, predominantly Microcystis sp., in contrast to Galon (where routine mitigation treatments effectively limit bloom development) and Masu’ot Itzhak where algal growth is constrained by nutrients availability. The pH and DO measurements were performed using a YSI ProDSS Multi-Parameter Water Quality Meter on 4 June 2025.
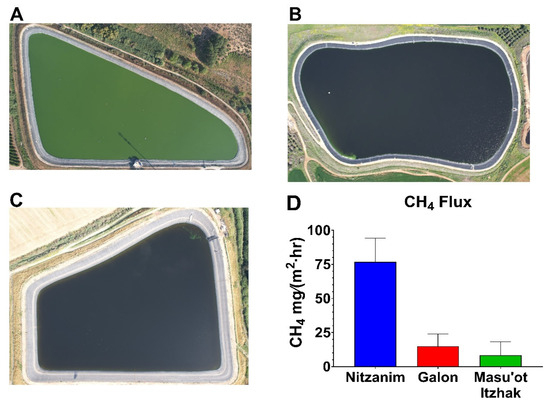
Figure 3.
Aerial photographs of the Nitzanim (A), Galon (B), and Masu’ot Itzhak (C) reservoirs, showing the relative phytoplankton (HCB) intensity, along with corresponding methane emission fluxes (D). Methane emissions were quantified using an ABB GLA131 laser-based gas analyzer connected to a custom-built floating flux chamber of known volume and surface area, measured in the middle of the waterbody.
2.2. Burial Efficiency (BE) of Organic Carbon
Notably, cyanobacteria produce large quantities and a wide variety of extracellular polysaccharides (EPS), which exert significant influence on their environment, including in biological desert soil crusts, rice paddies, and aquatic systems [29,30,31,32,33]. During intensive blooms, EPS facilitate cell-to-cell adhesion and colony formation, promoting the aggregation of phytoplankton into particulate organic carbon (POC) that sinks toward the sediments as “marine snow” in the oceans or “flocs” in freshwater bodies. The size of these particles impacts both their sedimentation rate [34] and internal oxygen dynamics; larger flocs exhibit slower oxygen diffusion, potentially leading to anoxic microenvironments within them. Consequently, anaerobic conditions may develop within the flocs delaying biomass degradation by bacterial respiration. It is not known, and worth exploring, whether anaerobic conditions stimulate methane formation in the flocs since they typically originate in the surface layer where the abundance of methanogens is likely much lower than in the sediments. In addition, the EPS also affect anaerobic decomposition, condensation of the sediment and mineralization [35,36] by aiding sediment compaction and maintaining structure. Their decomposition produces extracellular organic compounds that inhibits degrading bacterial communities delaying the breakdown of settled cyanobacterial biomass and methane formation, at least in rice fields [37,38,39].
2.3. Distinguishing Factors in Carbon BE: Lakes vs. Oceans
One of the long-standing dogmas in the field has been that dispersal, grazing by fish and zooplankton and bacterial respiration consumes a large portion of the aggregates, releasing most of the fixed CO2, while the particles sink to the bottom and during further degradation in the sediments [3,40]. This is indeed the case in the marine environment where only about 1% of the OC produced in the photic zones reach the sediments at >1 km depth [34,41,42] where it remains buried for geological timescale [34,43]. In contrast, detailed studies performed on fresh waterbodies, lakes and reservoirs, spanning various environmental condition, suggested a much larger BE than in the marine environment [26,44,45]. One example is a study performed by the US Geological Survey on 697 waterbodies in continental USA that concluded that the average BE in water reservoirs was 58% [46].
We must conclude that the long-standing dogma that “lakes’ OC is much less permanent than in oceans” doesn’t withstand empirical evaluation. The results obtained, particularly from eutrophic freshwater bodies show just the opposite. Naturally, the question arises—what distinguishes marine systems from freshwater environments as reflected by the much higher BE in the latter. To clarify this important point, it is essential to reveal the processes driving OC degradation within the waterbody which ultimately determine the OC fraction that reach and then buried in sediments. Briefly, the primary factors influencing these processes are the duration of exposure to oxygen, temperature, and the biomass density.
2.3.1. Exposure to Oxygen and the Temperature Impact
Seminal studies by Sebastian Sobek and colleagues [26,40,45] demonstrated that the BE of POC is strongly influenced by duration of exposure to oxygen during descent to the sediments and by temperature. Prolonged exposure to O2 and higher temperatures result in lower BE, primarily due to bacterial respiration which consumes OC and nutrients released from lysing cells. Since the oceans are much deeper than lakes the aggregates sinking to the sediments are subjected to grazing and bacterial respiration for a considerably longer duration than in lakes. Further, in shallow lakes, OC particles often reach anaerobic conditions in the sediments within a few meters from the water surface much faster than in the ocean [40,47]. In addition, the massive biomass often observed in eutrophic freshwater lakes, with substantial amount of EPS, forms larger flocs than marine snow. As mentioned, the larger the flocs are the slower their bacterial degradation is.
Furthermore, the strong currents characteristic of oceanic continental shelves where nutrient inputs fuel intensive algal blooms transport substantial amounts of oxygen to deeper coastal layers, thereby enhancing OC degradation. Ultimately, in addition to the effects of temperature and oxygen [40,41], large spatial variability was observed in the amount of OC reaching specific sediment sites [45] likely reflecting the impact of currents and changing wind directions on local algal abundance. In densely populated waterbodies and/or high turbidity, the depth of the photic zone (where net oxygen production occurs) is attenuated, particularly in shallow lakes where turbulence by wind or currents may raise sediment particles [48]. Consequently, anoxic condition may develop within a short distance from the water surface [17].
2.3.2. Cell-Density and Phytoplankton Biomass
As indicated, the biomass density is an important parameter affecting the oxygen gradient profile and hence OC degradation. Although blooms intensity increased significantly in the open, oligotrophic, ocean during the last century [49], the overall cell density of phytoplankton biomass is rather low, equivalent to approximately 0.1–0.3 mg chlorophyll a/m3. It may reach 5–10 mg chlorophyll a/m3 during spring-summer blooms mostly near coastal nutrient-rich areas. In contrast, the cell density in eutrophic freshwater bodies can easily exceed 300–500 mg chlorophyll/m3, particularly during HCB that form scums on the water surface [50,51] and https://portal.edirepository.org/nis/mapbrowse?scope=edi&identifier=1756&revision=2 (accessed on 20 July 2025). Chlorophyll extraction methodologies, along with recent advances in remote sensing technologies, have made it easier and more accessible to assess its concentrations across large areas. Where evaluations of carbon sequestration are required, conversion factors for estimating dry matter from chlorophyll concentrations [52,53] can be employed. However, remote sensing primarily assesses surface phytoplankton populations, while their spatial (including three-dimensional) and temporal distribution is, in most cases, highly variable. Therefore, ground-truthing is essential to accurately calibrate remote sensing measurements. It is timely to develop robust models that integrate remote sensing data with in situ measurements to capture the spatial and 3D distribution of phytoplankton, enabling comprehensive, whole lake biomass assessments. Alternatively, biomass can be estimated by systematically collecting and analyzing samples from multiple locations and depths. However, this process is labor-intensive, costly, and subject to inherent logistical limitations.
2.4. Sequestration of OC in the Marine and Freshwater Bodies
Geologically speaking, it is widely recognized that sediments derived from phytoplankton biomass have played a significant role in the formation of kerogen, a key intermediate in the transformation of OC into natural gas and other fossil fuels. This highlights their crucial contribution to the global carbon cycle over geological timescales. For instance, a recent review [54] proposed a model describing cyanobacterial proliferation and the preservation of organic matter, emphasizing their critical role in OC burial throughout Earth’s history.
This perspective raises a fundamental question: to what extent can the permanence of OC in a given waterbody be predicted based on its environmental conditions and biological activity. The concept of “burial permanence”—the length of time that OC remains sequestered in sediments is inherently complex as it involves understanding and measuring the dynamic processes that govern the stability and longevity of OC [55]. Radiometric dating of sediment cores using isotopes such as 14C, 137Cs, and 210Pb is commonly employed to assess the permanence. These analyses provide a detailed reconstruction of the waterbody’s depositional history and have been published in numerous peer-reviewed studies [44,56,57,58,59,60,61], governmental agencies reports (e.g., USGS SIR 2004-5184) and large-scale research initiatives like New Zealand’s Lake380 project (https://ourlakesourfuture.co.nz/, accessed on 20 July 2025). Naturally, the preservation of OC in the sediment is strongly influenced by local environmental conditions. In all cases we are aware of, sediment cores show a pattern of rapid OC degradation during the first few years, followed by a much slower breakdown phase, highlighting a stabilization process in the deeper sediment layers.
The reader is encouraged to explore the Lake380 project available on their website and emerging publications thereof. The data represents one of the most extensive and detailed waterbody analyses, over a range of ambient condition, conducted globally. The sediment cores collected as part of this effort offer invaluable data on both contemporary and historical water quality, encompassing a broad range of biotic and abiotic parameters. In particular, the use of pigment extraction and DNA sequencing along sediment cores serves as a powerful approach for distinguishing between, and reconstructing of algal, shrub and tree-originated biomass over time. Interestingly, the algal originated OC remains fairly constant over tens of thousands of years. This is probably due to various processes taking place in the sediment, where the buried OC undergoes physiochemical processes such as condensation, mineralization, and compaction, ultimately transforming into stable hydrocarbon structures, commonly known as kerogen [37,38,39,54]. Altogether, offering strong evidence for the capacity of freshwater systems to sequester OC over long periods.
