Effectiveness of High-Solid Loading Treatments to Enhance Nutrient and Antioxidant Bioavailability in Codium tomentosum
Abstract
1. Introduction
2. Materials and Methods
2.1. High-Solids Loading Water and Alkaline Autoclave Treatment
2.2. Enzymatic Hydrolysis Following High-Solids Water and Alkaline Autoclave Pretreatment
2.3. Proximate Composition
2.4. Scanning Electron Microscopy
2.5. Data Analysis
3. Results
3.1. Effects of High-Solids Loading Water and Alkaline Autoclave Hydrolysis Treatment
3.2. Effects of Enzymatic Hydrolysis Following High-Solids Water and Alkaline Autoclave Pretreatment
3.3. Microscopic Structural Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaOH | Sodium hydroxide |
| NAT | Natugrain® TS-Feed Enzyme from BASF |
| H2O | Water-autoclaved |
| Alk | Alkaline autoclaved |
| H2OEnz | Water-enzymatic autoclaved |
| AlkEnz | Alkaline-enzymatic autoclaved |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
References
- Arzeno-Soltero, I.B.; Saenz, B.T.; Frieder, C.A.; Long, M.C.; DeAngelo, J.; Davis, S.J.; Davis, K.A. Large global variations in the carbon dioxide removal potential of seaweed farming due to biophysical constraints. Commun. Earth Environ. 2023, 4, 185. [Google Scholar] [CrossRef]
- Wu, J.; Keller, D.P.; Oschlies, A. Carbon dioxide removal via macroalgae open-ocean mariculture and sinking: An Earth system modeling study. Earth Syst. Dyn. 2023, 14, 185–221. [Google Scholar] [CrossRef]
- Fernandes, H.; Martins, N.; Vieira, L.; Salgado, J.M.; Castro, C.; Oliva-Teles, A.; Belo, I.; Peres, H. Pre-treatment of Ulva rigida improves its nutritional value for European seabass (Dicentrarchus labrax) juveniles. Algal Res. 2022, 66, 102803. [Google Scholar] [CrossRef]
- del Río, P.G.; Domínguez, E.; Domínguez, V.D.; Romaní, A.; Domingues, L.; Garrote, G. Third generation bioethanol from invasive macroalgae Sargassum muticum using autohydrolysis pretreatment as first step of a biorefinery. Renew. Energy 2019, 141, 728–735. [Google Scholar] [CrossRef]
- Sousa, C.; Sousa-Pinto, I.; Oliveira, I.; Marinho, G.S. Seasonal variation in the composition and antioxidant potential of Codium tomentosum and Ulva lacinulata produced in a land-based integrated multi-trophic aquaculture system. J. Appl. Phycol. 2025, 37, 1557–1572. [Google Scholar] [CrossRef]
- Healy, L.E.; Zhu, X.; Pojić, M.; Sullivan, C.; Tiwari, U.; Curtin, J.; Tiwari, B.K. Biomolecules from Macroalgae—Nutritional Profile and Bioactives for Novel Food Product Development. Biomolecules 2023, 13, 386. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved glucose recovery from durian peel by alkaline-catalyzed steam pretreatment. PeerJ 2021, 9, e12026. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Morris, S.M.; Steege, L.; Robinson, J.; Bavington, C. Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations. J. Mar. Sci. Eng. 2021, 9, 1082. [Google Scholar] [CrossRef]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Gomes-Dias, J.S.; Romaní, A.; Teixeira, J.J.; Rocha, C.M.R. Valorization of Seaweed Carbohydrates: Autohydrolysis as a Selective and Sustainable Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 17143–17153. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Ben Messaoud, G.; Marzouki, M.N.; Spiess, A.; Smaali, I. The stranded macroalga Ulva lactuca as a new alternative source of cellulose: Extraction, physicochemical and rheological characterization. J. Clean. Prod. 2019, 234, 1421–1427. [Google Scholar] [CrossRef]
- do Canto, V.P.; Thompson, C.E.; Netz, P.A. Polyurethanases: Three-dimensional structures and molecular dynamics simulations of enzymes that degrade polyurethane. J. Mol. Graph. Model. 2019, 89, 82–95. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Schiener, P.; Stanley, M.S.; Black, K.D.; Green, D.H. Assessment of saccharification and fermentation of brown seaweeds to identify the seasonal effect on bioethanol production. J. Appl. Phycol. 2016, 28, 3009–3020. [Google Scholar] [CrossRef]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G.H. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. Corrigendum to “A review of macroalgae production, with potential applications in biofuels and bioenergy” [Renew Sustain Energy Rev 54 (2016) 473–481]. Renew. Sustain. Energy Rev. 2018, 96, 526. [Google Scholar] [CrossRef]
- Ben Yahmed, N.; Carrere, H.; Marzouki, M.N.; Smaali, I. Enhancement of biogas production from Ulva sp. by using solid-state fermentation as biological pretreatment. Algal Res. 2017, 27, 206–214. [Google Scholar] [CrossRef]
- Chen, H.; Xia, A.; Zhu, X.; Huang, Y.; Zhu, X.; Liao, Q. Hydrothermal hydrolysis of algal biomass for biofuels production: A review. Bioresour. Technol. 2022, 344, 126213. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Macroalgae biorefineries as a sustainable resource in the extraction of value-added compounds. Algal Res. 2023, 69, 102954. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Marine Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Shiva Climent Barba, F.; Rodríguez-Jasso, R.M.; Sukumaran, R.K.; Ruiz, H.A. High-solids loading processing for an integrated lignocellulosic biorefinery: Effects of transport phenomena and rheology—A review. Bioresour. Technol. 2022, 351, 127044. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment--a review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Bao, J. High solids loading pretreatment: The core of lignocellulose biorefinery as an industrial technology—An overview. Bioresour. Technol. 2023, 369, 128334. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Bamouh, A.; Kadmiri, I.M. Biostimulants Derived from Moroccan Seaweeds: Seed Germination Metabolomics and Growth Promotion of Tomato Plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Valentão, P.; Trindade, P.; Gomes, D.; Guedes de Pinho, P.; Mouga, T.; Andrade, P.B. Codium tomentosum and Plocamium cartilagineum: Chemistry and antioxidant potential. Food Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Glencross, B.D. A feed is still only as good as its ingredients: An update on the nutritional research strategies for the optimal evaluation of ingredients for aquaculture feeds. Aquac. Nutr. 2020, 26, 1871–1883. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Ravebtós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Ang, C.Y.; Yong, A.S.K.; Azad SAl Lim, L.S.; Zuldin, W.H.; Lal, M.T.M. Valorization of Macroalgae through Fermentation for Aquafeed Production: A Review. Fermentation 2021, 7, 304. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviosson, G.O.; Karlsson, E.N. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, H.B.; Reddy, N. A review on dielectric properties of biofiber-based composites. Adv. Compos. Hybrid. Mater. 2018, 1, 635–648. [Google Scholar] [CrossRef]
- Synytsya, A.; Copíková, J.; Kim, W.J.; Park, Y.I. Cell Wall Polysaccharides of Marine Algae. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 543–590. [Google Scholar] [CrossRef]
- Fernández, P.V.; Arata, P.X.; Ciancia, M. Polysaccharides from Codium Species: Chemical Structure and Biological Activity. Their Role as Components of the Cell Wall. Adv. Bot. Res. 2014, 71, 253–278. [Google Scholar] [CrossRef]
- Percivale, E.; Mcdowellrh, R.H. Algal Polysaccharides. Methods Plant Biochem. 1990, 2, 523–547. [Google Scholar] [CrossRef]
- Barszcz, M.; Paradziej-Łukowicz, J.; Taciak, M.; Tuśnio, A.; Staśkiewicz Muszyńska-Furas, B.; Lewandowska, A.; Pastuszewska, B.; Skomiał, J. Growth performance and physiological parameters of conventional and specified pathogen-free rats fed autoclaved diets with different protein sources. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1116–1126. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. The nutritional significance of “dietary fibre” analysis. Anim. Feed. Sci. Technol. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Martins-Vieira, J.C.; Torres-Mayanga, P.C.; Lachos-Perez, D. Hydrothermal Processing of Lignocellulosic Biomass: An Overview of Subcritical and Supercritical Water Hydrolysis. BioEnergy Res. 2022, 16, 1296–1317. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.-K. Seaweed polysaccharides and their potential biomedical applications. Starch Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Wojdyło, A.; Figiel, A.; Yang, B.; Laaksonen, O.; Foste, M.; Vilu, R.; Viiard, E. Fiber modification of brewers’ spent grain by autoclave treatment to improve its properties as a functional food ingredient. LWT 2021, 149, 111877. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V. Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: What is next? Algal Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- Lee Jye Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Chang, J.; Yin, Q.; Huang, W.; Liu, Y.; Dang, X.; Gao, T.; Lu, F. Effect of physicochemical pretreatments plus enzymatic hydrolysis on the composition and morphologic structure of corn straw. Renew. Energy 2019, 138, 502–508. [Google Scholar] [CrossRef]
