Effects of Artesunate on the Growth and Chlorophyll Fluorescence of the Cyanobacterium Microcystis aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Cultures
2.2. Experimental Conditions
2.3. Measurements of Cell Density
2.4. Determination of Chlorophyll Fluorescence Parameters
2.5. Statistical Analysis
3. Results
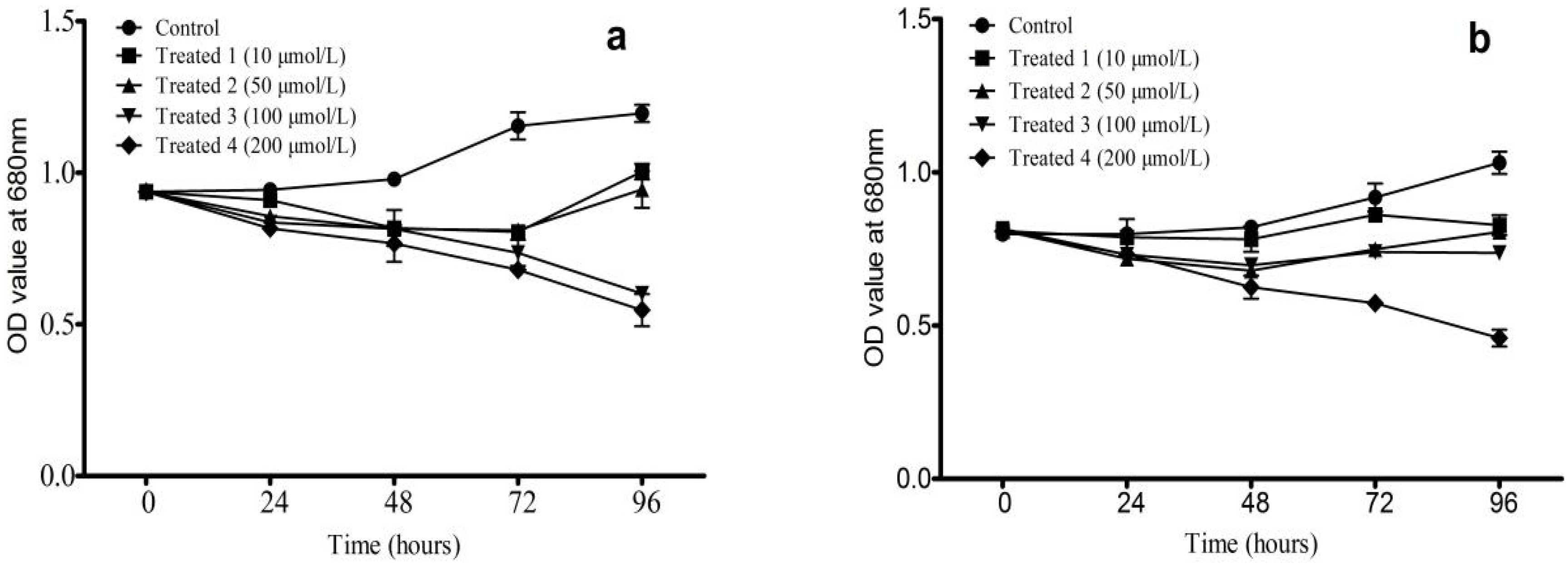
3.1. Effects of Artesunate on M. aeruginosa Growth
3.2. Effects of Artesunate on Chlorophyll Fluorescence Parameters
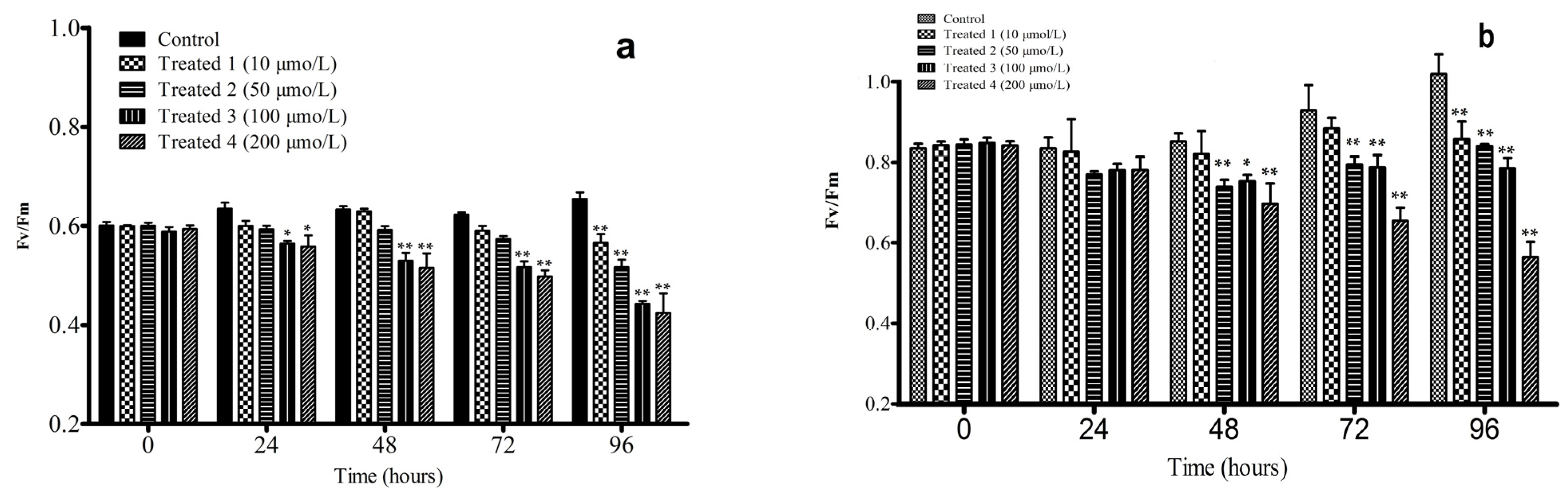
3.2.1. Effects of Artesunate on Fv/Fm
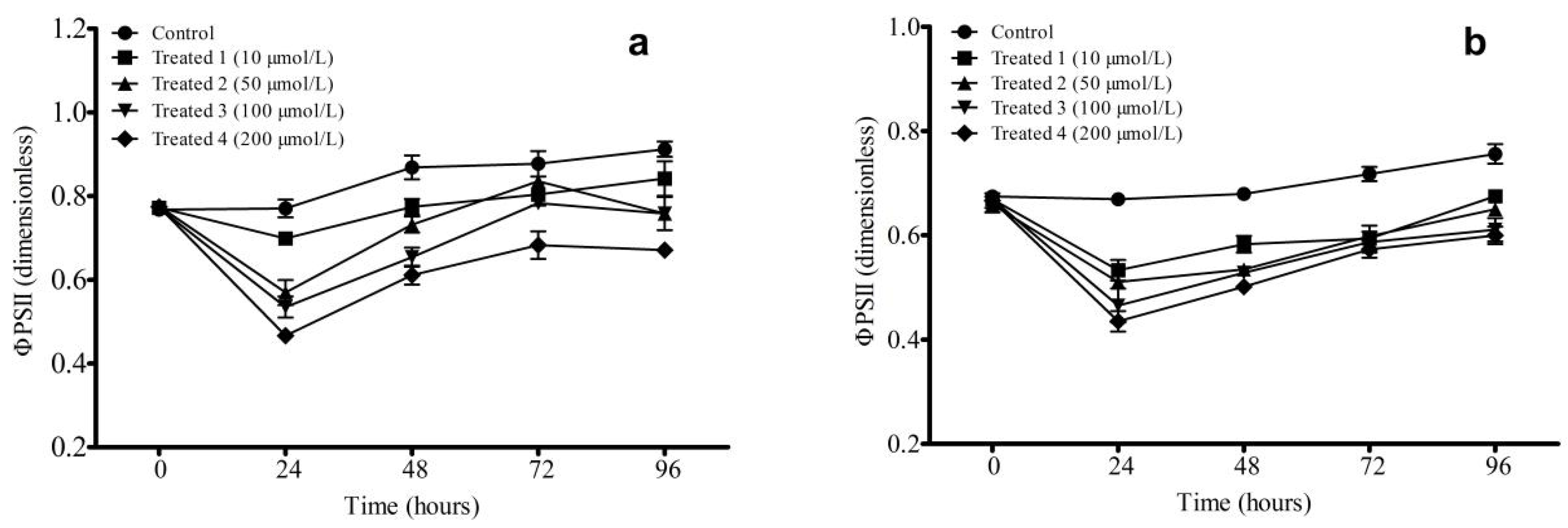
3.2.2. Change in φPSII
4. Discussion
4.1. Differential Inhibition of Toxigenic and Non-Toxigenic Strains
4.2. Time-Dependent Weakening of Inhibitory Effects
4.3. May Mechanisms of Artemisinin’s Algicidal Action
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I- chemical control methods. Harmful Algae 2021, 109, 102099. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.X.; Xu, C.; Shu, W.Q. Progress of research on potential chemoprotectants against microcystins. J. Environ. Health 2010, 27, 169–171. [Google Scholar]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): Abrief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jia, Y.; Qin, B.; Li, R.; Carmichael, W.W.; Gan, N.; Xu, H.; Shan, K.; Sukenik, A. Harmful cyanobacterial blooms: Biological traits, mechanisms, risks, and control strategies. Annu. Rev. Environ. Resour. 2023, 48, 123–147. [Google Scholar] [CrossRef]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef]
- Tu, Y.Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Zhang, T.J.; Wang, Y.F.; Liu, D. Historical story on natural medicinal chemistry: Artemisinin-a milestone of traditional Chinese medicine study. Chin. Tradit. Herb. Drugs 2016, 19, 3351–3361. [Google Scholar]
- Portugaliza, H.P.; Miyazaki, S.; Geurten, F.J.A.; Pell, C.; Rosanas, U.A.; Janse, C.J.; Cortés, A. Artemisinin exposure at the ring or trophozoite stage impacts Plasmodium falciparum sexual conversion differently. Elife 2020, 9, e60058. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Li, D.; Hu, S. Effects of artemisinin sustained-release granules on mixed alga growth and microcystins production and release. Environ. Sci. Pollut. Res. 2015, 22, 18637–18644. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Z. Effects of dihydroartemisinin and artemether on the growth, chlorophyll fluorescence, and extracellular alkaline phosphatase activity of the cyanobacterium Microcystis aeruginosa. PLoS ONE 2016, 11, e0164178. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Adugbo, S.E.; Ekanem, U.; Brisibe, F.; Figueira, G.M. Controlling bruchid pests of stored cowpea seeds with dried leaves of Artemisia annua and two other common botanicals. Afr. J. Biotechnol. 2011, 10, 9586–9592. [Google Scholar] [CrossRef]
- Khosravi, R.; Sendi, J.J.; Ghadamyari, M.; Yezdani, E. Effect of sweet wormwood Artemisia annua crude leaf extracts on some biological and physiological characteristics of the lesser mulberry pyralid Glyphodes pyloalis. J. Insect Sci. 2011, 11, 156. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R. A study on the toxicity of a medical plant, Artemisia annua L. (Asteracea) extracts to the sunn pest, Eurygaster integriceps puton (Hemiptera scutelleridae). J. Plant Prot. Res. 2010, 50, 79–85. [Google Scholar] [CrossRef]
- Shekari, M.; Sendi, J.J.; Etebari, K.; Zibaee, A.; Shadparvar, A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull. (Coleoptera: Chrysomellidae). Pestic. Biochem. Physiol. 2008, 91, 66–74. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Maggi, M.E.; Mangeaud, A.; Carpinella, M.C.; Ferrayoli, C.G.; Valladares, G.R.; Palacios, S.M. Laboratory evaluation of Artemisia annua L. extract and artemisinin activity against Epilachna paenulata and Spodoptera eridania. J. Chem. Ecol. 2005, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Durden, K.; Sellars, S.; Cowell, B.; Brown, J.J.; Pszczolkowski, M.A. Artemisia annua extracts, artemisinin and 1,8-cineole, prevent fruit infestation by a major, cosmopolitan pest of apples. Pharm. Biol. 2011, 49, 563–568. [Google Scholar] [CrossRef][Green Version]
- Duke, S.O.; Vaughn, K.C.; Croom, E.M.; ElSohly, H.N. Artemisinin, a constituent of annual wormwood (Artemisia annua), is a selective phytotoxin. Weed Sci. 1987, 35, 499–505. [Google Scholar] [CrossRef]
- Lydon, J.; Teasdale, J.R.; Chen, P.K. Allelopathic activity of annual wormwood (Artemisia annua) and the role of artemisinin. Weed Sci. 1997, 45, 807–811. [Google Scholar] [CrossRef]