2.5. Can Mitigation of HCBs Be Used for Substantial Carbon Burial?
Phytoplankton blooms in eutrophic freshwater systems hold considerable potential for OC sequestration [62]. However, their long-term effectiveness and sustainability remain subjects of active debate. Under business-as-usual conditions, a substantial fraction of bloom-derived OC is mineralized in the water column (Figure 1), thereby limiting BE. In contrast, prolonged exposure to mild oxidative stress has been shown to trigger a programmed cell death (PCD)-like response in cyanobacteria, with mortality rates reaching up to 99% [5,63,64,65,66]. The resulting biomass rapidly aggregates into large flocs that sink to the sediment. As reported by Sobek and colleagues [26,40,45] and discussed in Section 2.2, larger flocs and faster sedimentation rates are strongly correlated with higher BE because they reduce OC degradation by bacterial respiration. A key hypothesis worth testing is that mitigation treatments inducing massive bloom collapse may also create localized anoxia in bottom waters, further limiting microbial respiration. Interestingly, PCD-induced rapid collapse appears to minimize cyanotoxin release into the surrounding waters [63,67,68], though the mechanism is not yet understood. One possible explanation is that many intact cells are captured within the sinking flocs, carrying intracellular toxins and nutrients with them to the sediment bed—a possibility that requires experimental examination.
Over longer timescales, the removal of toxic cyanobacteria facilitates phytoplankton succession, restoring biodiversity and supporting renewed oxygenic photosynthesis even after dramatic declines in cyanobacterial abundance. Another clear benefit of mitigation-induced bloom collapse is the marked improvement in water clarity, which enhances light penetration and thus photosynthetic oxygen production throughout the water column. Taken together, these processes suggest that BE under treated conditions may significantly exceed that under untreated, baseline scenarios.
Researchers involved in carbon harvesting from phytoplankton biomass may utilize commercial systems that measure Total Organic Carbon (TOC) via combustion and CO2 detection, followed by acidification to quantify Total Inorganic Carbon (TIC) enabling full TIC/TOC profiling across depths and locations. Remote sensing offers a complementary approach by estimating chlorophyll-a as a proxy for biomass. However, assessing carbon flux to sediments post-mitigation remains challenging due to the spatial and temporal variability in HCB biomass, influenced by factors like wind mixing, stratification, light, and nutrients. Addressing this requires an integrated strategy combining remote sensing, in situ measurements, and modeling as mentioned in Section 2.3.2, above. While chlorophyll-a accounts for 0.6–1.6% of dry biomass [69] under laboratory conditions, field values may vary.
3. The Fate of Inorganic Carbon in the Marine- and Fresh-Waterbodies
3.1. The CO2-Concentrating Mechanisms (CCMs)
Cyanobacteria and algae have evolved sophisticated CCMs that enable them to accumulate Dissolved Inorganic Carbon (DIC) within their cells, particularly under CO2-limiting conditions [70,71,72,73,74]. They can thus be regarded as DIC containing bags, intracellular levels as high as 50 mM DIC were recorded [72]. These mechanisms allow the organisms to elevate the internal CO2 concentration in close proximity to their carboxylating enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which is predominantly localized within carboxysomes in cyanobacteria and pyrenoids in algae. This adaptation enables them to overcome the large gap between Rubisco’s relatively low affinity for CO2 and the low concentration of dissolved CO2 in equilibrium with the atmosphere, particularly in cyanobacteria, where the Km(CO2) of Rubisco is approximately tenfold higher than ambient levels. The size and dynamics of the internal DIC pool are strongly influenced by environmental factors, especially the CO2 concentration during growth and light intensity [70,72,75], and they vary among species and even among strains, including Microcystis sp. [76,77].
Seminal studies by Elke Dittmann, Martin Hagemann, and colleagues [78,79,80] revealed a non-canonical CCM in toxic Microcystis sp., where a significant part of Rubisco is located outside the carboxysomes. This distribution results in a less efficient CCM compared to the classical model cyanobacteria studied previously. Additionally, microcystin, a toxic secondary metabolite produced by Microcystis sp., has been shown to bind Rubisco [81]. Fluctuations in extracellular microcystin levels such as those occurring during cell lysis under stress affect the cytoplasmic localization of Rubisco [79]. These findings support earlier hypotheses [82] and provide a mechanistic framework for how cells might sense extracellular microcystin levels and adjust their physiology to cope with environmental stress, thereby enhancing ecological fitness.
A recent study [83] further demonstrated that a similar non-canonical CCM may also operate in the filamentous cyanobacterium Nostoc punctiforme. Here, a poorly functioning CCM necessitates a reliance on heterotrophic bacteria for CO2 supply under limiting DIC conditions, a surprising dependency that could explain the difficulty in isolating axenic cultures of various cyanobacteria and suggests that elevated ambient CO2 levels may be critical for their isolation and cultivation. Finally, a quantitative analysis of the internal DIC (and calcite) pool is essential to fully understand the operation and regulation of non-canonical CCMs and their effect on intracellular DIC accumulation [72]. In addition to enhancing the apparent photosynthetic affinity for CO2, the substantial intracellular accumulation of DIC plays a pivotal role in calcification processes across various algal groups.
3.2. Calcification Processes
The example in Figure 2 shows that during HCBs, intense photosynthetic activity in the upper water layers, where light is most available, is often accompanied by pronounced alkalization. As CO2 is rapidly depleted and fixed through photosynthesis, pH levels can rise above 10, indicating a substantial departure of carbonate chemistry from equilibrium with atmospheric CO2 (Figure 2A). Elevated DO concentrations in surface waters (Figure 2B), far exceeding equilibrium values with air, further confirm that this alkalization is driven by vigorous photosynthesis. Such conditions promote calcium carbonate precipitation both in the water column and on the surfaces of algal species with mucilaginous sheaths, which create localized supersaturation and facilitate calcite deposition [84,85,86,87,88,89,90].
Intracellular CaCO3 accumulation in bloom-forming Microcystis spp. has been documented [87,91,92], though quantification of calcite content under different environmental conditions remains incomplete [89,93,94,95,96,97,98]. Given the remarkable ability of cyanobacterial cells to accumulate DIC through their CCM [72], they can effectively be regarded as DIC-bearing entities. This highlights an unexplored potential for employing molecular tools to further enhance DIC accumulation and, consequently, carbon sequestration [99,100]. Promising genetic targets for such interventions include bicarbonate transporters (SbtA, BCT1, and BicA) as well as the NDH-1 complexes that facilitate CO2 recapture from leaking pools [71,101]. Noticeably, the abundance of calcium ions may be limiting calcite formation in freshwater bodies. Nevertheless, while the release of genetically modified organisms into natural environments remains subject to strict regulatory constraints, testing their capacity for enhanced DIC accumulation and cycling [102] under controlled or simulated bloom conditions could provide valuable insights into their potential utility for large-scale carbon capture applications.
Calcification in aquatic systems involves the reaction of Ca2+ with HCO3− to form calcium carbonate, releasing CO2 (and water, Figure 1). As mentioned, this process can lead to substantial CO2 emissions from both freshwater and marine environments [89,90,96,103,104]. However, the accumulation of calcium carbonate in coral or coastal reefs and freshwater sediments suggests a net uptake of DIC over time, as atmospheric CO2 dissolves in water to replenish bicarbonate consumed during calcification. In freshwater bodies, significant calcification often occurs in areas with high alkalinity, hard water or during substantial phytoplankton blooms where intensive CO2 uptake raises the pH to values where most of the DIC is in the form of carbonate, promoting calcite precipitation [105]. The Sea of Galilee (Lake Kinneret), a stratified hard-water lake, provides a notable example. It was estimated that over 52,000 tons of calcite settles into the sediment during the spring blooms of the dinoflagellate Peridinium gatunense [93,106]. Mineralization processes within the sediment also lead to carbonate formation particularly during intensive algal blooms [107]. Here, calcification is driven by the high level of dissolved CO2 consequent on intensive bacterial respiration. It is estimated that carbonate formation through mineralization at the sediment accounts for 3–8% of the deposited OC. However, distinguishing this carbonate from that settling onto the sediments from the upper water layer remains challenging [35]. Attempts to resolve this issue using δ13C would be complicated by the disequilibrium between CO2 and bicarbonate species at both the sediment surface and in the photic zone. This disequilibrium, driven by a substantial pH gradient, significantly shifts the δ13C values of these species away from the equilibrium value of approximately 7‰ [108].
Naturally, the amount of carbon buried through calcification is strongly influenced by various factors, with calcium, magnesium and proton concentrations playing a particularly significant role. These concentrations vary widely between different lakes and depth in the water column (Figure 2A) and affect both calcification and dissolution processes. Unfortunately, detailed analyses of the amount of carbonate reaching the sediments, whether due to calcification in the epilimnion or mineralization of OC, and its subsequent fate, including the extent of dissolution [104], remain scarce. Direct measurements of descending carbonates using sediment traps and cores provide valuable insights, though these techniques are labor intensive and costly with substantial variation observed among replicates. Alternatively, an assessment of calcium and magnesium cycles within the waterbody through measurements of their levels in the epilimnion, hypolimnion, as well as inflowing and outgoing streams throughout the year offers a robust approach to evaluate calcification and dissolution dynamics. In the case of Lake Kinneret, analysis [109] revealed that approximately one-third of the calcite formed during the spring bloom undergoes dissolution, while the remaining portion remains buried (Nishri, personal communication). Such assessments are, in our view, crucial for evaluating carbon fluxes in aquatic environments, understanding their role in the global carbon cycle, and exploring potential pathways for effective carbon sequestration (Figure 1).
3.3. A Challenge: Can We Use Changing pH to Assess the Amount of Atmospheric CO2 Dissolution in Freshwater Bodies?
Dissolution of atmospheric CO2 to replace that consumed during OC formation is commonly referred to as the “biological pump” (see Section 2). In a similar vein, the accumulation of calcite and other carbonate minerals in sediments, driven by water alkalization as described above, may be regarded as a complementary abiotic process. The extent of pH increase during intensive CO2 uptake by photosynthesis (Figure 2A) is strongly influenced by the alkalinity of the waterbody and the chemical composition of its buffering system. Higher alkalinity results in a smaller pH rise for a given amount of CO2 removed. In principle, alkalinity can therefore be used to estimate the amount of CO2 required to return the pH across a given range, including to pre-bloom conditions, assuming equilibrium among DIC species throughout the process. However, this equilibrium-based assumption can be misleading. Unlike oceanic systems, eutrophic lakes often exhibit significant disequilibrium among DIC species, driven by dynamic biological and chemical processes, most notably the intensive removal of CO2 during photosynthesis and the precipitation of carbonates at elevated pH (Figure 1). The latter process is especially pronounced when calcium and magnesium ions are sufficiently available leading to effective removal of DIC from the water column further complicating efforts to model or quantify DIC speciation accurately (Figure 1). To address this complexity, we propose a time-resolved modeling approach based on observed pH dynamics, incorporating non-equilibrium processes that may provide a more accurate framework to estimate CO2 fluxes.
