Members of the Order Mastogloiales Sensu Cox Belong to the Different Evolutionary Lineages of Diatoms: Phylogenetic Resolutions and Descriptions of New Types of Pore Occlusions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Strain Isolation, and Culturing
2.2. Slide Preparation and Microscopy
2.3. DNA Extraction and Amplification
2.4. Phylogram Construction
2.5. Taxonomy and Terminology
3. Results
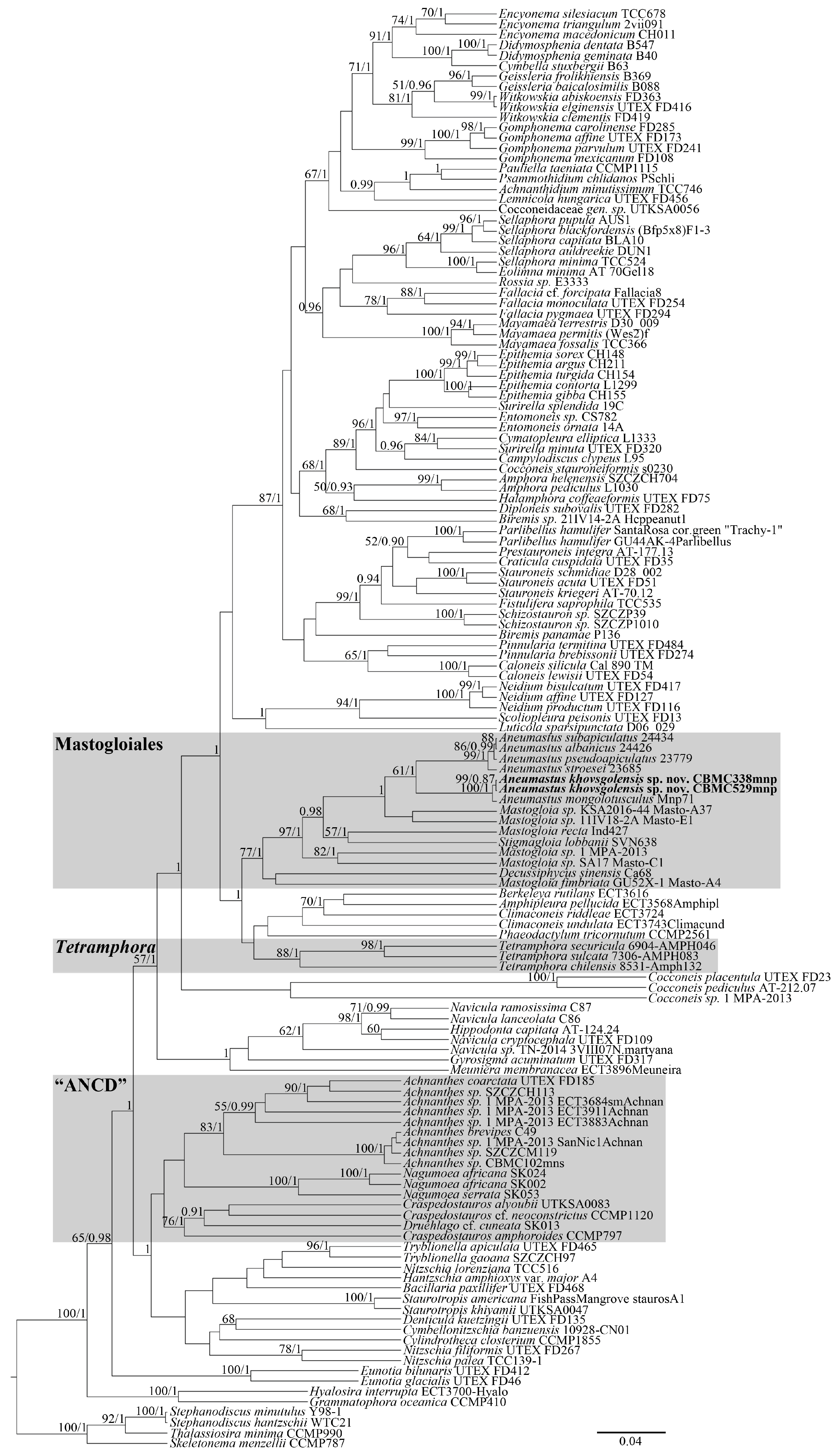
3.1. Molecular Analysis
3.2. Morphological Analysis of Pore Occlusions
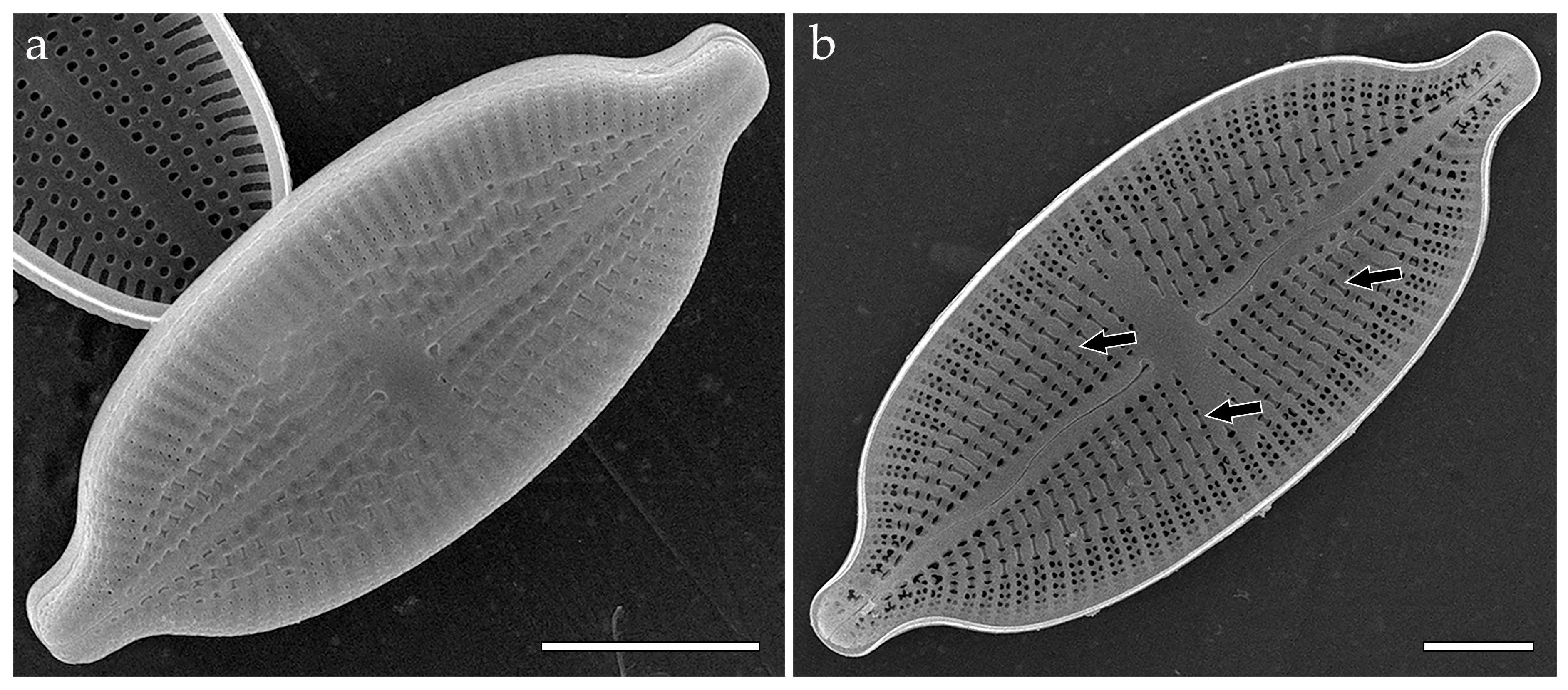
- colanderus-directus (pl. colanderus-directa)—This is a type of occlusion covering a pseudolocula in a cavitate valve (on its inner side), consisting of a flat or domed silica flap, with four–six (sometimes only one or more than four) openings. The occlusion is situated directly below the outer opening of the pseudolocula. Typical for Mastogloia danseyi (Thwaites) Thwaites ex W. Smith (generitype), Mastogloia angulata F.W. Lewis, Mastogloia cebuensis A. Mann, Mastogloia gibbosa Brun, Mastogloia lacustris (Grunow) Grunow, Mastogloia latecostata Hustedt, Mastogloia peracuta Janisch, Mastogloia sturdyi ([3] (Figures 44 and 46–49); [12] (Figures 53–57); [49] (Figure 27); [63] (Figures 1 and 52)), and Mastogloia recta (Figure 4). Also typical for Paramastogloia ([34] (Figure 2) {Paramastogloia cubana—generitype}), and Mastoneis ([34] (Figure 3), {Mastoneis biformis–generitype});
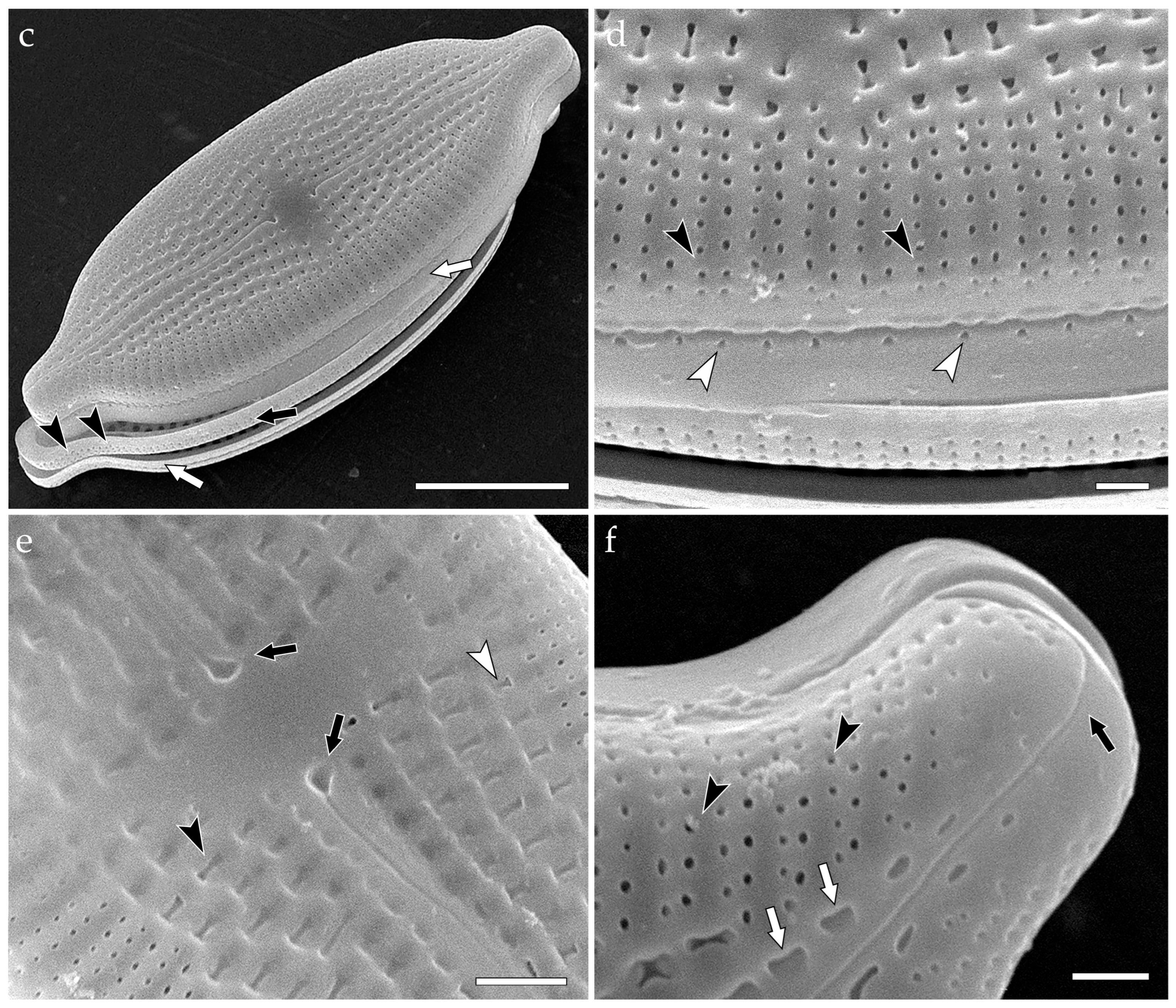
- colanderus-obliquus (pl. colanderus-obliqua)—This is a type of occlusion covering a pseudolocula in a cavitate valve (on its inner side), consisting of a silica flap with one or more perforations. The occlusion is situated asymmetrically in relation to the outer aperture, not lying directly under, due to the oblique shape of the cavity. The occlusion is sometimes bordered by four papillae. Typical for Mastogloia emarginata Hustedt, Mastogloia lineata Cleve & Grove, Mastogloia rimosa Cleve ([3] (Figures 40–43)) and Stigmagloia ([53] (Figures 24–29 and 36–38) {Stigmagloia lobbanii—generitype}; Figure 5);
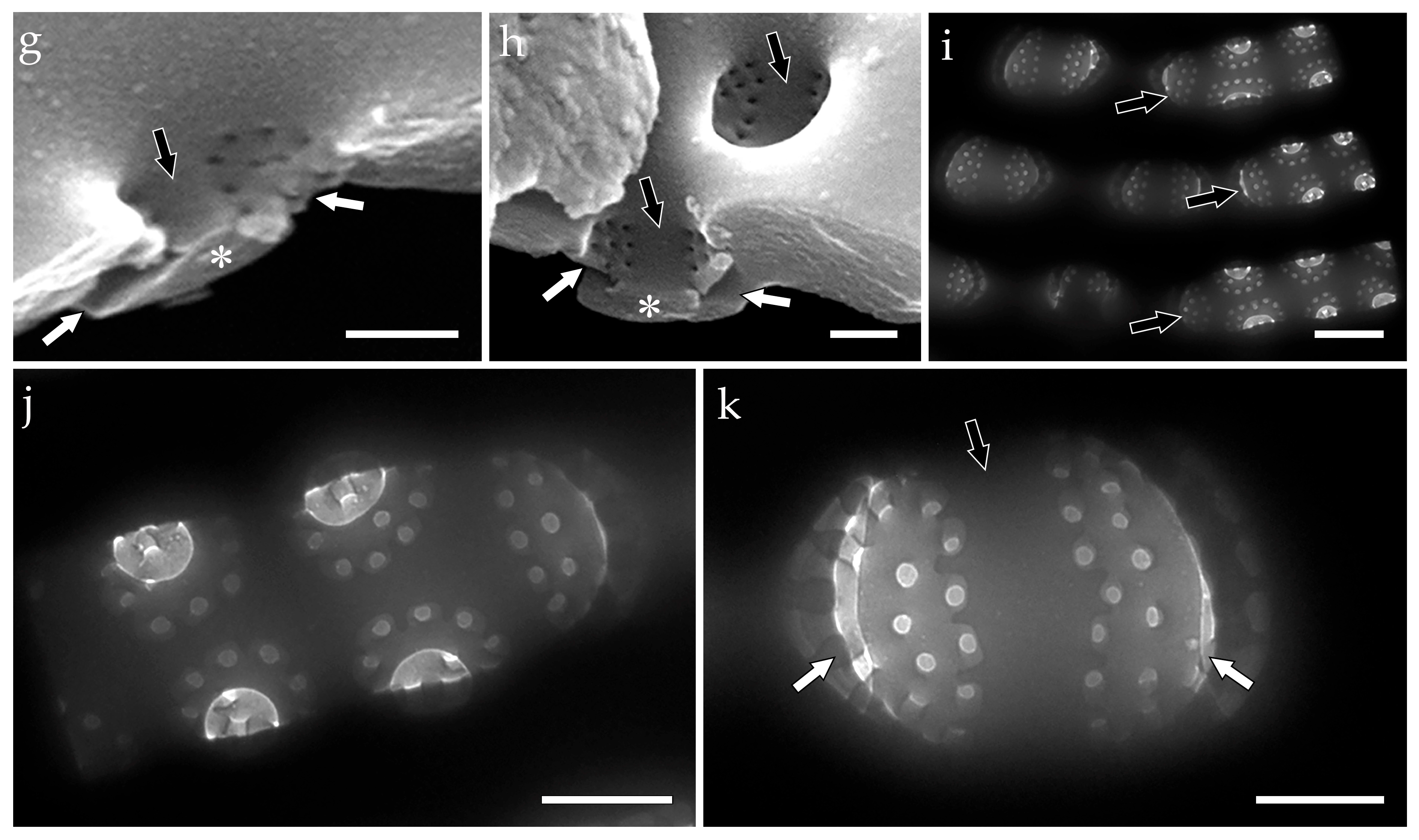
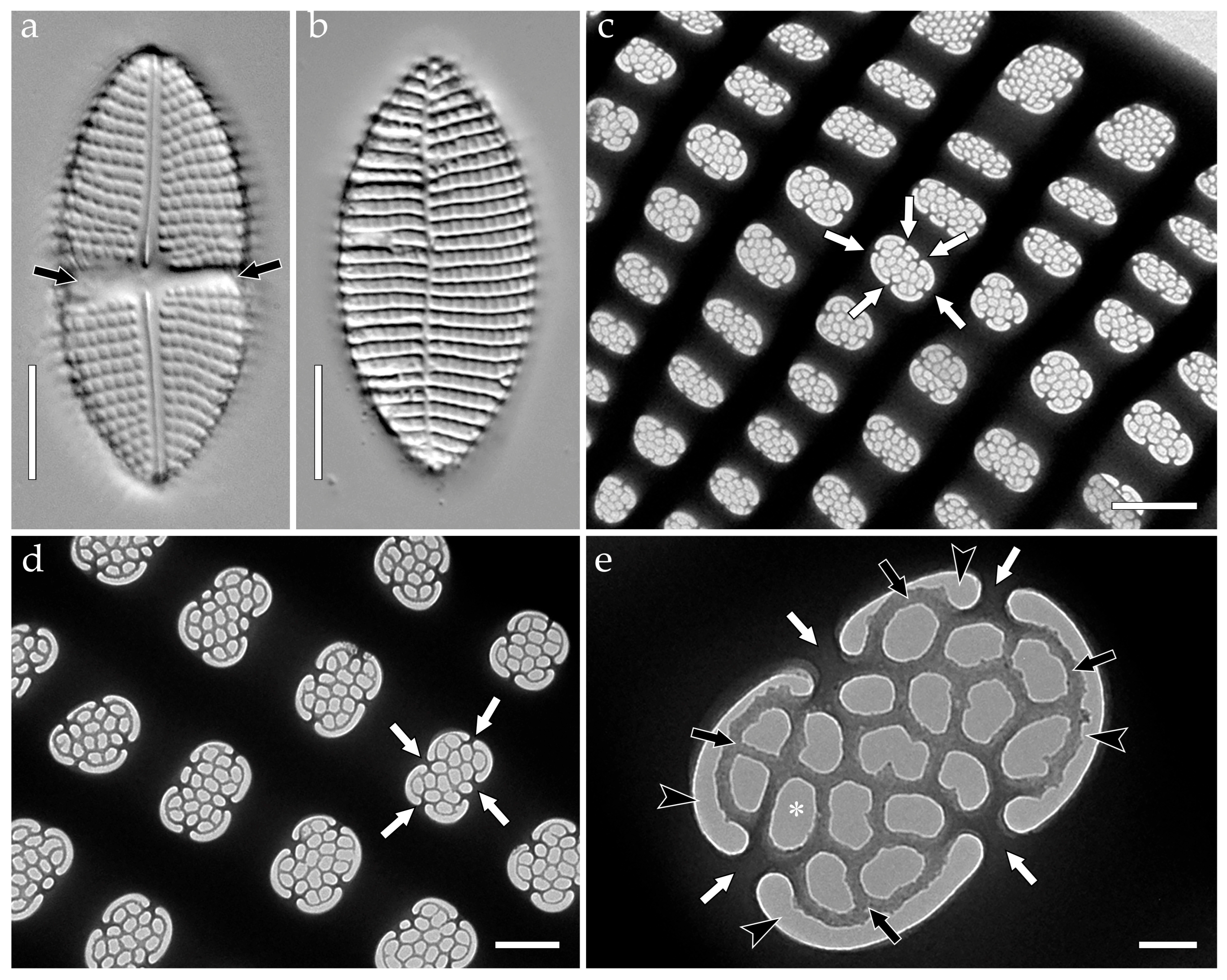
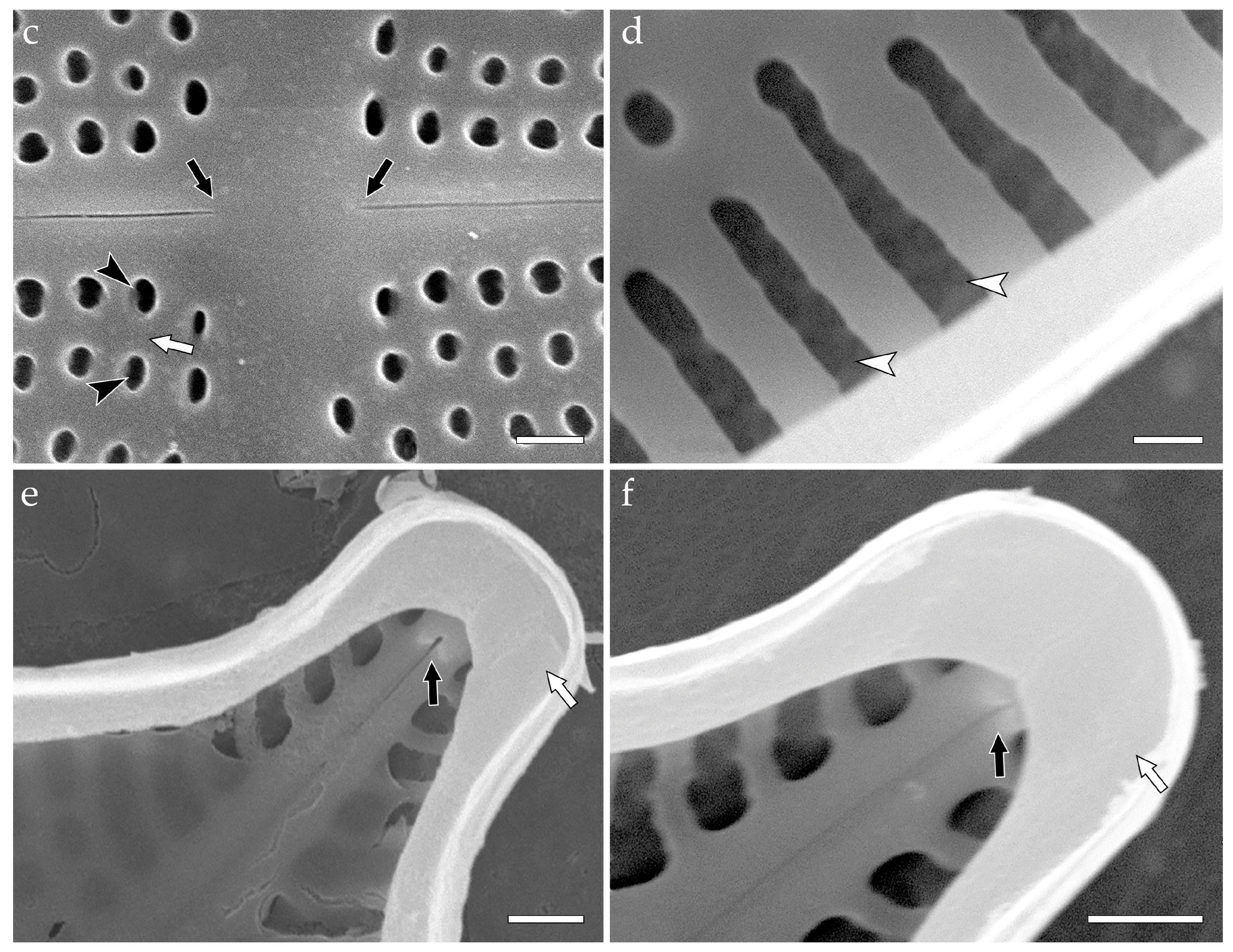
- colanderus-bifurcus (pl. colanderus-bifurca)—This is a type of occlusion covering a pseudolocula in a cavitate valve (on its inner side), with four, sometimes two, or six–eight openings arranged in two groups and separated by an interrupting layer of silica. Wide silica interruptions alternate with narrow junctions so that the wide interruption lies below the outer aperture. Each group of openings proceeds to different, adjacent outer apertures of pseudoloculi. In the transverse section, the silica interruption between the openings looks mushroom-shaped, and the cavity looks bifurcated. Narrow junctions are sometimes equipped with T-shaped costae. Typical for Mastogloia chersonensis A.W.F. Schmidt, Mastogloia corallum Paddock & Kemp, Mastogloia goesii (Cleve) Cleve, Mastogloia elegans Lewis, Mastogloia labuensis var. lanceolata Hustedt, Mastogloia neomauritiana Paddock & Kemp, Masogloia umbra Paddock & Kemp ([3] (Figures 45, 50 and 51); [63] (Figures 6, 16, 24, 33 and 42)), and Aneumastus ([4] (Plate 114, Figures 2 and 4) {Aneumastus tusculus (Ehrenberg) D.G. Mann & Stickle—generitype}; [29] (Figures 18,19, 22 and 23); [51] (Figures 16–19); Figure 2);