- Gabhane, J.; Prince William, S.P.M.; Vaidya, A.N.; Mahapatra, K.; Chakrabarti, T. Influence of heating source on the efficacy of lignocellulosic pretreatment—A cellulosic ethanol perspective. Biomass Bioenergy 2011, 35, 96–102. [Google Scholar] [CrossRef]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Sherif, S.A.; Dawoud, G.T.M.; Ghareeb, M.M. Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rendiconti Lincei 2016, 27, 665–672. [Google Scholar] [CrossRef]
- Zeng, G.; You, H.; Wang, K.; Jiang, Y.; Bao, H.; Du, M.; Chen, B.; Ai, N.; Gu, Z. Semi-simultaneous Saccharification and Fermentation of Ethanol Production from Sargassum horneri and Biosorbent Production from Fermentation Residues. Waste Biomass Valorization 2020, 11, 4743–4755. [Google Scholar] [CrossRef]
- Kooren, R.; Sumithra, T.G.; Jaseera, K.V.; Sunithakumari, K.; Hasan, S.; Sayooj, P.; Kaladharan, P. A comparative study on pre-treatment methods for enhanced saccharification from tropical seaweeds to aid in bioethanol production. Aquat. Bot. 2023, 184, 103594. [Google Scholar] [CrossRef]
- Greetham, D.; Adams, J.M.; Du, C. The utilization of seawater for the hydrolysis of macroalgae and subsequent bioethanol fermentation. Sci. Rep. 2020, 10, 9728. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Tahar, A.; Zghida, H.; Pereira, D.T.; Korbee, N.; Treichel, H.; Figueroa, F.; Achour, L. Biochemical Composition and Alkaline Extraction Optimization of Soluble Bioactive Compounds from the Green Algae Caulerpa cylindraceae. Mar. Drugs 2025, 23, 208. [Google Scholar] [CrossRef]
- Suhartini, S.; Rohma, N.A.; Elviliana; Hidayat, N.; Sunyoto, N.M.S.; Mardawati, E.; Kasbawati; Mascruhin, N.; Idrus, S.; Fitria; et al. Comparison of acid and alkaline pre-treatment on methane production from empty palm oil fruit bunches (OPEFB): Effect on characteristics, digester performance, and correlation of kinetic parameters. Renew. Energy 2023, 215, 119099. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Comparative study on chemical pretreatment methods for improving enzymatic digestibility of crofton weed stem. Bioresour. Technol. 2008, 99, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- El-Din, N.G.S.; Shaltout, N.A.; Ghazal, M.A.; Ali, A.E.; Beltagy, D.M. Chemical pretreatment of Ulva fasciata cell wall for enhancing biodiesel yield and properties. Egypt. J. Aquat. Biol. Fish. 2020, 24, 103–125. [Google Scholar] [CrossRef]
- Sindhu, R.; Pandey, A.; Binod, P. Chapter 4 - Alkaline Treatment. Pretreat. Biomass Process. Technol. 2015, 51–60. [Google Scholar] [CrossRef]
- Pan-utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-Assisted Extraction and Antioxidant Potential of Valuable Protein from Ulva rigida Macroalgae. Life 2023, 13, 86. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae(1). J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Wijesekara, I.; Lang, M.; Marty, C.; Gemin, M.-P.; Boulho, R.; Douzenel, P.; Wickramasinghe, I.; Bedoux, G.; Bourgougnon, N. Different extraction procedures and analysis of protein from Ulva sp. in Brittany, France. J. Appl. Phycol. 2017, 29, 2503–2511. [Google Scholar] [CrossRef]