When HCB is abruptly terminated (e.g., through a mitigation treatment) the cessation of photosynthetic CO2 uptake permits dissolved CO2 in the water to re-equilibrate with the atmosphere, typically resulting in a gradual decline in pH toward pre-bloom levels. The rate of atmospheric CO2 dissolution is influenced by abiotic drivers such as temperature, wind, turbulence, and vertical mixing. Additional contributors to the rising CO2 concentration include the balance between respiratory CO2 production (B) and the photosynthetic uptake (C, notably, C is expected to decrease after a mitigation treatment, leading to alterations in the other fluxes), and shifts in the distribution of DIC species as the system rebalances with changing pH (D). The relationships between these components can be described as:
where (A) is the amount of CO2 required to reduce the pH over a given range and E is the net amount of atmospheric CO2 dissolved in the water towards the new steady state reached following the mitigation treatment.
A = (B − C) + D + E
A key challenge is whether we can quantitatively distinguish between the contributions of each of these processes, in order to retrieve the net amount of CO2 dissolved from the atmosphere following bloom collapse. Potential approaches include measuring the initial rate of pH decline in sealed water containers (excluding air exchange) may provide insight into non-atmospheric CO2 contributions (e.g., from respiration and internal redistribution of DIC species). Quick time points measurements are critical since prolonged incubation can result in substantial pH reduction by microbial respiration alone, especially where oxygen and organic carbon are available. A more robust method involves quantifying DIC directly by acidifying collected samples, which converts all carbonate species to CO2 for measurement. This bypasses the reliance on pH-alkalinity modeling alone. We acknowledge that spatial heterogeneity, both horizontal and vertical, and temporal fluctuations in pH and alkalinity (see Figure 2A) are inherent to the dynamics of bloom development and collapse. These variations are also reflected in local DO levels (Figure 2B), which integrate the combined effects of photosynthesis, respiration, and gas exchange with the atmosphere. To account for these variabilities, representative sampling across depth profiles, followed by volumetric integration over the entire waterbody, is recommended.
4. The Methane Enigma
The escalating methane (CH4) level in the atmosphere is a matter of growing concern due to its projected impact on global warming, surpassing CO2 (per mol) by approximately 30-fold. It is now firmly established that waterbodies constitute significant contributors to atmospheric CH4 (Figure 1 and [110,111,112,113,114,115,116,117,118]). Large spatial and temporal variability in methane (CH4) emissions has been reported in studies assessing fluxes from freshwater systems. For example, Hounshell and colleuges [119] and Waldo et al. [120], using the eddy covariance approach, showed that under business-as-usual conditions, eutrophic lakes release between 1.3 and 51.4 g CH4 m−2 yr−1. This technique, which measures vertical gas fluxes (such as CO2 and CH4) by monitoring high-frequency fluctuations in gas concentration, wind speed, and direction above the ecosystem, provides valuable insights into real-time emission dynamics. However, its application is currently limited to relatively small and homogeneous waterbodies, where the spatial variability in turbulence and gas exchange can be adequately captured. Buoyant flow chambers can also be used to measure CH4 emissions through detection of its infrared absorption bands or by gas chromatography [121]. However, due to their small surface area, a major challenge is capturing the fraction of CH4 that escapes via ebullition directly into the atmosphere. Addressing this requires multiple spatial measurements and subsequent modeling to estimate the total CH4 flux across the water surface. For a more detailed mechanistic understanding of CH4 production and consumption dynamics (see below), it is advisable to conduct simultaneous measurements of dissolved CH4 and O2 concentrations, ideally complemented by redox potential profiling, along the water column.
4.1. Methane Biosynthesis
The mechanisms of CH4 biosynthesis and degradation are being resolved with recent findings challenging longstanding paradigms and potentially unveiling new strategies to mitigate CH4 emissions. Biogenic CH4 is primarily produced under anaerobic conditions by several archaea groups known as methanogens. They can combine CO2 and hydrogen to form CH4 in a reaction that consumes the OC produced within the lake, as well as from allochthonous inputs from its surroundings. Additional important CH4 producing pathways include methylotrophic bacteria that can convert methanol to methane and CH4 production by phytoplankton under oxic condition (discussed below). In addition to O2 level, CH4 formation is also affected by temperature though in some casees less than expected due to the presence of various methanogens whose optimal activities vary across a wide temperature range.
Biogenic CH4 has the lowest isotopic carbon ratio compared to other natural sources because it is extremely depleted in 13C [122]. Its δ13C value, typically ranges from −50‰ to −110‰ (relative to the Vienna Pee Dee Belemnite standard, VPDB), is the main diagnostic tool that distinguish it from other CH4 sources. This highly depleted isotopic signature is due to the preferential use of the lighter carbon isotope (12C) by methanogenic archaea during microbial methanogenesis, particularly in the reduction of CO2 or acetate to CH4 via the two main pathways involved. However, studies utilizing CH4 to assess the efficacy of CH4-derived carbon as a substrate for phytoplankton [123,124] should bear in mind that 13C discrimination also occurs during CO2 fixation, and that the CO2-concentrating mechanism in phytoplankton raises the utilization of 13C by the carboxylating enzyme [72].
The Methane Paradox
Until recently, the prevailing belief was that under business-as-usual conditions, most CH4 produced in anoxic sediments by archaea (see Section 6, below) is consumed by methanotrophs in the overlying oxic layers. This paradigm was supported by laboratory experiments and studies in small-scale aquatic systems, but recent research challenges this view. A growing body of evidence indicates that various phytoplankton inhabiting the photic, oxygenated water layers, including cyanobacteria, can produce significant amounts of CH4 during photosynthesis [112,125,126,127,128,129,130,131,132,133]. This aligns with earlier studies on aerobic CH4 biosynthesis by bacteria [134].
Collectively, these findings contribute to the recognition of the “methane paradox,” wherein CH4 concentrations in the oxic surface layers of lakes and coastal ocean regions rise toward the top water layer and can surpass levels expected at saturation with air [124,132,135]. The specific organisms involved in freshwater and oceanic methane production are being identified [131,135,136], and the exact metabolic pathways are under intensive investigation. Recent studies have demonstrated that CH4 formation is light- and photosynthesis-dependent, though the precise mechanisms remain poorly understood [131]. We hypothesize that CH4 production by photosynthetic organisms under oxic conditions may serve as a “safety valve” for dissipating excess reducing equivalents generated in the light energy harvesting machinery under high illumination or other stress conditions. If so, the inactivation of genes involved in CH4 biosynthesis would be expected to increase cellular sensitivity to high illumination and to alter both the redox balance and the turnover dynamics of photosynthetic components.
Stable carbon and hydrogen isotope analyses suggest that methylated compounds such as methylphosphonate (MP), methylamine, and methionine serve as precursors for oxic CH4 production. This process involves a gene cluster (phn) that is widespread in filamentous cyanobacteria and also present in certain strains of toxic Microcystis sp., such as those collected during a large bloom in Dianchi Lake, China [137], emphasizing the role of cyanobacteria in oxic methane formation. Complicating matters further, studies suggest that MP released by lysing cyanobacteria is utilized by bacteria as a phosphate source, thereby releasing CH4 in the photic zone [137]. Mutations in the C-P lyase phosphonate degradation pathway of the marine bacterium Pseudomonas stutzeri disabled its ability to produce methane [135]. Currently, accurate assessments of the relative contributions of cyanobacteria and algal species to CH4 formation and utilization in the upper aerobic zone are lacking, highlighting the need for genome screenings, identification of potentially involved genes [130] and physiological flux analyses.
4.2. Methane Consumption
Methane is consumed by methanotrophic microorganisms, which utilize it as both a carbon and a reducing power source, thereby mitigating its emission to the atmosphere. The activation of the stable C-H bond in CH4 is thermodynamically unfavorable, requiring a high redox potential and the transfer of eight electrons [138]. Methane oxidation proceeds via two main pathways: aerobic and anaerobic. These pathways differ significantly in their enzymatic mechanisms, the organisms involved, and their sensitivity to environmental factors such as temperature and redox potential. In both systems, CH4 is oxidized by forms of methane monooxygenase (MMO) or analogous enzymes, each exhibiting distinct kinetic properties. In aerobic methanotrophs, MMOs utilize molecular oxygen as the terminal oxidant. Here, the eight-electron transfer is mediated by specific components of the MMO complex, which catalyzes the conversion of CH4 to methanol, a key step in the metabolism of CH4 by aerobic methanotrophs. During anaerobic methane oxidation, CH4 is ultimately converted to CO2 and water through the action of microbial consortia, typically involving anaerobic methanotrophic archaea in partnership with sulfate-reducing bacteria. Here, alternative oxidants such as Fe3+ or copper, and in marine systems, sulfate or nitrate, often serve as terminal electron acceptors for methanotrophic respiration. Where sulfate availability is limited, such as in freshwater lakes, alternative electron acceptors like nitrate, iron or manganese can be utilized. Theoretically, an ORP above approximately +400 mV is likely required to support meaningful anaerobic methanotrophic activity. For reference, the redox potentials of key electron acceptors used in anaerobic processes, Fe3+/Fe2+ and NO3−/NO2− are +770 mV and +940 mV, respectively. These alternative pathways enable energy generation that supports methane oxidation under oxygen-depleted conditions, contributing to CH4 removal even in anoxic zones. Hypothetically, an addition of coarse-grained iron ores (or nitrate), designed to sink to anoxic sediment layers, could enhance CH4 consumption by stimulating Fe3+—dependent methanotrophic activity and thereby reduce overall methane emissions. Repeated applications may be necessary to sustain elevated levels of iron-reducing methanotroph populations and maintain their metabolic associations that enable the process. It is important to note that in most aquatic systems studied to date, nitrate is often depleted in the oxic (photic) zone but remains abundant in deeper, anoxic layers of the water column. Therefore, measuring the redox potential at the sediment-water interface is recommended to assess the potential for anaerobic CH4 consumption and of the impact of iron fertilization on anaerobic methane oxidation. In contrast, the redox potential required for aerobic CH4 oxidation is provided directly by oxygen, acting as the terminal electron acceptor during bacterial respiration. Thus, where possible, simultaneous measurements of ORP and DO are highly recommended to better understand the methanotrophic potential and to evaluate strategies for enhancing methane removal in aquatic systems.