3.3. Morphological Characteristics of Selected Species from the Order Mastogloiales
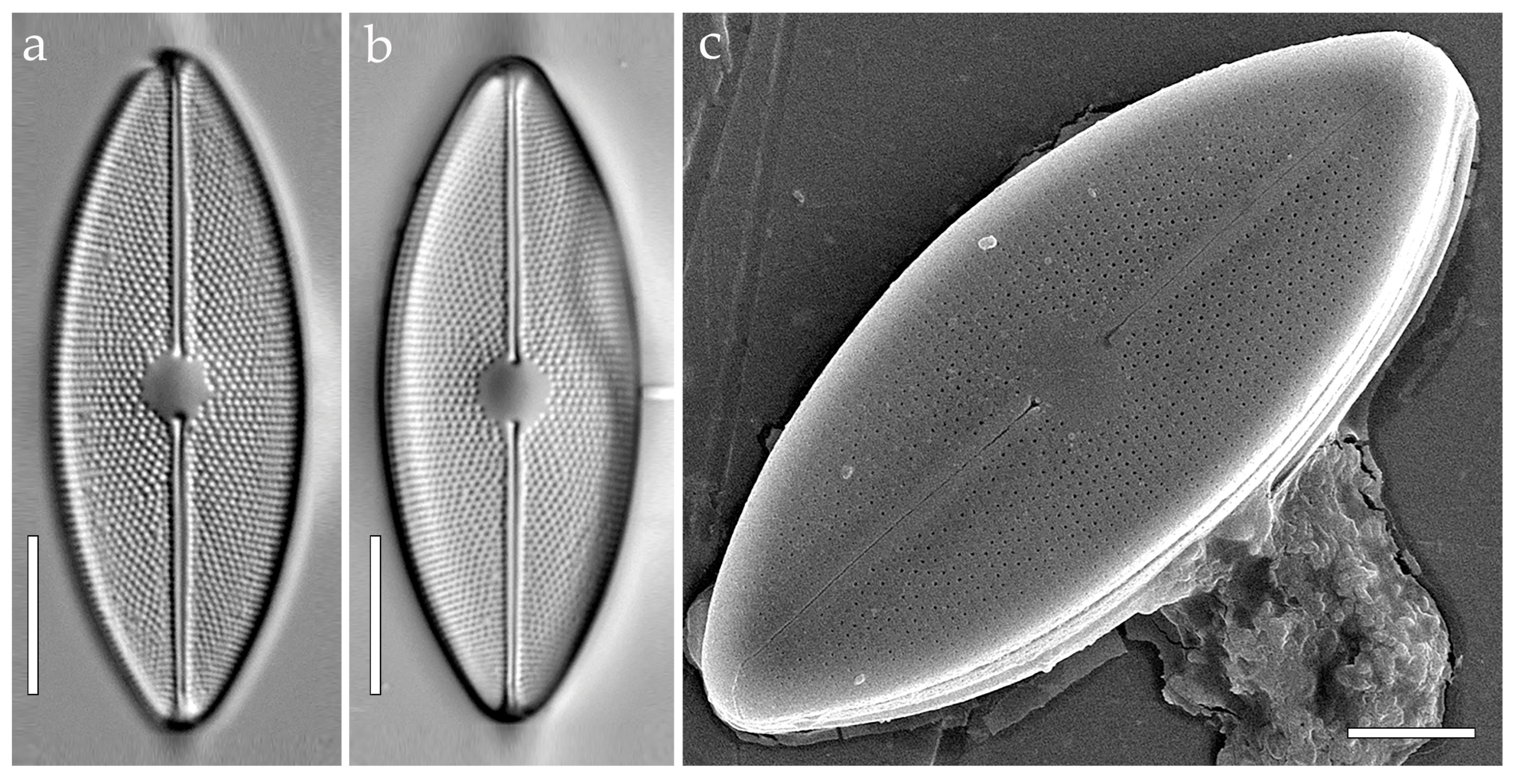
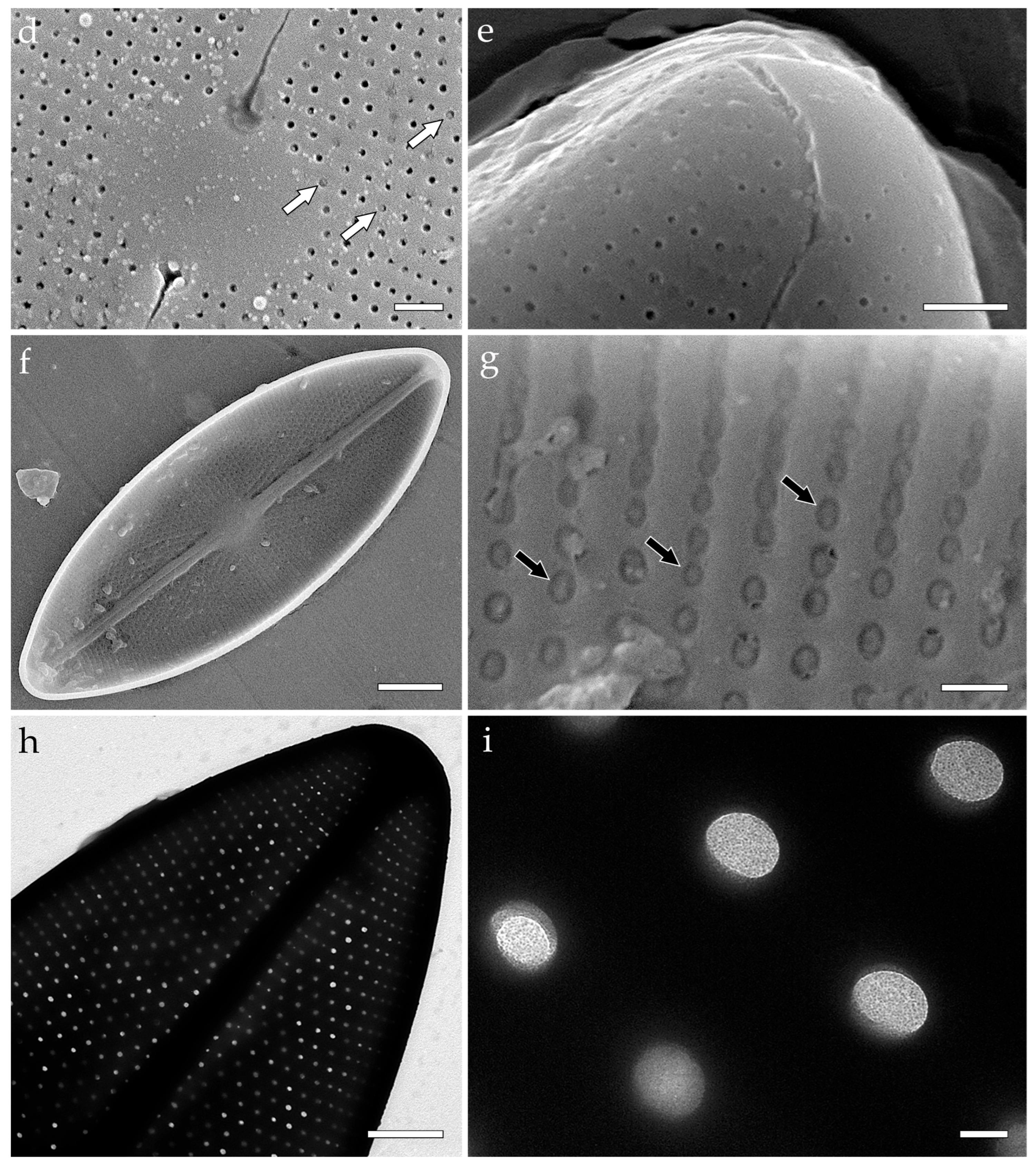
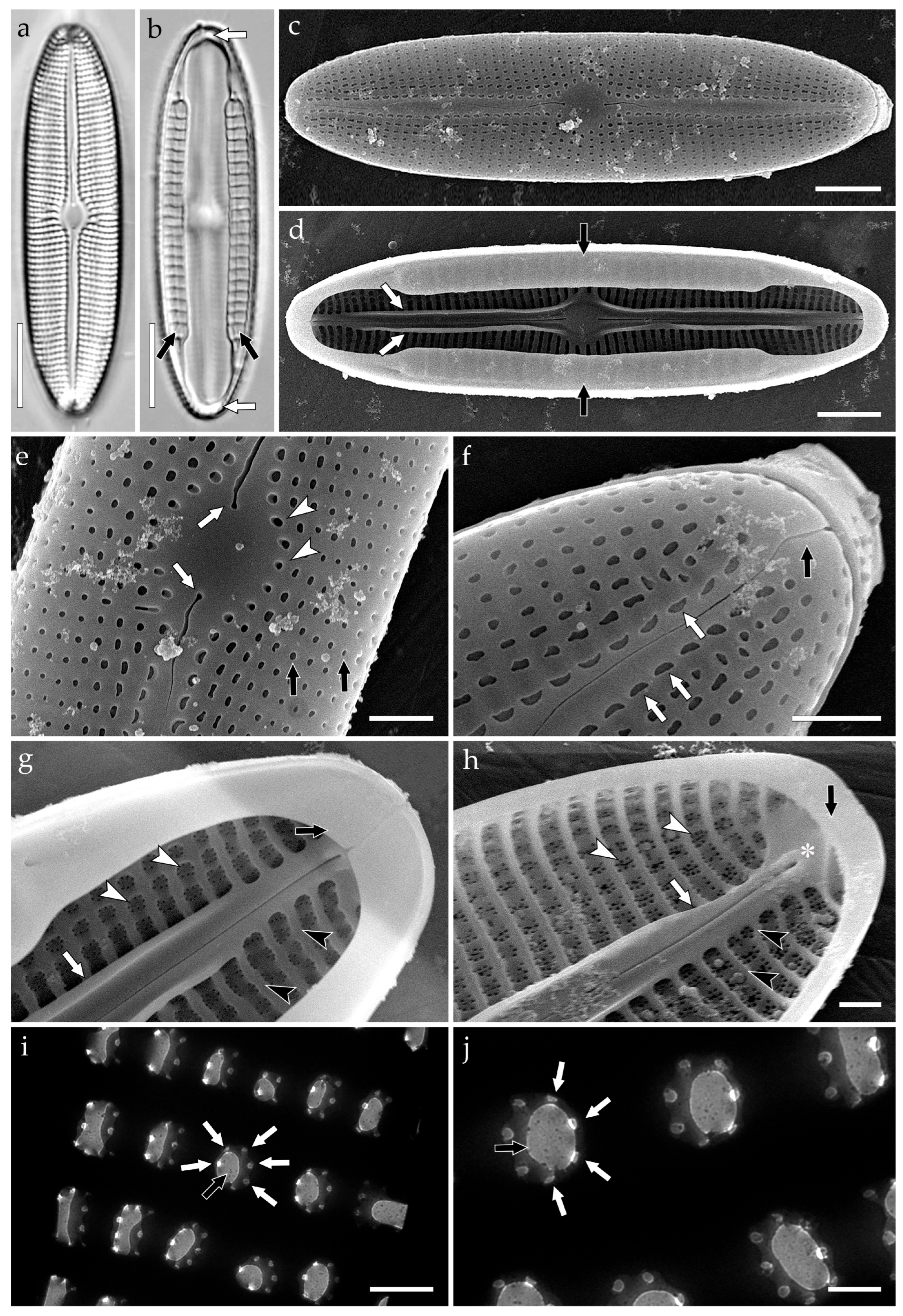
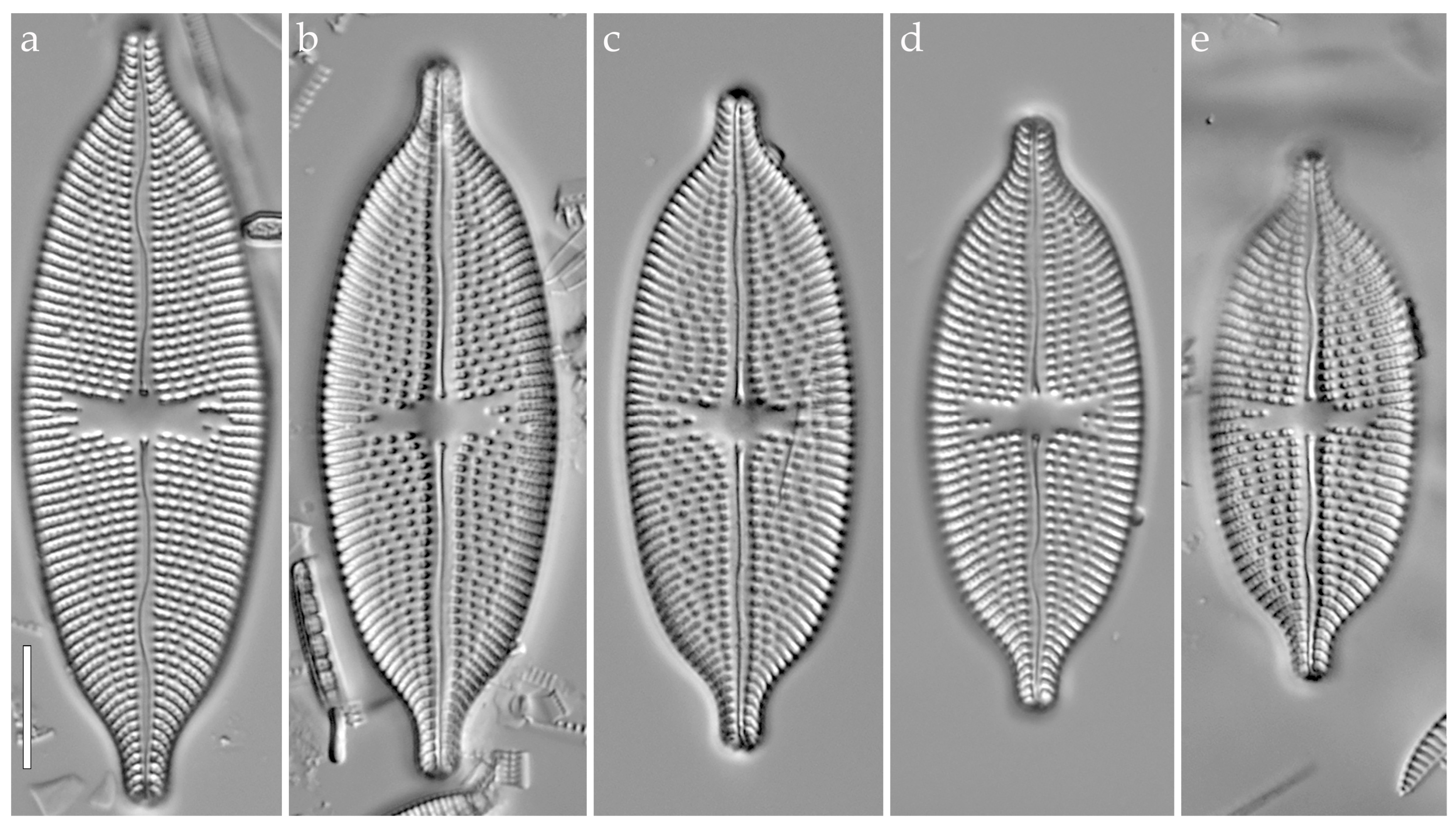
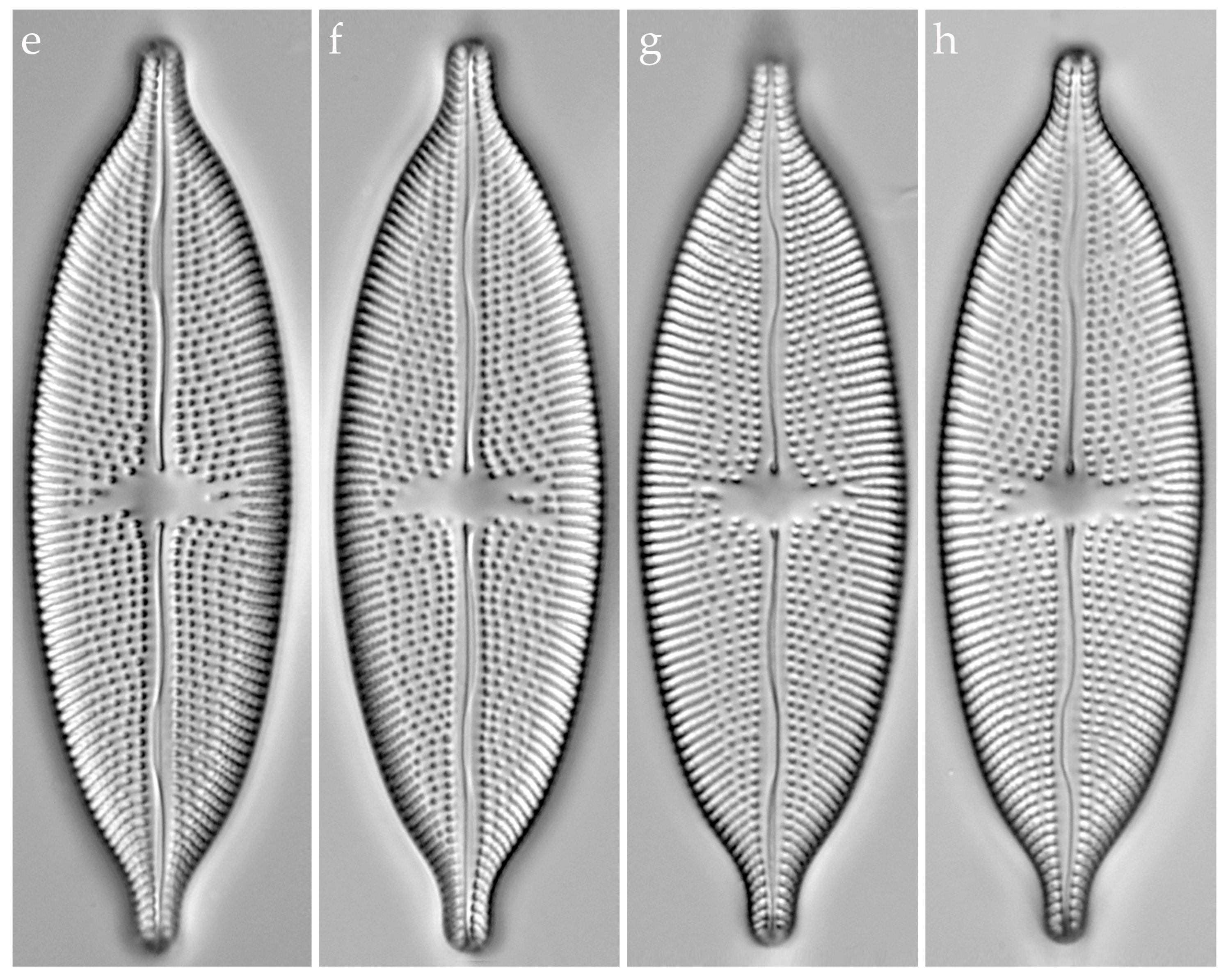
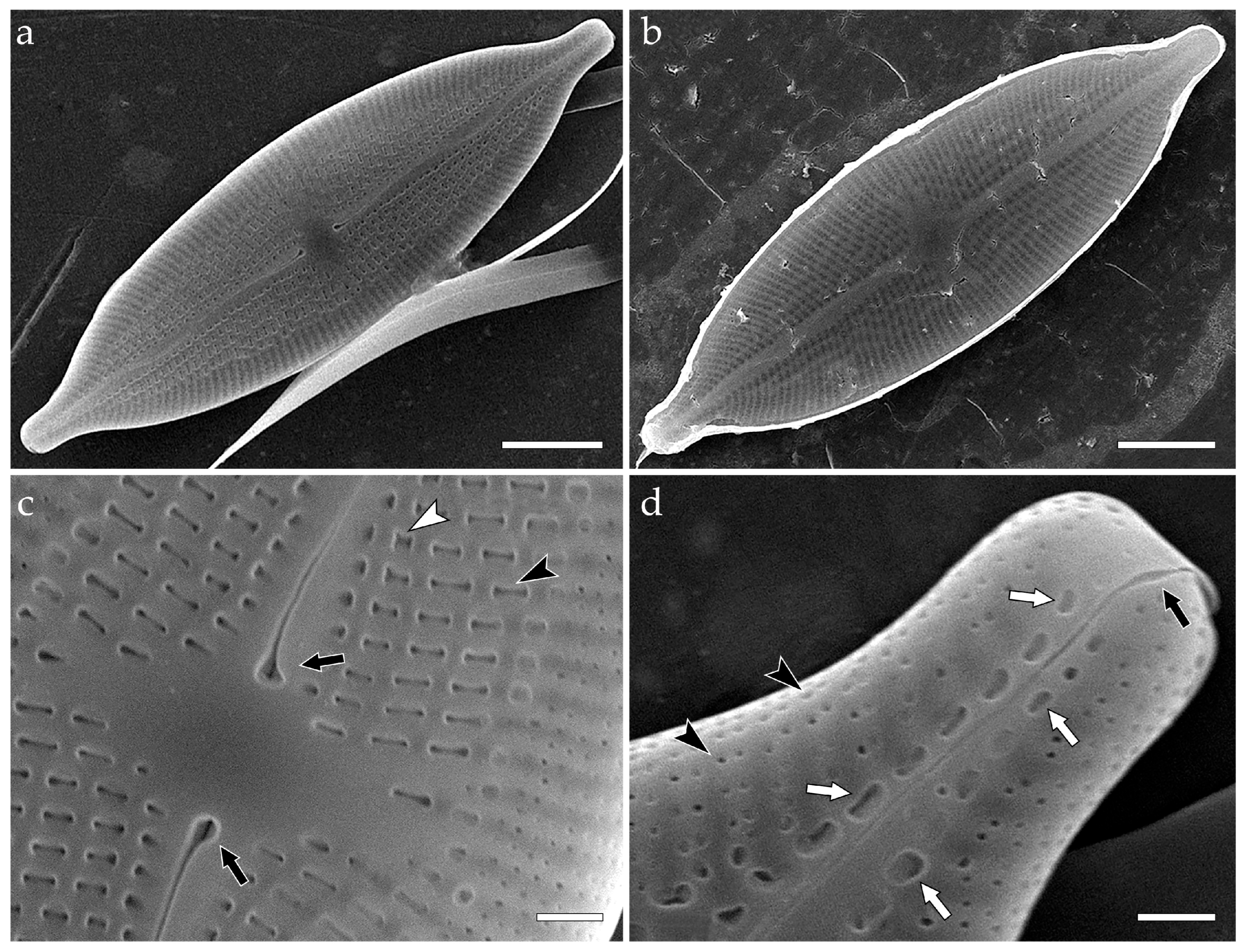
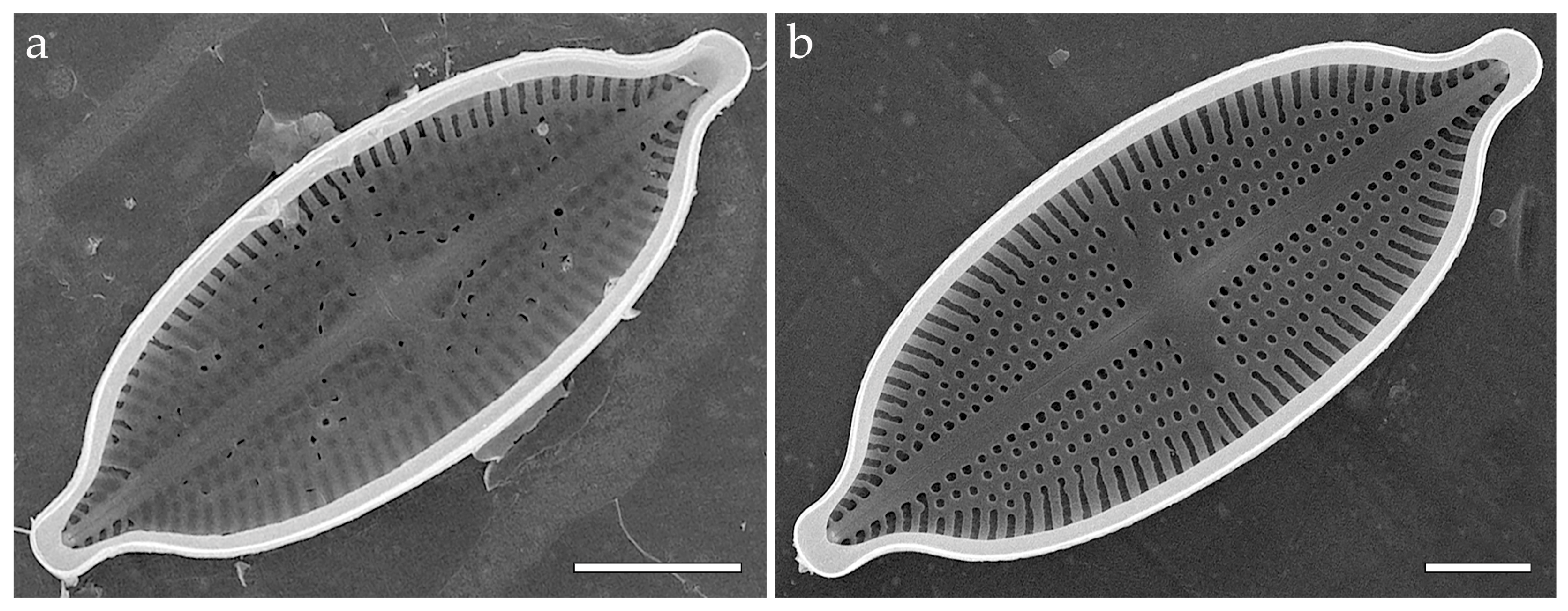
3.3.1. Aneumastus mongolotusculus (Figure 2)
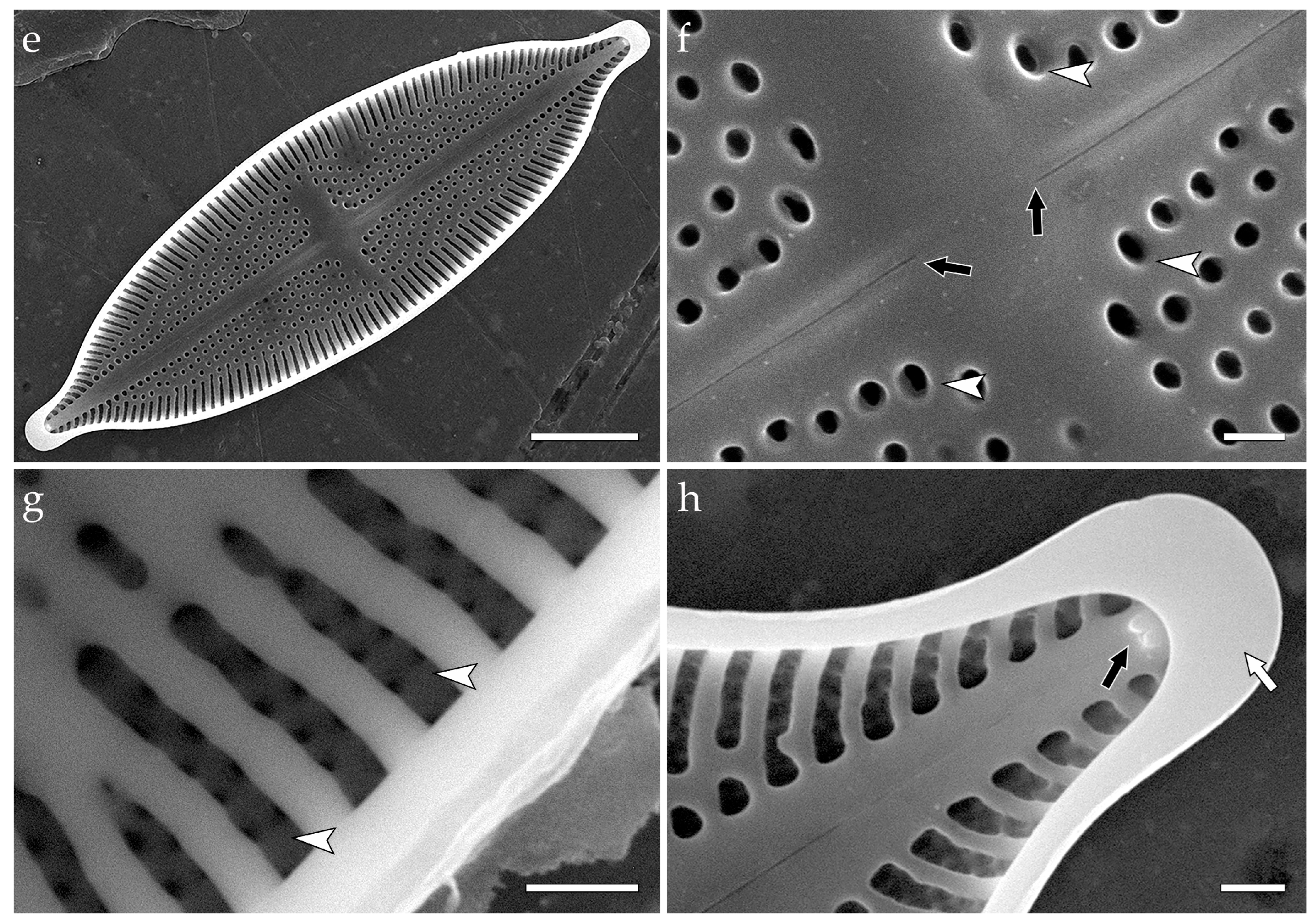
3.3.2. Decussiphycus sinensis (Figure 3)
3.3.3. Mastogloia recta (Figure 4)
3.3.4. Stigmagloia lobbanii (Figure 5)
3.3.5. Achnanthes sp. (Figure 6)
3.4. Description of the New Species
4. Discussion
4.1. Comparison of Aneumastus khovsgolensis sp. nov. with Similar Taxa
4.2. Phylogenetic Resolution of the Order Mastogloiales
4.2.1. Molecular Phylogenetics of Mastogloiales
4.2.2. Morphological Characteristic of Mastogloiales with Particular Reference to the Structure of Pore Occlusions
- M. elegans-type (also typical for M. goesii, M. corallum, M. neomauritiana, M. umbra)—a pseudoloculus with a single large external opening corresponding to four small internal openings, grouped by pairs ([3] (Figures 1, 6, 16, 24, 33 and 42));
- Mastogloia sturdyi-type—a loculus with a single large external opening and four small internal openings, perforating “in pairs into different loculi, to either side of the walls of the loculi” ([3] (Figure 52));
- Mastogloia cocconeiformis Grunow-type—a loculus (sensu Paddock & Kemp) in a cavitate valve with a single external and a single internal opening, occluded by a velum-like structure amidst the two openings. The velum-like structure is “saucer-shaped”, lying at an acute angle in relation to the inner opening ([2] (Figure 1); [3] (Figure 35));
- Mastogloia frickei Hustedt-type—an alveola (sensu Paddock & Kemp) in a cavitate valve, with a single lateral external opening and several squarish internal openings, which are arranged in a transverse row ([3] (Figure 36));
- Mastogloia biocellata (Grunow) G. Novarino & A.R. Muftah-type—an alveola (sensu Paddock & Kemp) in a cavitate valve, with a single lateral external opening and a single squarish internal opening ([3] (Figure 37));
- Mastogloia sp. 1-type—an alveolus in a cavitate valve, with a single round external opening and small internal openings. A pair of internal openings corresponds to an external opening, lying “directly below” the outer aperture. Two layers of the valve are firmly attached to each other ([3] (Figure 38));
- Mastogloia sp. 2-type—a pseudoloculus in a cavitate valve, with a single internal and a single internal opening. Both openings are more or less round, one directly under another ([3] (Figure 39));
- Mastogloia lineata-type—a pseudoloculus in a cavitate valve, with a single, large, rectangular external opening and one or two small, round internal openings. The inner openings are situated asymmetrically in relation to the outer aperture, not lying directly under (due to the shape of the cavity) ([3] (Figure 40));
- Mastogloia sp. 3-type—a pseudoloculus in a cavitate valve, with a single, slit-like external opening and one or two small, round internal openings. The inner openings are situated asymmetrically in relation to the outer aperture, not lying directly under (due to the shape of the cavity) ([3] (Figure 41));