- Salim, M.H.; Kassab, Z.; Ablouh Ehoussaine Sehaqui, H.; Aboulkas, A.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Manufacturing of macroporous cellulose monolith from green macroalgae and its application for wastewater treatment. Int. J. Biol. Macromol. 2022, 200, 182–192. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Filipigh, A.; Beltrán, S.; Riaño, P. Enzymatic hydrolysis of the industrial solid residue of red seaweed after agar extraction: Extracts characterization and modelling. Food Bioprod. Process. 2021, 126, 356–366. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.S. A concise review of the bioactivity and pharmacological properties of the genus Codium (Bryopsidales, Chlorophyta). J. Appl. Phycol. 2022, 34, 2827. [Google Scholar] [CrossRef]
- Duncan, S.M.; Schilling, J.S. Carbohydrate-hydrolyzing enzyme ratios during fungal degradation of woody and non-woody lignocellulose substrates. Enzyme Microb. Technol. 2010, 47, 363–371. [Google Scholar] [CrossRef]
- Priskila Rahael, K.; Penina Tua Rahantoknam, S.; Kahfi Hamid, S.; Anindia, F. Manufacture of solid soap based on crude papain enzyme and antioxidant from papaya. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012048. [Google Scholar] [CrossRef]
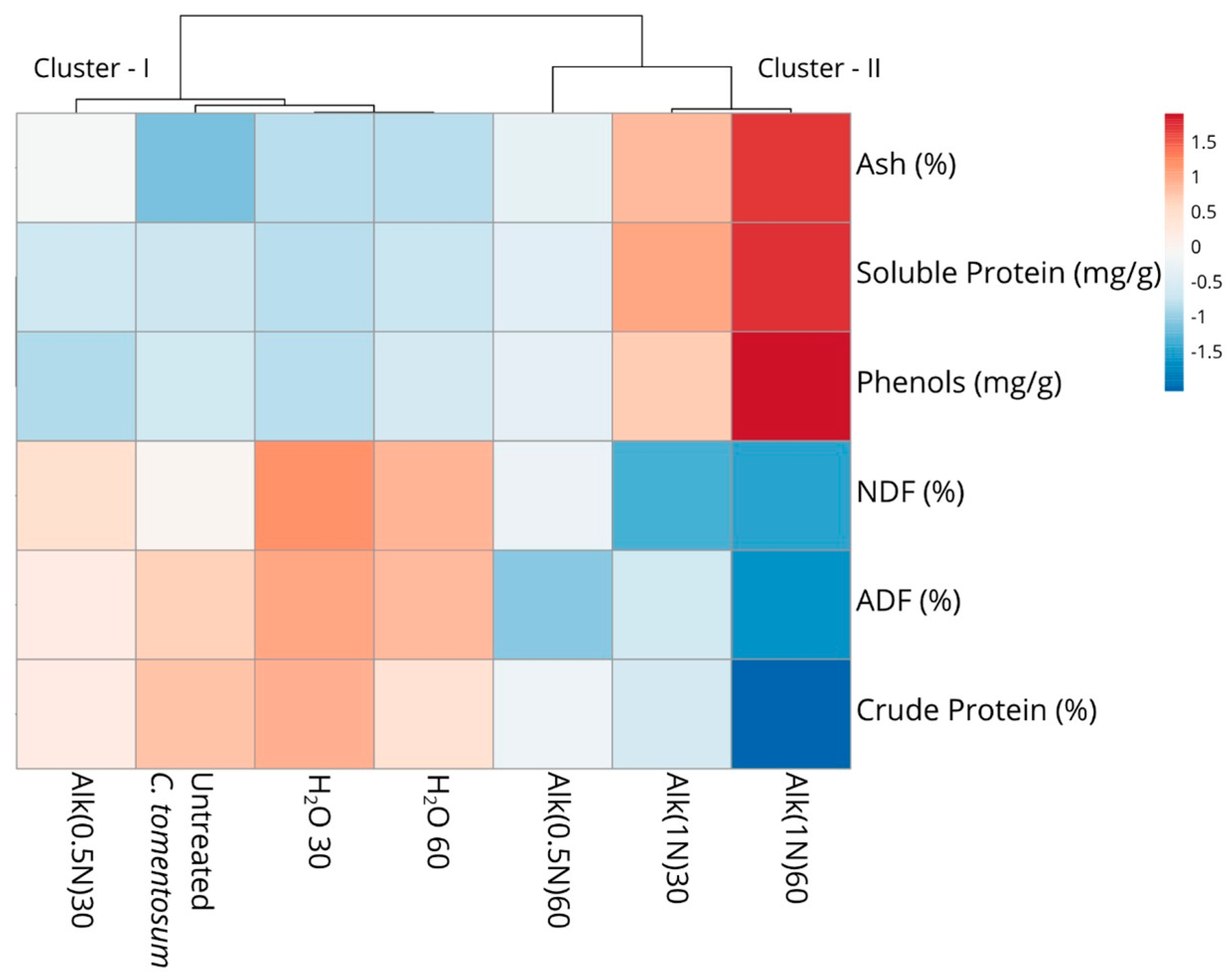


| Treatment | Time | Solvent | NDF (%) | ADF (%) | Crude Protein (%) | Soluble Protein (mg/g) | Phenols (mg/g) | Ash (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | — | — | 22.04 ± 0.4 | 6.35 ± 0.4 | 21.22 ± 0.8 | 4.10 ± 0.1 | 0.92 ± 0.0 | 36.51 ± 0.8 | ||||||
| H2O-30 | 30 min | H2O | 29.08 ± 0.7 | 7.01 ± 0.2 c | 21.44 ± 0.3 | 3.85 ± 0.1 | 0.69 ± 0.2 | 38.94 ± 1.4 a | ||||||
| Alk(0.5N)30 | 0.5N NaOH | 25.03 ± 0.6 | 5.57 ± 0.7 b,B | 20.26 ± 0.5 | 4.18 ± 0.2 | 0.65 ± 0.2 | 44.28 ± 0.5 b | |||||||
| Alk(1N)30 | 1N NaOH | 14.39 ± 0.7 | 4.22 ± 0.4 a,B | 19.10 ± 1.6 | 7.36 ± 0.5 | 2.46 ± 0.2 | 51.23 ± 2.4 c | |||||||
| H2O-60 | 60 min | H2O | 27.46 ± 0.7 | 6.71 ± 0.9 b | 20.63 ± 0.4 | 4.03 ± 0.0 | 0.95 ± 0.1 | 38.86 ± 0.1 a | ||||||
| Alk(0.5N)60 | 0.5N NaOH | 20.65 ± 0.6 | 3.41 ± 0.3 a,A | 19.66 ± 0.3 | 4.59 ± 0.4 | 1.26 ± 0.1 | 42.58 ± 1.1 a | |||||||
| Alk(1N)60 | 1N NaOH | 13.58 ± 0.2 | 2.50 ± 0.3 a,A | 16.77 ± 0.3 | 8.68 ± 1.4 | 3.86 ± 1 | 57.24 ± 2.5 b | |||||||
| Non-orthogonal contrast 1 | ||||||||||||||
| NDF | ADF | Crude Protein | Soluble Protein | Phenols | Ash | |||||||||
| 1. Untreated vs. H2O 30 | 0.000 | 0.140 | 0.726 | 0.624 | 0.055 | 0.041 | ||||||||
| 2. Untreated vs. H2O 60 | 0.000 | 0.403 | 0.353 | 0.893 | 0.842 | 0.046 | ||||||||
| 3. Untreated vs. Alk(0.5N)30 | 0.023 | 0.088 | 0.139 | 0.873 | 0.027 | 0.000 | ||||||||
| 4. Untreated vs. Alk(0.5N)60 | 0.253 | 0.000 | 0.022 | 0.340 | 0.066 | 0.000 | ||||||||