As previously noted, cyanobacteria are increasingly recognized as significant contributors to CH4 production. However, despite CH4 being a potential source of both carbon and reducing power and despite cyanobacteria’s capacity to support eight electron transfer processes such as nitrogen fixation, they appear to lack the MMO enzyme necessary for cleaving the stable C-H bond in CH4. To the best of our knowledge, no published studies have demonstrated the growth of axenic cyanobacterial cultures using CH4 as the sole carbon source. Nevertheless, growing interest in cyanobacteria-methanotroph interactions has led to co-culture studies that reveal enhanced CH4 oxidation in the presence of cyanobacteria. This enhancement is likely attributable to increased local oxygen availability provided by cyanobacterial photosynthesis. Conversely, methanotroph presence has been associated with accelerated cyanobacterial growth [123,139]. The precise metabolic contribution of methanotrophs to cyanobacterial physiology remains uncertain. In our opinion, given the highly efficient CCM characteristic of cyanobacteria, it seems unlikely that the observed benefits arise solely from respiratory CO2 release by methanotrophs, as has been previously suggested. This hypothesis could be further evaluated by experimentally increasing the ambient CO2 concentration.
In the absence of extensive data from freshwater systems, insights may be drawn from studies on cyanobacteria–methanotroph interactions in rice paddy soil microcosms. In such systems, inoculation with cyanobacteria has been shown to reduce CH4 emissions by up to 90%, with outcomes strongly dependent on the cyanobacterial species used. For instance, Nostoc sp. and Calothrix sp. were found to influence different methanotrophic communities, leading to variable outcomes in CH4 flux [37], These emerging studies reveal a more complex and nuanced picture of CH4 dynamics within microbial consortia, underscoring the importance of species-specific interactions, possibly allelopathic mediated by secondary metabolites, in shaping these processes. Continued investigation of these relationships is essential for elucidating their ecological significance and for identifying potential applications in CH4 mitigation strategies.
4.3. Can HCB Mitigation Reduce Methane Emissions?
Naturally, the net flux of CH4 emissions from waterbodies is governed by the balance between its production and consumption. The significant variability observed in CH4 emission fluxes across different eutrophic lakes likely reflects differences in local conditions and various parameters affecting this balance. Studies have shown that cyanobacterial blooms can enhance methane emissions through multiple pathways. For instance, research on Lake Tai, China, showed that regions with dense cyanobacterial populations emitted far more CH4 compared to low-density areas, 867 vs. 3.4 μg CH4 m−2 min−1, respectively, indicating a strong correlation between bloom intensity and methane production [139]. This is an important observation, as it may point to a potential route for decreasing global methane emissions from eutrophic lakes through proper management and control of HCBs.
Three water reservoirs located in the northwestern Negev region of Israel were selected to examine this possibility—Nitzanim (5 ha, Figure 3A) and Galon (6 ha, Figure 3B) are filled with tertiary treated recycled nutrient-rich water, whereas Masu’ot Itzhak (8 ha, Figure 3C) is supplied with water collected from occasional winter floods in a nearby stream. Aerial photographs taken by drone clearly show a dense phytoplankton population in Nitzanim, while phytoplankton presence is barely visible in Galon and Masu’ot Itzhak. Routinely mitigation treatment applied in Galon Reservoir effectively suppressed the HCB that are typically observed in Nitzanim. This difference is further supported by the higher surface pH (Figure 2A) and DO concentrations (Figure 2B) in Nitzanim, likely resulting from intensive photosynthetic activity associated with the denser HCB population. Additionally, the steeper vertical decline in both pH and DO in Nitzanim suggests stronger light attenuation, and thus reduced oxygen production at depth, caused by the bloom’s density.
Measurements of methane flux (Figure 3D) revealed substantially higher CH4 emissions in Nitzanim compared to the other two reservoirs. It remains to be determined whether the lower emissions observed in Galon and Masu’ot Itzhak are primarily due to reduced CH4 production by cyanobacteria or to a lower availability of organic carbon in the sediments, which serves as a substrate for methanogenesis. Nevertheless, these preliminary findings are encouraging and underscore the need for further research to elucidate how local environmental factors shape the balance between methane production and consumption, ultimately influencing net emissions. Further, the emerging correlation between eutrophic conditions, bloom intensity, and methane release suggests that bloom-targeted mitigation strategies, such as those employed in Galon Reservoir, may offer an effective approach to reduce CH4 (and N2O?, see below) emissions from eutrophic waterbodies.
5. Nitrous Oxide in Freshwater Bodies
In addition to its detrimental effects on the ozone layer, nitrous oxide (N2O) is a potent GHG, with a global warming potential approximately 270 times greater than that of CO2. In recent years, freshwater lakes have been recognized as significant sources of atmospheric N2O emissions [22,140,141,142]. Cyanobacteria and microalgae have been implicated in N2O biosynthesis in aquatic environments [143,144,145,146].
The primary contributors to N2O production in waterbodies are microbial processes, particularly ammonia oxidation and denitrification. In oxic waters, ammonia-oxidizing Archaea and Bacteria release N2O as a byproduct during the oxidation of ammonia to nitrite. Under oxygen-depleted conditions, N2O also serves as a key intermediate in the denitrification pathway, in which nitrate and nitrite are sequentially reduced to molecular nitrogen (N2), often coupled with the oxidation of organic matter.
Phytoplankton blooms, and mitigation treatments thereof, can significantly influence N2O dynamics by altering the structure and extent of oxic-anoxic transition zones (Figure 2D), such as at the sediment-water interface or within stratified water columns. These zones are critical sites for microbial nitrogen transformations, and shifts in redox gradients can determine whether the dominant end-product of nitrogen metabolism is benign N2 or climate-active N2O.
Despite its environmental significance, mechanistic understanding of N2O synthesis and degradation in aquatic systems is poorly understood. Recent research is unfolding the complex interplay of biotic and abiotic pathways that govern N2O fluxes [147]. Gaining a deeper understanding of these mechanisms is essential for improving nitrogen cycle models in eutrophic waterbodies and developing strategies to mitigate N2O emissions from inland waters. Interestingly, recent studies have shown that sunlight can drive abiotic N2O production in both freshwater and marine systems. This occurs primarily through photochemical reduction of nitrate to nitrite, followed by subsequent reactions leading to N2O formation [148]. However, the precise mechanisms and global relevance of this pathway are still under investigation.
Of note, some methanotrophs are capable of anaerobic methane oxidation using nitrate or nitrite as electron acceptors, effectively reducing one GHG (CH4) while potentially producing another, far more harmful (N2O) through a synergistic metabolic process. As with methane, it remains to be experimentally determined whether mitigation treatments targeting algal blooms which can significantly reduce organic matter and alter redox dynamics, will also lead to measurable reductions in N2O emissions to the atmosphere, as expected.
6. Concluding Remarks
While mitigation strategies targeting HCBs hold considerable promise for reducing GHGs emissions, a more comprehensive understanding of their impacts, underlying mechanisms, and environmental constraints remains essential. Beyond the specific biogeochemical pathways involved, three overarching mechanisms appear to link HCB management to the mitigation of GHG emissions from lakes:
- 1.
- GHG reduction: Elevated cyanobacterial biomass is linked to excess GHG emissions; thus, effective bloom control that decreases overall blooms is likely to reduce these fluxes.
- 2.
- Biomass sinking and carbon burial: Some mitigation approaches promote rapid sinking of cyanobacterial biomass, enhancing burial of organic and inorganic carbon in sediments and effectively removing carbon from the atmosphere.
- 3.
- Community composition shift: bloom-forming cyanobacteria possess physiological traits that favor GHG production. Shifting community composition toward non-cyanobacterial dominance may reduce emissions, regardless of the specific mitigation technique applied.
As noted above, both organic and inorganic carbon sequestration, as well as CO2 and CH4 emissions, vary substantially among water bodies, while information on N2O emissions remains scarce. Ongoing research aims to study GHG fluxes under diverse environmental conditions to better quantify freshwater contributions to global warming and to identify key controlling factors. Seminal studies by Tan, Zhu, and Grasset and colleagues [149,150,151] have provided robust models for simulating CH4 and carbon dynamics, including temperature-dependent variations in their relative fluxes. These tools enable quantitative assessment of the environmental and biological factors shaping carbon cycling and will help refine mitigation strategies and guide the development of targeted, science-based interventions across aquatic ecosystems.
Finally, recent insights into the modification of methyl–coenzyme M reductase, the key enzyme in archaeal methanogenesis [152], provide a valuable framework linking environmental drivers to methanogen physiology and isotopic signatures of biogenic methane. In this framework, factors such as temperature, pressure, substrate availability, and isotopic gradients interact through partitioning of the available ΔG among enzymatic steps. These interactions collectively determine enzymatic reversibility, growth rates, and isotopic outcomes. It remains to be seen whether such mechanistic understanding, and potential manipulation of ΔG partitioning, can be harnessed to more effectively control GHG emissions under natural conditions.