- Mastogloia emarginata-type—a pseudoloculus in a cavitate valve, with a single, large, round external opening and multiple small internal openings. The inner openings are situated asymmetrically in relation to the outer aperture, not lying directly under (due to the shape of the cavity). The walls of the pseudoloculus are “overhanging” ([3] (Figure 42));
- Mastogloia rimosa-type—a pseudoloculus in a cavitate valve, with a crescent, somewhat sunk external opening and a single round internal opening. The inner opening is situated asymmetrically in relation to the outer aperture, not lying directly under (due to the shape of the cavity). The internal opening is surrounded by four papillae ([3] (Figure 43));
- Mastogloia peracuta-type—a pseudoloculus in a cavitate valve, with a large, round external opening and one, sometimes two, minute internal openings. Pseudolocular walls are equally developed ([3] (Figure 44));
- M. elegans-type—a pseudoloculus in a cavitate valve, with a single, large, round or elongated external opening and usually four small internal openings. Inner openings are grouped in pairs, proceeding to different (adjacent) external openings. ([3] (Figure 45));
- Mastogloia cebuensis-type—a pseudoloculus in a cavitate valve, with a single, large, round or rectangular opening and (usually) six internal openings. External and internal openings correspond to the same pseudoloculus ([3] (Figure 46));
- Mastogloia latecostata-type—a pseudoloculus in a cavitate valve, with a large quadrate external opening and small internal openings, which are arranged in pairs. Each internal opening in a pair corresponds to adjacent (not the same) external apertures. Pseudolocular walls are asymmetrically developed ([3] (Figure 47));
- Mastogloia gibbosa-type—a pseudoloculus in a cavitate valve, with a large, round or elongated external opening and four, six, or more small internal openings. External and internal openings correspond to the same pseudoloculus ([3] (Figure 48));
- Mastogloia angulata-type—a pseudoloculus in a cavitate valve, with a large, round external opening and multiple small internal openings, forming a domed velum-like structure ([3] (Figure 49));
- Mastogloia labuensis var. lanceolata-type—a pseudoloculus in a cavitate valve, organized the same way as M. elegans-type, but equipped with a costa beneath the transapical wall of pseudolocula ([3] (Figure 50));
- Mastogloia chersonensis-type—a pseudoloculus in a cavitate valve, organized the same way as Mastogloia labuensis var. lanceolata-type, but differentiated by transapical shape of external opening, greater number of inner openings (six–eight vs. four–six) and thicker interstriae with costae ([3] (Figure 51));
- Aneumastus—pseudoloculi are occluded with colanderus-bifurca, i.e., in each pseudolocula external opening is somewhat hourglass-shaped, represented by a shallow rectangular depression. The depression is narrow to wide in the same valve or in different species. On the inside, small round perforations are organized in two groups (each with more than six perforations) separated by an apically elongated silica interruption. Each group of perforations within a single pair corresponds to different outer openings. In the transverse section, the silica interruption between the openings looks mushroom-shaped (formed by the silica interruption and two overhanging walls of the adjacent external apertures), and the cavity looks bifurcated ([4] (Plate 114, Figures 2 and 4); [29] (Figures 18,19, 22 and 23); [51] (Figures 16–19); Figure 2c–k;