- Delabays, N.; Slacanin, I.; Bohren, C. Herbicidal potential of artemisinin and allelopathic properties of Artemisia annua L: From the laboratory to the field. J. Plant Dis. Protect. 2008, 21, 317–322. [Google Scholar]
- Panamanik, R.C.; Chikkaswamy, B.K.; Roy, D.G.; Panamanik, A.; Kumar, V. Effects of biochemicals of Artemisia annua in plants. J. Phytol. Res. 2008, 21, 11–18. [Google Scholar]
- Jessing, K.K.; Cedergreen, N.; Jensen, J.; Hansen, H.C. Degradation and ecotoxicity of the biomedical drug artemisinin in soil. Environ. Toxicol. Chem. 2009, 28, 701–710. [Google Scholar] [CrossRef]
- Ni, L.; Acharya, K.; Ren, G. Preparation and characterization of anti-algal sustained-release granules and their inhibitory effects on algae. Chemosphere 2013, 91, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Yisa, A.G.; Chia, M.A.; Sha’aba, R.I.; Gauje, B.; Gadzama, I.M.K.; Oniye, S.J. Risk assessment of the antibiotic amoxicillin on non-toxin-producing strains and toxin-producing strains of Microcystis. Environ. Sci. Pollut. Res. 2023, 30, 56398–56409. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Qin, B.; Han, Y.H.; Yang, S.Y. Sulfamethoxazole and sulfaisoxazole stress on growth, photosynthesis, cell structure and oxidation system in Microcystis aeruginosa. Algal Res. 2025, 87, 103880. [Google Scholar] [CrossRef]
- Endo, H.; Moriyama, H.; Okumura, Y. Photoinhibition and photoprotective responses of a brown marine macroalga acclimated to different light and nutrient regimes. Antioxidants 2023, 12, 357. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, P.A.; Zhuang, B.W. The stomatal conductance and Fv/Fm as the indicators of stress tolerance of avocado seedlings under short-term waterlogging. Agronomy 2022, 12, 1084. [Google Scholar] [CrossRef]
- He, Y.C.; Chen, Y.; Tao, H.M.; Zhou, X.F.; Liu, J.; Liu, Y.H.; Yang, B. Secondary metabolites from cyanobacteria: Source, chemistry, bioactivities, biosynthesis and total synthesis. Phytochem. Rev. 2025, 24, 483–525. [Google Scholar] [CrossRef]
- Babica, P.; Bláha, L.; Marsálek, B. Exploring the natural role of microcystins—A review of effects on photoautotrophic organisms. J. Phycol. 2010, 42, 9–20. [Google Scholar] [CrossRef]