Author Contributions
A.K. and M.H. conceived, designed and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Hebrew University of Jerusalem and BlueGreen Water Technologies.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Ami Nishri for many fruitful discussions, and Hans Paerl, Elke Dittmann, Boaz Lazar and Martin Hagemann for their valuable comments and suggestions on the draft. We are also grateful to Gad Weiss and Alon Goldman from BlueGreen Water Technologies (https://bluegreenwatertech.com/, accessed on 20 July 2025) for providing the resources and technical support that made the experiments presented in Figure 2 and Figure 3 possible.
Conflicts of Interest
Author Moshe Harel is CSO at BlueGreen Water Solutions Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, Y.; Hou, X.; Qin, B.; Kuster, T.; Qu, F.; Chen, N.; Paerl, H.W.; Zheng, C. Harmful algal blooms in inland waters. Nat. Rev. Earth Environm. 2024, 5, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Qin, B.; Zhang, Q.; Paerl, H.W.; Van Dam, B.; Jeppesen, E.; Zeng, C. Global lake phytoplankton proliferation intensifies climate warming. Nat. Commun. 2024, 15, 10572. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, J.; Chen, D.; Thiery, W.; Mercado-Bettín, D.; Xiong, L.; Xia, J.; Woolway, R.I. Enhanced heating effect of lakes under global warming. Nat. Commun. 2025, 16, 3954. [Google Scholar] [CrossRef]
- Greenwood, E.E.; Lauber, T.; van den Hoogen, J.; Donmez, A.; Bain, R.E.S.; Johnston, R.; Crowther, T.W.; Julian, T.R. Mapping safe drinking water use in low- and middle-income countries. Science 2024, 385, 784–790. [Google Scholar] [CrossRef]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Aquatic Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef]
- Gantar, M.; Berry, J.P.; Thomas, S.; Wang, M.; Perez, R.; Rein, K.S. Allelopathic activity among Cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol. Ecol. 2008, 64, 55–64. [Google Scholar] [CrossRef]
- Oberhaus, L.; Briand, J.-F.; Humbert, J.-F. Allelopathic growth inhibition by the toxic, bloom-forming cyanobacterium Planktothrix rubescens. FEMS Microbiol. Ecol. 2008, 66, 243–249. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Hu, J.; An, G.; Wang, C. Deciphering the joint intracellular and extracellular regulatory strategies of toxigenic Microcystis to achieve intraspecific competitive advantage: An integrated multi-omics analysis with novel allelochemicals identified. Water Res. 2025, 283, 123774. [Google Scholar] [CrossRef]
- Harel, M.; Weiss, G.; Lieman-Hurwitz, J.; Gun, J.; Lev, O.; Lebendiker, M.; Temper, V.; Block, C.; Sukenik, A.; Zohary, T.; et al. Interactions between Scenedesmus and Microcystis may be used to clarify the role of secondary metabolites. Environ. Microbiol. Rep. 2013, 5, 97–104. [Google Scholar] [CrossRef]
- Vardi, A.; Schatz, D.; Beeri, K.; Motro, U.; Sukenik, A.; Levine, A.; Kaplan, A. Dinoflagellate-cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr. Biol. 2002, 12, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Eshkol, R.; Livne, A.; Hadas, O.; Rom, M.; Tchernov, D.; Vardi, A.; Kaplan, A. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): A novel allelopathic mechanism. Limnol. Oceanogr. 2002, 47, 1656–1663. [Google Scholar] [CrossRef]
- Cai, P.; Wu, H.; Pan, M.; Cao, G.; Wu, X.; Tian, C.; Wang, C.; Xiao, B. Eutrophication amplifies Microcystis response to increasing winter temperatures, intensifying winter blooms. J. Appl. Phycol. 2025, 1–13. [Google Scholar] [CrossRef]
- Cai, P.; Cai, Q.; He, F.; Huang, Y.; Tian, C.; Wu, X.; Wang, C.; Xiao, B. Flexibility of Microcystis Overwintering Strategy in Response to Winter Temperatures. Microorganisms 2021, 9, 2278. [Google Scholar] [CrossRef]
- Brunberg, A.-K.; Blomqvist, P. Benthic overwintering of Microcystis colonies under different environmental conditions. J. Plank. Res. 2002, 24, 1247–1252. [Google Scholar] [CrossRef]
- Reinl, K.L.; Harris, T.D.; North, R.L.; Almela, P.; Berger, S.A.; Bizic, M.; Burnet, S.H.; Grossart, H.-P.; Ibelings, B.W.; Jakobsson, E.; et al. Blooms also like it cold. Limnol. Oceanogr. Lett. 2023, 8, 546–564. [Google Scholar] [CrossRef]
- Bressac, M.; Laurenceau-Cornec, E.C.; Kennedy, F.; Santoro, A.E.; Paul, N.L.; Briggs, N.; Carvalho, F.; Boyd, P.W. Decoding drivers of carbon flux attenuation in the oceanic biological pump. Nature 2024, 633, 587–593. [Google Scholar] [CrossRef]
- Jiao, N.Z.; Luo, T.W.; Chen, Q.R.; Zhao, Z.; Xiao, X.L.; Liu, J.H.; Jian, Z.M.; Xie, S.C.; Thomas, H.; Herndl, G.J.; et al. The microbial carbon pump and climate change. Nat. Rev. Microbiol. 2024, 22, 408–419. [Google Scholar] [CrossRef]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Hogberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef]
- DelSontro, T.; Beaulieu, J.J.; Downing, J.A. Greenhouse gas emissions from lakes and impoundments: Upscaling in the face of global change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nature Commun. 2019, 10, 1375. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Waldo, S.; Balz, D.A.; Barnett, W.; Hall, A.; Platz, M.C.; White, K.M. Methane and carbon dioxide emissions from reservoirs: Controls and upscaling. J. Geophys. Res. Biogeos. 2020, 125, e2019JG005474. [Google Scholar] [CrossRef]
- Sakaguchi, J.; Nakayama, K.; Komai, K.; Kubo, A.; Shimizu, T.; Omori, J.; Uno, K.; Fujii, T. Carbon dioxide uptake in a eutrophic stratified reservoir: Freshwater carbon sequestration potential. Heliyon 2023, 9, e20322. [Google Scholar] [CrossRef]
- Sobek, S.; Durisch-Kaiser, E.; Zurbrügg, R.; Wongfun, N.; Wessels, M.; Pasche, N.; Wehrli, B. Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source. Limnol. Oceanogr. 2009, 54, 2243–2254. [Google Scholar] [CrossRef]
- Wang, S.; Nie, X.; Li, Z.; Ran, F.; Yang, C.; Xiao, T. Quantification of sedimentary organic carbon sources in a landeriverelake continuum combined with multi-fingerprint and un-mixing models. Intnl. J. Sedim. Res. 2024, 39, 230–242. [Google Scholar] [CrossRef]
- Du, Z.; Wang, L.; Xie, S.; Yang, J.; Yan, F.; Li, C.; Ding, M.; Zhang, Y.; Ding, X.; Xiao, C. Carbon dioxide and methane fluxes in different waterbodies in Inexpressible Island, Ross Sea, East Antarctica. Mar. Pollut. Bull. 2025, 213, 117703. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Weiss, G.; Daniel, E.; Wilenz, A.; Hadas, O.; Sukenik, A.; Sedmak, B.; Dittmann, E.; Braun, S.; Kaplan, A. Casting a net: Fibres produced by Microcystis sp. in field and laboratory populations. Environ. Microbiol. Rep. 2012, 4, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Franco-Morgado, M.; Amador-Espejo, G.G.; Pérez-Cortés, M.; Gutiérrez-Uribe, J.A. Microalgae and cyanobacteria polysaccharides: Important link for nutrient recycling and revalorization of agro-industrial wastewater. Appl. Food Res. 2023, 3, 100296. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, F.; Wang, H.; Shen, C.; Yu, K. Meta-analysis of abiotic conditions affecting exopolysaccharide production in cyanobacteria. Metabolites 2025, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Peaudecerf, F.J.; Fernandez, V.I.; Behrendt, L.; Lee, K.S.; Stocker, R. Sinking enhances the degradation of organic particles by marine bacteria. Nat. Geosci. 2021, 14, 775–780. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, S.; Wang, M. Biomimetic mineralization for carbon capture and sequestration. Carbon Capt. Sci. Technol. 2024, 13, 100257. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, P.; Zhu, W.; Jing, C.; He, J.; Yang, X.; Zhou, L.; Zheng, Z. Effects of cyanobacterial accumulation and decomposition on the microenvironment in water and sediment. J. Soils Sedim. 2020, 20, 2510–2525. [Google Scholar] [CrossRef]
- Pérez, G.; Krause, S.M.B.; Bodelier, P.L.E.; Meima-Franke, M.; Pitombo, L.; Irisarri, P. Interactions between cyanobacteria and methane processing microbes mitigate methane emissions from rice soils. Microorganisms 2023, 11, 2830. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, M.; Song, L.; Chen, X.; Zhang, Z.; Gao, N. Characteristics of extracellular organic matters and the formation potential of disinfection by-products during the growth phases of M. aeruginosa and Synedra sp. Environ. Sci. Poll. Res. 2022, 29, 14509–14521. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, H.; Yu, J.; Su, Y.; Liu, Z. Geochemical characteristics of n-alkanes in sediments from oligotrophic and eutrophic phases of five lakes and potential use as paleoenvironmental proxies. CATENA 2023, 220, 106682. [Google Scholar] [CrossRef]