- Paramastogloia and Mastoneis—pseudoloculi are occluded with colanderus-directa, i.e., in each pseudolocula is a large, circular to rectangular external opening and four inner openings that are small and round. The external opening lies directly above the group of four inner openings. Within the foursome, the openings are situated equidistantly or grouped in pairs. The silica flap in between the inner openings is flat ([34] (Figures 2 and 3)).
4.2.3. The Emended Description of the Order Mastogloiales
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LM | Light microscopy |
| SEM | Scanning electron microscopy |
| TEM | Transmitting electron microscopy |
| rbcL | Gene-encoding large subunit of RuBisCO |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| 18S rRNA | Small subunit of the rRNA |
| DIC | Differential interference contrast |
| PCR | Polymerase chain reaction |
| BI | Bayesian inference |
| ML | Maximum likelihood |
| GTR | General time reversible |
| LB | Likelihood bootstrap |
| PP | Posterior probabilities |
References
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 1–747. [Google Scholar]
- Pennesi, C.; Poulin, M.; Totti, C. Phylogenetic relationships and biogeography of the diatom genus Mastogloia (Bacillariophyceae): Revision of the section ellipticae including the description of new taxa. Protist 2016, 167, 148–173. [Google Scholar] [CrossRef]
- Paddock, T.B.B.; Kemp, K.D. An illustrated survey of the morphological features of the diatom genus Mastogloia. Diatom Res. 1990, 5, 73–103. [Google Scholar] [CrossRef]
- Lange-Bertalot, H. Navicula sensu Stricto, 10 Genera Separated from Navicula sensu Lato, Frustulia. Diatoms of Europe; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2001; Volume 2, pp. 1–526. [Google Scholar]
- Edlund, M.B.; Brant, L.A.; Levkov, Z.; Nakov, T. An emended description of Decussata (Patrick) Lange-Bertalot & Metzeltin that includes protoplast organization and detailed valve and cingulum ultrastructure. Diatom Res. 2006, 21, 269–280. [Google Scholar] [CrossRef]
- Spaulding, S.A.; Akbulut, A.; Kociolek, J.P. A new diatom species of Aneumastus D.G. Mann & Stickle from Central Turkey. Diatom Res. 2003, 18, 149–160. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Stoermer, E.F. Observations on North American Gomphoneis (Bacillariophyceae). II. Descriptions and ultrastructure of two new species. Trans. Am. Microsc. Soc. 1986, 105, 141–151. [Google Scholar] [CrossRef]
- Cox, E.J.; Williams, D.M. Systematics of naviculoid diatoms: The interrelationships of some taxa with a stauros. European J. Phycol. 2000, 35, 273–282. [Google Scholar] [CrossRef]
- Cox, E. Craspedostauros gen. nov., a new diatom genus for some unusual marine raphid species previously placed in Stauroneis Ehrenberg and Stauronella Mereschkowsky. Eur. J. Phycol. 1999, 34, 131–147. [Google Scholar] [CrossRef]
- Cox, E.J.; Williams, D.M. Systematics of naviculoid diatoms (Bacillariophyta): A preliminary analysis of protoplast and frustule characters for family and order level classification. Syst. Biodivers. 2006, 4, 385–399. [Google Scholar] [CrossRef]