| 5. Untreated vs. Alk(1N)30 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | ||||||||
| 6. Untreated vs. Alk(1N)60 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||||
| Two-way ANOVA | Variable Source 2 | Solvent | ||||||||||||
| Solvent | Time | Interaction | H2O | Alk0.5 | Alk1 | |||||||||
| NDF | *** | * | ns | c | b | a | ||||||||
| ADF | *** | *** | * | b | a | a | ||||||||
| Crude Protein | *** | * | ns | b | b | a | ||||||||
| Soluble Protein | *** | ns | ns | a | a | b | ||||||||
| Phenols | *** | *** | ns | a | a | b | ||||||||
| Ash | *** | ns | * | a | b | c | ||||||||
| Treatment | Solvent | Enzyme | NDF (%) | ADF (%) | Crude Protein (%) | Soluble Protein (mg/g) | Phenols (mg/g) | Ash (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | H2O | — | 33.5 ± 0.3 | 8.69 ± 3.5 | 21.6 ± 0.7 | 4.48 ± 0.1 | 0.83 ± 0.1 | 40.4 ± 0.0 | ||||
| H2OEnz0.2% | 0.2% | 31.0 ± 1.7 | 6.56 ± 3.7 | 19.8 ± 0.4 | 3.99 ± 0.1 | 0.81 ± 0.1 | 38.1 ± 0.7 | |||||
| H2OEnz0.4% | 0.4% | 25.6 ± 9.3 | 4.74 ± 2.1 | 20.7 ± 0.5 | 3.92 ± 0.1 | 2.20 ± 0.5 | 39.6 ± 1.8 | |||||
| Alk | 1N NaOH | — | 18.1 ± 0.0 | 3.40 ± 0.3 | 18.4 ± 0.3 | 7.54 ± 0.4 | 0.83 ± 0.2 | 44.8 ± 0.0 | ||||
| AlkEnz0.2% | 0.2% | 17.3 ± 1.5 | 3.96 ± 1.3 | 16.6 ± 0.6 | 7.28 ± 0.7 | 0.90 ± 0.1 | 47.4 ± 0.5 | |||||
| AlkEnz0.4% | 0.4% | 17.4 ± 0.9 | 3.15 ± 0.2 | 17.3 ± 0.4 | 8.27 ± 1.2 | 3.10 ± 0.7 | 47.0 ± 1.5 | |||||
| Two-way ANOVA | Variable Source 1 | Enzyme | ||||||||||
| Solvent | Enzyme | Interaction | 0 | 0.2% | 0.4% | |||||||
| NDF | ** | ns | ns | - | - | - | ||||||
| ADF | ns | ns | ns | - | - | - | ||||||
| Crude Protein | *** | ** | ns | b | a | ab | ||||||
| Soluble Protein | *** | ns | ns | - | - | - | ||||||
| Phenols | ** | *** | ns | a | a | b | ||||||
| Ash | *** | ns | ns | - | - | - | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Oliveira, C.; Ferreira, M.; Belo, I.; Oliva-Teles, A.; Peres, H. Effectiveness of High-Solid Loading Treatments to Enhance Nutrient and Antioxidant Bioavailability in Codium tomentosum. Phycology 2025, 5, 69. https://doi.org/10.3390/phycology5040069
Ramos-Oliveira C, Ferreira M, Belo I, Oliva-Teles A, Peres H. Effectiveness of High-Solid Loading Treatments to Enhance Nutrient and Antioxidant Bioavailability in Codium tomentosum. Phycology. 2025; 5(4):69. https://doi.org/10.3390/phycology5040069
Chicago/Turabian StyleRamos-Oliveira, Catarina, Marta Ferreira, Isabel Belo, Aires Oliva-Teles, and Helena Peres. 2025. "Effectiveness of High-Solid Loading Treatments to Enhance Nutrient and Antioxidant Bioavailability in Codium tomentosum" Phycology 5, no. 4: 69. https://doi.org/10.3390/phycology5040069
APA StyleRamos-Oliveira, C., Ferreira, M., Belo, I., Oliva-Teles, A., & Peres, H. (2025). Effectiveness of High-Solid Loading Treatments to Enhance Nutrient and Antioxidant Bioavailability in Codium tomentosum. Phycology, 5(4), 69. https://doi.org/10.3390/phycology5040069