- Sobek, S.; Gudasz, C.; Koehler, B.; Tranvik, L.J.; Bastviken, D.; Morales-Pineda, M. Temperature dependence of apparent respiratory quotients and oxygen penetration depth in contrasting lake sediments. Geophys. Res. Biogeosci. 2017, 122, 3076–3087. [Google Scholar] [CrossRef]
- Pavia, F.J.; Anderson, R.F.; Lam, P.J.; Cael, B.B.; Vivancos, S.M.; Fleisher, M.Q.; Lu, Y.; Zhang, P.; Cheng, H.; Edwards, R.L. Shallow particulate organic carbon regeneration in the South Pacific Ocean. Proc. Natl. Acad. Sci. USA 2019, 116, 9753–9758. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Knauer, G.A.; Karl, D.M.; Broenkow, W.W. VERTEX: Carbon cycling in the Northeast Pacific. Deep Sea Res. 1987, 34, 267–285. [Google Scholar] [CrossRef]
- Dunne, J.P.; Sarmiento, J.L.; Gnanadesikan, A. A synthesis of global particle export from the surface ocean and cycling through the ocean interior and on the seafloor. Glob. Biogeochem. Cycl. 2007, 21, GB4006. [Google Scholar] [CrossRef]
- Anderson, N.J.; Heathcote, A.J.; Engstrom, D.R.; Globocarb Data Contributors. Anthropogenic alteration of nutrient supply increases the global freshwater carbon sink. Sci. Adv. 2020, 6, eaaw2145. [Google Scholar] [CrossRef]
- Mendonça, R.; Kosten, S.; Sobek, S.; Cardoso, S.J.; Figueiredo-Barros4, M.P.; Estrada, C.H.D.; Roland, F. Organic carbon burial efficiency in a large tropical hydroelectric reservoir. Biogeosci. Dis. 2015, 12, 18513–18540. [Google Scholar] [CrossRef]
- Clow, D.W.; Stackpoole, S.M.; Verdin, K.L.; Butman, D.E.; Zhu, Z.; Krabbenhoft, D.P.; Striegl, R.G. Organic carbon burial in lakes and reservoirs of the conterminous United States. Environ. Sci. Technol. 2015, 49, 7614–7622. [Google Scholar] [CrossRef]
- Kallistova, A.Y.; Kosyakova, A.I.; Rusanov, I.I.; Kadnikov, V.V.; Beletsky, A.V.; Koval, D.D.; Yusupov, S.K.; Zekker, I.; Pimenov, N.V. Methane production in a temperate freshwater lake during an Intense cyanobacterial bloom. Microbiology 2023, 92, 638–649. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Y.; Zhu, G.; Gao, G. Eutrophication control of large shallow lakes in China. Sci. Total Environ. 2023, 881, 163494. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, S.; Zhao, D.; Hu, C.; Xu, W.; Anderson, D.M.; Li, Y.; Song, X.P.; Boyce, D.G.; Gibson, L.; et al. Coastal phytoplankton blooms expand and intensify in the 21st century. Nature 2023, 615, 280–284. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef]
- Phlips, E.J.; Badylak, S.; Milbrandt, E.C.; Stelling, B.; Arias, M.; Armstrong, C.; Behlmer, T.; Chappel, A.; Foss, A.; Kaplan, D.; et al. Fate of a toxic Microcystis aeruginosa bloom introduced into a subtropical estuary from a flow-managed canal and management implications. J. Environm. Manag. 2025, 375, 124362. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.J.; MacIntyre, H.L.; Kana, T.M. Dynamic model of phytoplankton growth and acclimation: Responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 1997, 148, 187–200. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0521605199. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M.; Ma, S.; Su, J.; He, K.; Wang, H.; Zhang, S. From cyanobacteria to kerogen: A model of organic carbon burial. Precam. Res. 2023, 390, 107145–107159. [Google Scholar] [CrossRef]
- Dynarski, K.A.; Bossio, D.A.; Scow, K.M. Dynamic Stability of Soil Carbon: Reassessing the “Permanence” of Soil Carbon Sequestration. Front. Environ. Sci. 2020, 8, 514701. [Google Scholar] [CrossRef]
- Nakagawa, T.; Gotanda, K.; Haraguchi, T.; Danhara, T.; Yonenobu, H.; Brauer, A.; Yokoyama, Y.; Tada, R.; Takemura, K.; Staff, R.A.; et al. SG06, a fully continuous and varved sediment core from Lake Suigetsu, Japan: Stratigraphy and potential for improving the radiocarbon calibration model and understanding of late Quaternary climate changes. Quat. Sci. Rev. 2012, 36, 164–176. [Google Scholar] [CrossRef]
- Hassan, S.; Bali, B.m.S.; Muneer, W.; Ali, S.N.; Morthekai, P.; Wani, A.H.; Sabreena; Ganai, B.A. Deciphering source, degradation status and temporal trends of organic matter in a himalayan freshwater lake using multiproxy indicators, optically stimulated luminescence dating and time series forecasting. Sci. Total Environ. 2024, 957, 177618. [Google Scholar] [CrossRef]
- Zastepa, A.; Taranu, Z.E.; Kimpe, L.E.; Blais, J.M.; Gregory-Eaves, I.; Zurawell, R.W.; Pick, F.R. Reconstructing a long-term record of microcystins from the analysis of lake sediments. Sci. Total Environ. 2017, 579, 893–901. [Google Scholar] [CrossRef]
- Matsumoto, G.I.; Tani, Y.; Seto, K.; Tazawa, T.; Yamamuro, M.; Watanabe, T.; Nakamura, T.; Takemura, T.; Imura, S.; Kanda, H. Holocene paleolimnological changes in Lake Skallen Oike in the Syowa Station area of Antarctica inferred from organic components in a sediment core (Sk4C-02). J. Paleolimnol. 2010, 44, 677–693. [Google Scholar] [CrossRef]
- Engstrom, D.R.; Schottler, S.P.; Leavitt, P.R.; Havens, K.E. A reevaluation of the cultural eutrophication of Lake Okeechobee using multiproxy sediment records. Ecol. Appl. 2006, 16, 1194–1206. [Google Scholar] [CrossRef]
- Lee, S.; Block, B.; Jessup, B.; Salk, K.P. Estimates of Sediment Accumulation Rates and Bottom Core ages in Northeast Lakes, EPA/600/R-23/015; U.S. Environmental Protection Agency: Washington, DC, USA, 2024. [Google Scholar]
- Joarder, M.S.A.; Islam, M.S.; Hasan, M.H.; Sadman- Anjum, J.; Kabir, M.F.; Rashid, F.; Joarder, T.A. A comprehensive review of carbon dioxide capture, transportation, utilization, and storage: A source of future energy. Environ. Sci. Pollut. Res. 2025, 32, 9299–9332. [Google Scholar] [CrossRef]
- Zhou, T.R.; Cao, H.S.; Zheng, J.; Teng, F.; Wang, X.J.; Lou, K.; Zhang, X.H.; Tao, Y. Suppression of water-bloom cyanobacterium Microcystis aeruginosa by algaecide hydrogen peroxide maximized through programmed cell death. J. Hazard. Mat. 2020, 393, 122394. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Yu, S.M.; Hu, J.; Effiong, K.; Ge, Z.W.; Tang, T.; Xiao, X. Programmed cell death process in freshwater Microcystis aeruginosa and marine Phaeocystis globosa induced by a plant derived allelochemical. Sci. Total Environ. 2022, 838, 156055. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Li, J.; Li, T.; Sha, J.; Liu, J.; Li, L.; Dai, G.; Jia, Y.; Song, L. Unveiling the susceptibility mechanism of Microcystis to consecutive sub-lethal oxidative stress—Enhancing oxidation technology for cyanobacterial bloom control. J. Hazard. Mater. 2024, 480, 135993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zaman, F.; Jia, Y.; Huang, Y.; Li, T.; Bai, F.; Li, L.; Song, L.; Li, J. Harmful Cyanobacterial bloom control with hydrogen peroxide: Mechanism, affecting factors, development, and prospects. Curr. Poll. Rep. 2024, 10, 566–579. [Google Scholar] [CrossRef]
- Kinley-Baird, C.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar] [CrossRef]
- Sandrini, G.; Piel, T.; Xu, T.S.; White, E.; Qin, H.J.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harm. Algae 2020, 99, 101916. [Google Scholar] [CrossRef]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2003, 54, 609–622. [Google Scholar] [CrossRef]
- Burnap, R.L.; Hagemann, M.; Kaplan, A. Regulation of the CO2 concentrating mechanism in cyanobacteria. Life 2015, 5, 348–371. [Google Scholar] [CrossRef]
- Kaplan, A.; Reinhold, L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Biol. 1999, 50, 539–570. [Google Scholar] [CrossRef]
- Raven, J.A. Inorganic carbon concentrating mechanisms in relation to the biology of algae. Photosynth. Res. 2003, 77, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Lucius, S.; Hagemann, M. The primary carbon metabolism in cyanobacteria and its regulation. Front. Plant Sci. 2024, 15, 1417680. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; Matthijs, H.C.P.; Verspagen, J.M.H.; Muyzer, G.; Huisman, J. Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J. 2014, 8, 589–600. [Google Scholar] [CrossRef]
- Song, Y.F.; Qiu, B.S. The CO2-concentrating mechanism in the bloom-forming cyanobacterium Microcystis aeruginosa (Cyanophyceae) and effects of UVB radiation on its operation. J. Phycol. 2007, 43, 957–964. [Google Scholar] [CrossRef]
- Barchewitz, T.; Guljamow, A.; Meissner, S.; Timm, S.; Henneberg, M.; Baumann, O.; Hagemann, M.; Dittmann, E. Non-canonical localization of RubisCO under high-light conditions in the toxic cyanobacterium Microcystis aeruginosa PCC7806. Environ. Microbiol. 2019, 21, 4836–4851. [Google Scholar] [CrossRef]