- Cleve, P.T. Synopsis of the naviculoid diatoms. Part II. In Kongliga Svenska Vetenskapsak Akademiens Handlingar; R. Swedish Academy of Sciences: Stockholm, Sweden, 1895; Volume 27, pp. 1–219. [Google Scholar]
- Cox, E.J. Achnanthes sensu stricto belongs with genera of the Mastogloiales rather than with other monoraphid diatoms (Bacillariophyta). Eur. J. Phycol. 2006, 41, 67–81. [Google Scholar] [CrossRef]
- Cox, E.J. Coscinodiscophyceae, Mediophyceae, Fragilariophyceae, Bacillariophyceae (Diatoms). In Syllabus of Plant Families—A. Engler’s Syllabus der Pflanzenfamilien Part 2/1: Photoautotrophic Eukaryotic Algae. Glaucocystophyta, Cryptophyta, Dinophyta/Dinozoa, Haptophyta, Heterokontophyta/Ochrophyta, Chlorarachniophyta/Cercozoa, Euglenophyta/Euglenozoa, Chlorophyta, Streptophyta p.p., 13th ed.; Frey, W., Ed.; Borntraeger Science Publishers: Stuttgart, Germany, 2015; pp. 64–103. [Google Scholar]
- Medlin, L.K.; Kaczmarska, I. Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision. Phycologia 2004, 43, 245–270. [Google Scholar] [CrossRef]
- Sorhannus, U. Diatom phylogenetics inferred based on direct optimization of nuclear-encoded SSU rRNA sequences. Cladistics 2004, 20, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Bruder, K.; Medlin, L. Molecular assessment of phylogenetic relationships in selected species/genera in the naviculoid diatoms (Bacillariophyta). I. The genus Placoneis. Nova Hedwig. 2007, 85, 331. [Google Scholar] [CrossRef]
- Bruder, K.; Medlin, L.K. Morphological and molecular investigations of naviculoid diatoms. II. Selected genera and families. Diatom Res. 2008, 23, 283–329. [Google Scholar] [CrossRef]
- Bruder, K.; Medlin, L.K. Morphological and molecular investigations of naviculoid diatoms. III. Hippodonta and Navicula ss. Diatom Res. 2008, 23, 331–347. [Google Scholar] [CrossRef]
- Bruder, K.; Sato, S.; Medlin, L.K. Morphological and molecular investigations of naviculoid diatoms IV. Pinnularia vs. Caloneis. Diatom 2008, 24, 8–24. [Google Scholar]
- Rimet, F.; Kermarrec, L.; Bouchez, A.; Hoffmann, L.; Ector, L.; Medlin, L.K. Molecular phylogeny of the family Bacillariaceae based on 18S rDNA sequences: Focus on freshwater Nitzschia of the section Lanceolatae. Diatom Res. 2011, 26, 273–291. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Stepanek, J.G.; Lowe, R.L.; Johansen, J.R.; Sherwood, A.R. Molecular data show the enigmatic cave-dwelling diatom Diprora (Bacillariophyceae) to be a raphid diatom. Eur. J. Phycol. 2013, 48, 474–484. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Andreeva, S.A.; Gusev, E.S.; Kuznetsova, I.V.; Annenkova, N.V. Molecular phylogeny of monoraphid diatoms and raphe significance in evolution and taxonomy. Biol. Bull. 2016, 43, 398–407. [Google Scholar] [CrossRef]
- Ashworth, M.P.; Lobban, C.S.; Witkowski, A.; Theriot, E.C.; Sabir, M.J.; Baeshen, M.N.; Hajarah, N.H.; Baeshen, N.A.; Sabir, J.S.; Jansen, R.K. Molecular and morphological investigations of the stauros-bearing, raphid pennate diatoms (Bacillariophyceae): Craspedostauros EJ Cox, and Staurotropis TBB Paddock, and their relationship to the rest of the Mastogloiales. Protist 2017, 168, 48–70. [Google Scholar] [CrossRef]
- Ashworth, M.P.; Nakov, T.; Theriot, E.C. Revisiting Ross and Sims (1971): Toward a molecular phylogeny of the Biddulphiaceae and Eupodiscaceae (Bacillariophyceae). J. Phycol. 2013, 49, 1207–1222. [Google Scholar] [CrossRef]