- Guljamow, A.; Barchewitz, T.; Grosse, R.; Timm, S.; Hagemann, M.; Dittmann, E. Diel Variations of Extracellular Microcystin Influence the Subcellular Dynamics of RubisCO in Microcystis aeruginosa PCC 7806. Microorganisms 2021, 9, 1265. [Google Scholar] [CrossRef]
- Roy, S.; Guljamow, A.; Dittmann, E. Impact of temperature on the temporal dynamics of microcystin in Microcystis aeruginosa PCC7806. Front. Microbiol. 2023, 14, 1200816. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Borner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Boerner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Teikari, J.E.; Russo, D.A.; Heuser, M.; Baumann, O.; Zedler, J.A.Z.; Liaimer, A.; Dittmann, E. Competition and interdependence define interactions of Nostoc sp. and Agrobacterium sp. under inorganic carbon limitation. NPJ Biofilms Microbiom. 2025, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Distribution and functions of calcium mineral deposits in photosynthetic organisms. In Progress in Botany; Lüttge, U., Cánovas, F.M., Risueño, M.-C., Leuschner, C., Pretzsch, H., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 84, pp. 293–326. [Google Scholar] [CrossRef]
- Taylor, A.R.; Brownlee, C.; Wheeler, G. Coccolithophore cell biology: Chalking up progress. Annu. Rev. Mar. Sci. 2017, 9, 283–310. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, C.; Wang, Y. Unlocking the potential of microbially induced calcium carbonate precipitation (MICP) for hydrological applications: A beview of opportunities, challenges, and environmental considerations. Hydrology 2023, 10, 178. [Google Scholar] [CrossRef]
- Gaëtan, J.; Halary, S.; Millet, M.; Bernard, C.; Duval, C.; Hamlaoui, S.; Hecquet, A.; Gugger, M.; Marie, B.; Mehta, N.; et al. Widespread formation of intracellular calcium carbonates by the bloom-forming cyanobacterium Microcystis. Environ. Microbiol. 2023, 25, 751–765. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhao, L.; Li, G.K.; Zhu, C.; Xu, D.; Ji, J.F.; Chen, J. Assessment and selection of cyanobacterial strains for CO2 mineral sequestration: Implications for carbonation mechanism. Geomicrobiol. J. 2023, 40, 446–461. [Google Scholar] [CrossRef]
- Khan, H.; Marcé, R.; Laas, A.; Obrador, B. The relevance of pelagic calcification in the global carbon budget of lakes and reservoirs. Limnetica 2022, 41. [Google Scholar] [CrossRef]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef]
- Bruley, A.; Gaëtan, J.; Gugger, M.; Pancrace, C.; Millet, M.; Gaschignard, G.; Dezi, M.; Humbert, J.-F.; Leloup, J.; Skouri-Panet, F.; et al. Diel changes in the expression of a marker 1 gene and candidate genes for intracellular 2 amorphous CaCO3 biomineralization in 3 Microcystis. Peer Comm. J. 2024, 5, e18. [Google Scholar] [CrossRef]
- Mehta, N.; Gaëtan, J.; Giura, P.; Azaïs, T.; Benzerara, K. Detection of biogenic amorphous calcium carbonate (ACC) formed by bacteria using FTIR spectroscopy. Spect. Acta Part A Mol. Biomol. Spect. 2022, 278. [Google Scholar] [CrossRef]
- Fruchter, N.; Lazar, B.; Nishri, A.; Almogi-Labin, A.; Eisenhauer, A.; Be’eri Shlevin, Y.; Stein, M. 88Sr/86Sr fractionation and calcite accumulation rate in the Sea of Galilee. Geochim. Cosmochim. Acta 2017, 215, 17–32. [Google Scholar] [CrossRef]
- Escoffier, N.; Perolo, P.; Many, G.; Pasche, N.T.; Perga, M.-E. Fine-scale dynamics of calcite precipitation in a large hardwater lake. Sci. Total Environ. 2023, 864, 160699. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Jongsma, R.; Bakenhus, I.; Möckel, R.; Philipp, B.; Neu, T.R.; Wendt-Potthoff, K. Interaction of cyanobacteria with calcium facilitates the sedimentation of microplastics in a eutrophic reservoir. Water Res. 2021, 189, 116582. [Google Scholar] [CrossRef] [PubMed]
- Many, G.; Escoffier, N.; Perolo, P.; Bärenbold, F.; Bouffard, D.; Perga, M.-E. Calcite precipitation: The forgotten piece of lakes’ carbon cycle. Sci. Adv. 2024, 10, eado5924. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.E.; Eadie, B.J. Satellite observations of calcium carbonate precipitations in the Great Lakes. Limnol. Oceanogr. 1978, 23, 877–887. [Google Scholar] [CrossRef]
- Küchler-Krischun, J.; Kleiner, J. Heterogeneously nucleated calcite precipitation in Lake Constance. A short time resolution study. Aquatic Sci. 1990, 52, 176–197. [Google Scholar] [CrossRef]
- Jiang, H.B.; Cheng, H.M.; Gao, K.S.; Qiu, B.S. Inactivation of Ca2+/H+ exchanger in Synechocystis sp. strain PCC 6803 promotes cyanobacterial calcification by upregulating CO2-concentrating mechanisms. Appl. Environ. Microbiol. 2013, 79, 4048–4055. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Burnap, R.L. Action at a distance: The remarkable coupling of CO2 uptake to electron transfer in specialized cyanobacterial NDH-1 complexes. Proc. Natl. Acad. Sci. USA 2025, 122, e2511786122. [Google Scholar] [CrossRef]
- Tchernov, D.; Hassidim, M.; Luz, B.; Sukenik, A.; Reinhold, L.; Kaplan, A. Sustained net CO2 evolution during photosynthesis by marine microorganisms. Curr. Biol. 1997, 7, 723–728. [Google Scholar] [CrossRef]
- McGowan, H.; Lensky, N.; Abir, S.; Shaked, Y.; Wurgaft, E. Direct measurement of CO2 air-sea exchange over a desert Fringing coral rReef, Gulf of Eilat (Aqaba), Israel. J. Geophys. Res. Oceans 2022, 127, e2022JC018548. [Google Scholar] [CrossRef]
- Dean, W.E. The carbon cycle and biogeochemical dynamics in lake sediments. J. Paleolimnol. 1999, 21, 375–393. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Gann, E.R.; Martin, R.M.; Pound, H.L.; Krausfeldt, L.E.; Chaffin, J.D.; Wilhelm, S.W. Elevated pH Conditions Associated with Microcystis spp. Blooms Decrease Viability of the Cultured Diatom Fragilaria crotonensis and Natural Diatoms in Lake Erie. Front. Microbiol. 2021, 13, 837198. [Google Scholar] [CrossRef]
- Nishri, A.; Stiller, M.; Rimmer, A.; Geifman, Y.; Krom, M. Lake Kinneret (The Sea of Galilee): The effects of diversion of external salinity sources and the probable chemical composition of the internal salinity sources. Chem. Geol. 1999, 158, 37–52. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Q.; Gu, X.; Zhang, L.; Han, C.; Wang, Z. Burial or mineralization: Origins and fates of organic matter in the water–suspended particulate matter–sediment of macrophyte- and algae-dominated areas in Lake Taihu. Water Res. 2023, 243, 120414. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.R. Theoretical and experimental aspects of isotopic fractionation. Rev. Mineral. 1986, 16, 1–40. [Google Scholar]
- Katz, A.; Nishri, A. Calcium, magnesium and strontium cycling in stratified, hardwater lakes: Lake Kinneret (Sea of Galilee), Israel. Geochim. Cosmochim. Acta 2013, 105, 372–394. [Google Scholar] [CrossRef]
- Xu, X.; Wu, C.; Xie, D.; Ma, J. Sources, Migration, Transformation, and Environmental Effects of Organic Carbon in Eutrophic Lakes: A Critical Review. Int. J. Environ. Res. Public Health 2023, 20, 860. [Google Scholar] [CrossRef]
- Zhou, C.; Penga, Y.; Zhou, M.; Jiaa, R.; Bud, H.; Xua, X.; Chen, L.; Mag, J.; Kinouchi, B.; Wang, G. Cyanobacteria decay alters CH4 and CO2 produced hotspots along vertical sediment profiles in eutrophic lakes. Water Res. 2024, 265, 122319. [Google Scholar]
- Hartmann, I.F.; Günthel, M.; Klintzsch, T.; Kirillin, G.; Grossart, H.-P.; Keppler, F.; Isenbeck-Schröter, M. High Spatiotemporal Dynamics of Methane Production and Emission in Oxic Surface Water. Environ. Sci. Technol. 2020, 54, 1451–1463. [Google Scholar] [CrossRef]
- Rocher-Ros, G.; Stanley, E.; Loken, L.; Raymond, P.; Liu, S.; Sponseller, R. Global methane emissions from rivers and streams. Nature 2023, 621, 530–535. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Holgerson, M.A.; Raymond, P.A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016, 9, 222–226. [Google Scholar] [CrossRef]
- Townsend-Small, A.; Disbennett, D.; Fernandez, J.M.; Ransohoff, R.W.; Mackay, R.; Bourbonniere, R.A. Quantifying emissions of methane derived from anaerobic organic matter respiration and natural gas extraction in Lake Erie. Limnol. Oceanogr. 2016, 61, S356–S366. [Google Scholar] [CrossRef]
- Kh-Hriiziirou, M.; Khoiyangbam, R.S. Strategies for CH4 Emission Reduction from Lakes: Understanding the Key Driving Factors for CH4 Production and Lake Restoration. Int. J. Lakes Rivers 2024, 17, 109–124. [Google Scholar] [CrossRef]
- Lew, S.; Burandt, P.; Glińska-Lewczuk, K. Microbial Communities Drive Methane Fluxes From Floodplain Lakes—A Hydrological Gradient Perspective. Environ. Microbiol. 2025, 27, e70127. [Google Scholar] [CrossRef] [PubMed]