- Sabir, J.S.; Theriot, E.C.; Manning, S.R.; Al-Malki, A.L.; Khiyami, M.A.; Al-Ghamdi, A.K.; Sabir, M.J.; Romanovicz, D.K.; Hajrah, N.H.; Omri, A.E.; et al. Phylogenetic analysis and a review of the history of the accidental phytoplankter, Phaeodactylum tricornutum Bohlin (Bacillariophyta). PLoS ONE 2018, 13, e0196744. [Google Scholar] [CrossRef]
- Lobban, C.S.; Tharngan, B.G.; Ashworth, M.P. Four new Licmophora species (Licmophorales), with a review of valve characters and exploration of cingulum characters, including a new septum type. Diatom Res. 2018, 33, 187217. [Google Scholar] [CrossRef]
- Lobban, C.S.; Ashworth, M.P.; Camacho, T.; Lam, D.W.; Theriot, E.C. Revision of Ardissoneaceae (Bacillariophyta, Mediophyceae) from Micronesian populations, with descriptions of two new genera, Ardissoneopsis and Grunowago, and new species in Ardissonea, Synedrosphenia and Climacosphenia. PhytoKeys 2022, 208, 103. [Google Scholar] [CrossRef] [PubMed]
- Stelbrink, B.; Jovanovska, E.; Levkov, Z.; Ognjanova-Rumenova, N.; Wilke, T.; Albrecht, C. Diatoms do radiate: Evidence for a freshwater species flock. J. Evol. Biol. 2018, 31, 1969–1975. [Google Scholar] [CrossRef]
- Maltsev, Y.; Andreeva, S.; Podunay, J.; Kulikovskiy, M. Description of Aneumastus mongolotusculus sp. nov. (Bacillariophyceae, Mastogloiales) from Lake Hovsgol (Mongolia) on the basis of molecular and morphological investigations. Nova Hedwig. 2019, 148, 21–33. [Google Scholar] [CrossRef]
- Mironov, A.; Glushchenko, A.; Kezlya, E.; Maltsev, Y.; Iurmanov, A.; Liu, Y.; Kulikovskiy, M. Decussiphycus sinensis sp. nov. (Bacillariophyceae, Mastogloiales)—A new species described from China, with comments on phylogenetic position of the genus. PhytoKeys 2025, 254, 1–19. [Google Scholar] [CrossRef]
- Stepanek, J.G.; Kociolek, J.P. Re-examination of Mereschkowsky’s genus Tetramphora (Bacillariophyta) and its separation from Amphora. Diatom Res. 2016, 31, 123–148. [Google Scholar] [CrossRef]
- Mereschkowsky, C. Les types de l’endochrome chez les diatomées. Bot. Zap. 1903, 21, 1–106. [Google Scholar]
- Cleve, P.T. Synopsis of the naviculoid diatoms. Part I. Kongliga Svenska Vetenskapsak Akademiens Handlingar; R. Swedish Academy of Sciences: Stockholm, Sweden, 1894; Volume 26, pp. 1–194. [Google Scholar]
- Lobban, C.S. Marine apartectal (chamberless) Mastogloiaceae (Diatomeae: Bacillariales): Paramastogloia cubana gen. nov., sp. nov., new observations and emended diagnosis of Mastoneis, and comparison with Mastogloiopsis. Taxonomy 2025, 5, 24. [Google Scholar] [CrossRef]
- Stoermer, E.F. Polymorphism in Mastogloia 1. J. Phycol. 1967, 3, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.C.; Gibson, R.A. Ultrastructural studies on some Mastogloia (Bacillariophyceae) species belonging to the group Ellipticae. Bot. Mar. 1979, 32, 499–509. [Google Scholar] [CrossRef]
- Stephens, F.C.; Gibson, R.A. Ultrastructural studies of some Mastogloia (Bacillariophyceae) species belonging to the groups Undulatae, Apiculatae, Lanceolatae and Paradoxae. Phycologia 1980, 19, 143–152. [Google Scholar] [CrossRef]
- Stephens, F.C.; Gibson, R.A. Ultrastructure Studies on Some Mastogloia Species of the Group Inaequales (Bacillariophyceae) 1. J. Phycol. 1980, 16, 354–363. [Google Scholar] [CrossRef]