- Hounshell, A.G.; D’Acunha, B.M.; Breef-Pilz, A.; Johnson, M.S.; Thomas, R.Q.; Carey, C.C. Eddy covariance data reveal that a small freshwater reservoir emits a substantial amount of carbon dioxide and methane. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007091. [Google Scholar] [CrossRef]
- Waldo, S.; Beaulieu, J.J.; Barnett, S.W.; Balz, D.A.; Vanni, M.J.; Williamson, T.; Walker, J.T. Temporal trends in methane emissions from a small eutrophic reservoir: The key role of a spring burst. Biogeosciences 2021, 18, 5291–5311. [Google Scholar] [CrossRef]
- Jentzsch, K.; van Delden, L.; Fuchs, M.; Treat, C.C. An expert survey on chamber measurement techniques for methane fluxes. Earth Syst. Sci. Data Discuss. 2024, 2024, 1–59. [Google Scholar] [CrossRef]
- Whiticar, M. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. J. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Cerbin, S.; Pérez, G.; Rybak, M.; Wejnerowski, Ł.; Konowalczyk, A.; Helmsing, N.; Naus-Wiezer, S.; Meima-Franke, M.; Pytlak, Ł.; Raaijmakers, C.; et al. Methane-derived carbon as a driver for cyanobacterial growth. Front. Microbiol. 2022, 13, 837198. [Google Scholar] [CrossRef]
- Schroll, M.; Liu, L.; Einzmann, T.; Keppler, F.; Grossart, H.-P. Methane accumulation and its potential precursor compounds in the oxic surface water layer of two contrasting stratified lakes. Sci. Total Environ. 2023, 903, 166205. [Google Scholar] [CrossRef]
- Bižić, M.; Klintzsch, T.; Ionescu, D.; Hindiyeh, M.Y.; Günthel, M.; Muro-Pastor, M.A.; Eckert, W.; Urich, T.; Keppler, F.; Grossart, H.-P. Aquatic and terrestrial cyanobacteria produce methane. Sci. Adv. 2020, 6, eaax5343. [Google Scholar] [CrossRef]
- Hu, S.X.; Zhou, B.; Wang, Y.; Wang, Y.; Zhang, X.X.; Zhao, Y.; Zhao, X.Y.; Tang, X.X. Effect of CO2-induced seawater acidification on growth, photosynthesis and inorganic carbon acquisition of the harmful bloom-forming marine microalga, Karenia mikimotoi. PLoS ONE 2017, 12, e0183289. [Google Scholar] [CrossRef] [PubMed]
- Klintzsch, T.; Geisinger, H.; Wieland, A.; Langer, G.; Nehrke, G.; Bizic, M.; Greule, M.; Lenhart, K.; Borsch, C.; Schroll, M.; et al. Stable carbon isotope signature of methane released from phytoplankton. Geophys. Res. Lett. 2023, 50, e2023GL103317. [Google Scholar] [CrossRef]
- Bizic, M. Phytoplankton photosynthesis: An unexplored source of biogenic methane emission from oxic environments. J. Plankton Res. 2021, 43, 822–830. [Google Scholar] [CrossRef]
- Cheng, X.X.; Lian, J.C.; Liu, B.; Zhu, X.W.; Jin, Y.; Zhang, L.J.; Tan, F.X.; Wu, D.J.; Liang, H. Integrated ferrate and calcium sulfite to treat algae-laden water for controlling ultrafiltration membrane fouling: High-efficiency oxidation and simultaneous cell integrity maintaining. Chem. Engin. J. 2023, 461, 141880. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Hart, L.N.; Chase, E.E.; Natwora, K.E.; Obuya, J.A.; Olokotum, M.; Houghton, K.A.; Kiledal, E.A.; Achieng, D.; Barker, K.B.; et al. Molecular investigation of harmful cyanobacteria reveals hidden risks and niche partitioning in Kenyan Lakes. Harmful Algae 2024, 140, 102757. [Google Scholar] [CrossRef]
- Rao, Y.; Gao, G.; Berman-Frank, I.; Bizic, M.; Gao, K. Light-dependent methane production by a coccolithophorid may counteract its photosynthetic contribution to carbon dioxide sequestration. Commun. Earth Environ. 2024, 5, 695. [Google Scholar] [CrossRef]
- Khatun, S.; Iwata, T.; Kojima, H.; Fukui, M.; Aoki, T.; Mochizuki, S.; Naito, A.; Kobayashi, A.; Uzawa, R. Aerobic methane production by planktonic microbes in lakes. Sci. Total Environ. 2019, 696. [Google Scholar] [CrossRef]
- Fazi, S.; Amalfitano, S.; Venturi, S.; Pacini, N.; Vazquez, E.; Olaka, L.A.; Tassi, F.; Crognale, S.; Herzsprung, P.; Lechtenfeld, O.J.; et al. High concentrations of dissolved biogenic methane associated with cyanobacterial blooms in East African lake surface water. Commun. Biol. 2021, 4, 845. [Google Scholar] [CrossRef]
- Kamat, S.S.; Williams, H.J.; Dangott, L.J.; Chakrabarti, M.; Raushel, F.M. The catalytic mechanism for aerobic formation of methane by bacteria. Nature 2013, 497, 132–136. [Google Scholar] [CrossRef]
- Repeta, D.J.; Ferrón, S.; Sosa, O.A.; Johnson, C.G.; Repeta, L.D.; Acker, M.; DeLong, E.F.; Karl, D.M. Marine methane paradox explained by bacterial degradation of dissolved organic matter. Nat. Geosci. 2016, 9, 884–887. [Google Scholar] [CrossRef]
- Xu, H.L.; Li, H.; Tang, Z.Z.; Liu, Y.; Li, G.; He, Q. Underestimated methane production triggered by phytoplankton succession in river-reservoir systems: Evidence from a microcosm study. Water Res. 2020, 185, 116233. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, L.-Z.; Chen, M.-Y.; Teng, W.-K.; Zheng, L.-L.; Peng, L.; Lv, J.; Brand, J.J.; Hu, C.-X.; Han, B.-P.; et al. The widespread capability of methylphosphonate utilization in filamentous cyanobacteria and its ecological significance. Water Res. 2022, 217, 118385. [Google Scholar] [CrossRef]
- Dummer, N.F.; Willock, D.J.; He, Q.; Howard, M.J.; Lewis, R.J.; Qi, G.; Taylor, S.H.; Xu, J.; Bethell, D.; Kiely, C.J.; et al. Methane oxidation to methanol. Chem. Rev. 2023, 123, 6359–6411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Peng, Y.; Yua, M.; Deng, Y.; Chen, L.; Zhang, L.; Xua, X.; Zhang, S.; Yanb, Y.; Wang, G. Severe cyanobacteria accumulation potentially induces methylotrophic methane producing pathway in eutrophic lakes. Environ. Poll. 2022, 292, 118443. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, X.; Wang, D.; Yao, E.; Li, Y.; Huang, W.; Zhang, L.; Wang, J.; Zhong, J. Significant diurnal variations in nitrous oxide (N2O) emissions from two contrasting habitats in a large eutrophic lake (Lake Taihu, China). Environ. Res. 2024, 261, 119691. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Townsend-Small, A.; Zastepa, A.; Watson, S.B.; Brandes, J.A. Methane and nitrous oxide measured throughout Lake Erie over all seasons indicate highest emissions from the eutrophic Western Basin. J. Great Lakes Res. 2020, 46, 1604–1614. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Chen, B.; Zhang, Q.; Xu, N.; Paerl, H.W.; Wang, T.; Hong, W.; Penuelas, J.; Qian, H. Potential health risk assessment of cyanobacteria across global lakes. Appl. Environ. Microbiol. 2024, 90, e01936-24. [Google Scholar] [CrossRef]
- Weathers, P.J.; Niedzielski, J.J. Nitrous oxide production by cyanobacteria. Arch. Microbiol. 1986, 146, 204–206. [Google Scholar] [CrossRef]
- Fabisik, F.; Guieysse, B.; Procter, J.; Plouviez, M. Nitrous oxide (N2O) synthesis by the freshwater cyanobacterium Microcystis aeruginosa. Biogeosciences 2023, 20, 687–693. [Google Scholar] [CrossRef]
- Cai, S.; Lao, Q.; Chen, C.; Zhu, Q.; Chen, F. The impact of algal blooms on promoting in-situ N2O emissions: A case in Zhanjiang bay, China. J. Environ. Manag. 2024, 358, 120935. [Google Scholar] [CrossRef]
- Plouviez, M.; Shilton, A.; Packer, M.A.; Guieysse, B. Nitrous oxide emissions from microalgae: Potential pathways and significance. J. Appl. Phycol. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Roothans, N.; Pabst, M.; van Diemen, M.; Herrera Mexicano, C.; Zandvoort, M.; Abeel, T.; van Loosdrecht, M.C.M.; Laureni, M. Long-term multi-meta-omics resolves the ecophysiological controls of seasonal N2O emissions during wastewater treatment. Nat. Water 2025, 3, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Leon-Palmero, E.; Morales-Baquero, R.; Thamdrup, B.; Löscher, C.; Reche, I. Sunlight drives the abiotic formation of nitrous oxide in fresh and marine waters. Science 2025, 387, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yao, H.; Melack, J.; Grossart, H.-P.; Jansen, J.; Balathandayuthabani, S.; Sargsyan, K.; Leung, L.R. A lake biogeochemistry model for global methane emissions: Model development, site-level validation, and global applicability. J. Adv. Model. Earth Syst. 2024, 16, e2024MS004275. [Google Scholar] [CrossRef]
- Zhu, Y.; Purdy, K.J.; Martínez Rodríguez, A.; Trimmer, M. A rationale for higher ratios of CH4 to CO2 production in warmer anoxic freshwater sediments and soils. Limnol. Oceanograph. Lett. 2023, 8, 398–405. [Google Scholar] [CrossRef]
- Grasset, C.; Mesman, J.P.; Tranvik, L.J.; Maranger, R.; Sobek, S. Contribution of lake littoral zones to the continental carbon budget. Nature Geosci. 2025, 18, 747–752. [Google Scholar] [CrossRef]
- Gropp, J.; Bill, M.; Lloyd, M.K.; Stein, R.A.; Nayak, D.D.; Stolper, D.A. Modulation of methyl–coenzyme M reductase expression alters the isotopic composition of microbial methane. Science 2025, 389, 711–715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).