- Yohn, T.A.; Gibson, R.A. Marine diatoms of the Bahamas. II. Mastogloia Thw. ex Wm. Sm. species of the groups Decussatae and Ellipticae. Bot. Mar. 1982, 35, 41–53. [Google Scholar] [CrossRef]
- Yohn, T.A.; Gibson, R.A. Marine diatoms of the Bahamas III. Mastogloia Thw. ex Wm. Sm. species of the groups Inaequales, Lanceolatae, Sulcatae and Undulatae. Bot. Mar. 1982, 35, 277–288. [Google Scholar] [CrossRef]
- Simonsen, R. On some diatoms of the genus Mastogloia. Beih. Zur Nova Hedwig. 1990, 100, 121–142. [Google Scholar]
- Novarino, G. A note on the internal construction of the partectal ring of Mastogloia lanceolata. Diatom Res. 1987, 2, 213–217. [Google Scholar] [CrossRef]
- Novarino, G. Observations on the frustule architecture of Mastogloia smithii, with particular reference to the valvocopulae and its integration with the valve. Diatom Res. 1990, 5, 373–385. [Google Scholar] [CrossRef]
- Novarino, G.; Muftah, A.R. A description of Mastogloia biocellata stat. nov. from Qatari coastal waters. Diatom Res. 1991, 6, 337–344. [Google Scholar] [CrossRef]
- Novarino, G.; Muftah, A.R. Observations on the variability of the number of partecta in five species of Mastogloia. Diatom Res. 1992, 7, 103–108. [Google Scholar] [CrossRef]
- Pennesi, C.; Poulin, M.; De Stefano, M.; Romagnoli, T.; Totti, C. New insights to the ultrastructure of some marine Mastogloia species section Sulcatae (Bacillariophyceae), including M. neoborneensis sp. nov. Phycologia 2011, 50, 548–562. [Google Scholar] [CrossRef]
- Pennesi, C.; Poulin, M.; De Stefano, M.; Romagnoli, T.; Totti, C. Revision of the genus Mastogloia, section sulcatae through electron microscopy. In Proceedings of the Twenty-Second International Diatom Symposium, Abstracts, Ghent, Belgium, 26–31 August 2012. [Google Scholar]
- Pennesi, C.; Poulin, M.; Hinz, F.; Romagnoli, T.; De Stefano, M.; Totti, C. Comparison of two new species of Mastogloia (Bacillariophyceae) with other small members of section Ellipticae. Phytotaxa 2013, 126, 1–21. [Google Scholar] [CrossRef]
- Lee, S.S.; Gaiser, E.E.; Van De Vijver, B.; Edlund, M.B.; Spaulding, S.A. Morphology and typification of Mastogloia smithii and M. lacustris, with descriptions of two new species from the Florida Everglades and the Caribbean region. Diatom Res. 2014, 29, 325–350. [Google Scholar] [CrossRef]
- Pavlov, A.; Jovanovska, E.; Wetzel, C.E.; Ector, L.; Levkov, Z. Freshwater Mastogloia (Bacillariophyceae) taxa from Macedonia, with a description of the epizoic M. sterijovskii sp. nov. Diatom Res. 2016, 31, 85–112. [Google Scholar] [CrossRef]
- Udovič, M.G.; Šušnjara, M.; Kulaš, A.; Goreta, G.; Arapov, J.; Levkov, Z. One new species of Aneumastus DG Mann et Stickle (Bacillariophyceae) from Krka River, Croatia. Fottea Olomouc 2023, 23, 21–29. [Google Scholar] [CrossRef]
- Lobban, C.S.; Navarro, J.N. Mastogloiopsis biseriata, gen. et sp. nov., a diatom without partecta, is very similar to the Marginulatae group of Mastogloia. Nova Hedwig. 2012, 94, 251. [Google Scholar] [CrossRef]
- Kezlya, E.; Glushchenko, A.; Kapustin, D.; Maltsev, Y.; Doan-Nhu, H.; Kulikovskiy, M. Stigmagloia lobbanii gen. et sp. nov. (Bacillariophyceae, Mastogloiales), a new stigma-bearing diatom genus separated from Mastogloia. Phytotaxa 2024, 677, 49–65. [Google Scholar] [CrossRef]
- AlgaeBase. World-Wide Electronic Publication, University of Galway. Available online: https://www.algaebase.org/ (accessed on 24 September 2025).
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide c1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Daugbjerg, N.; Andersen, R.A. A molecular phylogeny of the heterokont algae based on analyses of chloroplast-encoded rbcL sequence data. J. Phycol. 1997, 33, 1031–1041. [Google Scholar] [CrossRef]
- Ruck, E.C.; Theriot, E.C. Origin and evolution of the canal raphe system in diatoms. Protist 2011, 162, 723–737. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Paddock, T.B.B.; Kemp, K.D. A description of some new species of the genus Mastogloia with further observations on M. elegans and M. goessii. Diatom Res. 1988, 3, 109–121. [Google Scholar] [CrossRef]
- Stancheva, R.; Temniskova, D. Observations on Decussata hexagona (Torka) Lange-Bertalot (Bacillariophyta) from Holocene sediments in Bulgaria. Nova Hedwig. 2016, 82, 237–246. [Google Scholar] [CrossRef]
- Cox, E.J. Pore occlusions in raphid diatoms-a reassessment of their structure and terminology, with particular reference to members of the Cymbellales. Diatom 2004, 20, 33–46. [Google Scholar]
- Thomas, E.W.; Stepanek, J.G.; Kociolek, J.P. Historical and current perspectives on the systematics of the ‘enigmatic’ diatom genus Rhoicosphenia (Bacillariophyta), with single and multi-molecular marker and morphological analyses and discussion on the monophyly of ‘monoraphid’ diatoms. PLoS ONE 2016, 11, e0152797. [Google Scholar] [CrossRef]
- Kulikovskiy, M.; Maltsev, Y.; Andreeva, S.; Glushchenko, A.; Gusev, E.; Podunay, Y.; Ludwig, T.V.; Tusset, E.; Kociolek, J.P. Description of a new diatom genus Dorofeyukea gen. nov. with remarks on phylogeny of the family Stauroneidaceae. J. Phycol. 2019, 55, 173–185. [Google Scholar] [CrossRef]
- Kulikovskiy, M.; Glushchenko, A.; Kezlya, E.; Kuznetsova, I.; Kociolek, J.P.; Maltsev, Y. The genus Pinnularia Ehrenberg (Bacillariophyta) from the Transbaikal area (Russia, Siberia): Description of seven new species on the basis of morphology and molecular data with discussion of the phylogenetic position of Caloneis. Plants 2023, 12, 3552. [Google Scholar] [CrossRef]
- Li, J.; Mironov, A.; Jiang, Y.; Liang, J.; Kociolek, J.P.; Maltsev, Y.; Fan, Y.; Liu, Y.; Kulikovskiy, M. Molecular and morphological investigations of two new species in Qinia and Cymbella (Bacillariophyceae: Cymbellales) from China. PLoS ONE 2014, 19, e0314880. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.; Glushchenko, A.; Maltsev, Y.; Genkal, S.; Kuznetsova, I.; Kociolek, J.P.; Liu, Y.; Kulikovskiy, M. Reassessment of pore occlusion in some diatom taxa with re-evaluation of Placoneis Mereschkowsky (Bacillariophyceae: Cymbellales) and description of two new genera. PeerJ 2024, 12, e17278. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.; Maltsev, Y.; Kociolek, J.P.; Kezlya, E.; Liu, Y.; Kulikovskiy, M. Description of Encyonemataceae fam. nov. and Witkowskiaceae fam. nov. (Bacillariophyceae, Cymbellales) based on molecular and morphological analyses. Sci. Rep. 2024, 14, 31045. [Google Scholar] [CrossRef]
- Mann, D.G. An ontogenetic approach to diatom systematics. In Proceedings of the 7th International Diatom Symposium, Philadelphia, PA, USA, 22–27 August 1982. [Google Scholar]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Levkov, Z.; Krstic, S.; Metzeltin, D.; Nakov, T. Diatoms of Lakes Prespa and Ohrid, about 500 taxa from ancient lake system. Iconogr. Diatomol. 2007, 16, 1–613. [Google Scholar]
- Glushchenko, A.; Kulikovskiy, M.; Okhapkin, A.; Kociolek, J.P. Aneumastus laosica sp. nov. and A. genkalii sp. Nov.—Two New Diatom Species from Laos (Southeast Asia) with Comments on the Biogeography of the Genus. Cryptogam. Algol. 2017, 38, 183–199. [Google Scholar] [CrossRef]
- Trentin, R.; Moschin, E.; Duarte Lopes, A.; Schiaparelli, S.; Custódio, L.; Moro, I. Molecular, morphological and chemical diversity of two new species of Antarctic Diatoms, Craspedostauros ineffabilis sp. nov. and Craspedostauros zucchellii sp. nov. J. Mar. Sci. Eng. 2022, 10, 1656. [Google Scholar] [CrossRef]
- Sugawara, K.; Suzuki, H.; Kamiya, M.; Osada, K.; Witkowski, A. Morphology and molecular phylogeny of the marine diatom genus Nagumoea (Bacillariophyceae) from Japan. Phycol. Res. 2023, 71, 182–192. [Google Scholar] [CrossRef]
- Sugawara, K.; Kamiya, M.; Osada, K.; Suzuki, H. Molecular phylogeny of the marine diatom genus Druehlago (Bacillariophyceae) from Japan. Phycol. Res. 2024, 72, 231–237. [Google Scholar] [CrossRef]
- Hendey, N.I. The structure of the diatom cell wall as revealed by the electron microscope. J. Quekett Microsc. Club 1959, 5, 147–175. [Google Scholar]





| Samples from Fresh Waterbodies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample № | Slide № | Strain № | Locality | Coordinates | Substratum | t., °C | pH | µS/cm | Date of Collection |
| Mon58 | 09487 (wild), 10105 (strain) | CBMC338mnp | Southeastern shore of Lake Khovsgol, Khovsgol aimak, Mongolia | 50.62634 °N, 100.50109 °E | Benthos | 17.1 | 8.30 | 248 | 24 July 2024 |
| Mon59 | 09488 (wild), 10206 (strain) | CBMC529mnp | Southeastern shore of Lake Khovsgol, Khovsgol aimak, Mongolia | 50.62592 °N, 100.50087 °E | Epilithon, washing from silty stones | 17.6 | 8.51 | 210 | 24 July 2024 |
| Mn267 | 02937 | Mnp71 | Eastern shore of Lake Khovsgol, Khovsgol aimak, Mongolia | 50.80500 °N, 100.43972 °E | Benthos | 21 | 9.54 | 178 | 19 July 2015 |
| THHN 2014043 | 09153 | Ca68 | Unnamed stream, northern slope Wuzhishan Mountain, Hainan Province, China | 18.98150 °N, 109.68540 °E | Epilithon, washing from silty stones | 26.7 | 7.64 | 60 | 12 July 2014 |
| I277 | 04185 | Ind427 | Mahalona River, Sulawesi Island, Indonesia | 2.65694 °S, 121.52957 °E | Benthos | 18.8 | 8.79 | 184 | 24 September 2015 |
| Samples from saline waterbodies | |||||||||
| Sample № | Slide № | Strain № | Locality | Coordinates | Substratum | t., °C | Salinity, ‰ | Date of collection | |
| Mn183 | 03319 | CBMC102mns | Northeastern shore of Lake Oigon, Zavkhan aimak, Mongolia | 49.21344 °N, 96.64444 °E | Phytoplankton | 23.3 | 39 | 14 July 2015 | |
| NTs65 | 09255 | SVN638 | Shore of South China Sea, Khánh Hòa Province, Nha Trang, Vietnam | 12.20755 °N, 109.21541 °E | Epilithon | 29.2 | 34 | 3 April 2018 | |
| Type of Population | Sample | Slide | Length | Width | Striae (/10 μm) | Uniseriate Pseudoloculi (/10 μm) |
|---|---|---|---|---|---|---|
| Wild Wild Cultured | Mon58 | 09487 | 37.6–62.0 | 15.8–19.4 | 12 | 8 |
| Mon59 | 09488 | 43.5–63.8 | 17.0–20.3 | 12 | 8 | |
| Mon58 | 10105 | 41.6–43.7 | 16.5–17.3 | 12–13 | 8 | |
| Cultured | Mon59 | 10206 | 68.5–72.4 | 20.3–21.0 | 12 | 8 |
| A. khovsgolensis sp. nov. | A. tusculus (Type) | A. mongolotusculus | |
|---|---|---|---|
| Valve outline | Linear-elliptic to elliptic, abruptly narrowing towards the apices | Broadly linear-elliptic, gradually narrowing towards the apices | Broadly linear-elliptic |
| Morphology of apices | Obtusely rostrate | More or less rostrate, shoulder-like | Abruptly protracted, more or less capitate |
| Valve apices width, µm | 3.2–3.8 | 3.9–4.5 * | 2.9–3.2 |
| Valve length, µm | 37.6–72.4 | 51–67 * | 42–61 |
| Valve width, µm | 15.8–21.0 | 19–22 * | 16–19 |
| Striae density, in 10 µm | 12–13 | 10 * | 11–12 |
| Reference | This study | [4] | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, A.; Glushchenko, A.; Genkal, S.; Kezlya, E.; Maltsev, Y.; Nergui, S.; Kulikovskiy, M. Members of the Order Mastogloiales Sensu Cox Belong to the Different Evolutionary Lineages of Diatoms: Phylogenetic Resolutions and Descriptions of New Types of Pore Occlusions. Phycology 2025, 5, 68. https://doi.org/10.3390/phycology5040068
Mironov A, Glushchenko A, Genkal S, Kezlya E, Maltsev Y, Nergui S, Kulikovskiy M. Members of the Order Mastogloiales Sensu Cox Belong to the Different Evolutionary Lineages of Diatoms: Phylogenetic Resolutions and Descriptions of New Types of Pore Occlusions. Phycology. 2025; 5(4):68. https://doi.org/10.3390/phycology5040068
Chicago/Turabian StyleMironov, Andrei, Anton Glushchenko, Sergey Genkal, Elena Kezlya, Yevhen Maltsev, Soninkhishig Nergui, and Maxim Kulikovskiy. 2025. "Members of the Order Mastogloiales Sensu Cox Belong to the Different Evolutionary Lineages of Diatoms: Phylogenetic Resolutions and Descriptions of New Types of Pore Occlusions" Phycology 5, no. 4: 68. https://doi.org/10.3390/phycology5040068
APA StyleMironov, A., Glushchenko, A., Genkal, S., Kezlya, E., Maltsev, Y., Nergui, S., & Kulikovskiy, M. (2025). Members of the Order Mastogloiales Sensu Cox Belong to the Different Evolutionary Lineages of Diatoms: Phylogenetic Resolutions and Descriptions of New Types of Pore Occlusions. Phycology, 5(4), 68. https://doi.org/10.3390/phycology5040068