- Fu, C.J.; Wang, X.Y.; Yu, J.; Cui, H.; Hou, S.N.; Zhu, H. From winter dormancy to spring bloom: Regulatory mechanisms in Microcystis aeruginosa post-overwintering recovery. Water Res. 2024, 269, 122807. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Qin, N.; Zhao, W.; Huang, H. Time dependence effect of metal toxicology and application in WQC derivation of main water basins in China. Ecol. Indic. 2025, 170, 113049. [Google Scholar] [CrossRef]
- Kathol, M.; Azme, A.; Saifur, S.; Aich, N.; Saha, R. Unique adaptations of a photosynthetic microbe Rhodopseudomonas palustristo the toxicological effects of perfluorooctanoic acid. Env. Sci. Adv. 2025, 4, 1191–1197. [Google Scholar] [CrossRef]
- Zhou, L.H.; Zheng, T.L.; Wang, X.; Ye, J.L.; Tian, Y.; Hong, H.S. Effect of five chinese traditional medicines on the biological activity of a red-tide causing alga-Alexandrium tamarense. Harmful Algae 2007, 6, 354–360. [Google Scholar] [CrossRef]
- Chia, M.A.; Micheline, K.C.A.; Lorenzi, A.S.; Bittencourt-Oliveira, M.D.C. Does anatoxin-a influence the physiology of Microcystis aeruginosa and Acutodesmus acuminatus under different light and nitrogen conditions? Environ. Sci. Pollut. Res. Int. 2016, 23, 23092–23102. [Google Scholar] [CrossRef] [PubMed]
- Mikula, P.; Zezulka, S.; Jancula, D.; Marsalek, B. Metabolic activity and membrane integrity changes in Microcystis aeruginosa—New findings on hydrogen peroxide toxicity in cyanobacteria. Eur. J. Phycol. 2012, 47, 195–206. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.J.; Chia, W.N.; Loh, C.C.Y.; Li, Z.; Lee, Y.M. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111–10122. [Google Scholar] [CrossRef] [PubMed]
| Species Classification | Species and Stage | Endpoint | Effect Formulation or Concentration | Estimated or Given Artemisinin Concentrations | Source |
|---|---|---|---|---|---|
| Insect | Cowpea bruchid, Callosobruchus maculatus | Significantly increased mortality | 2.5 g leaves/250 g cowpea seeds | 0.1–4 μg/g | [14] |
| Lesser mulberry pyralid, Glyphodes pyloalis 4th instar larvae | LC50 by topical application | 0.33 mg leaf/mL | 0.033–1.32 mg/L | [15] | |
| Sunn pest (Eurygaster Integriceps) | LC50 | 32% (24 h) and 17% (48 h), methanolic extract | 9.6–380 μg/mL (24 h) and 50–200 mg/L (48 h) | [16] | |
| Elm leaf Beetle, Xanthogaleruca luteola Mull. | LC50 | 48% (24 h) and 44% (48 h), methanolic extract | 14.4–576 μg/mL (24 h) and 13.2–528 mg/L (48 h) | [17] | |
| Small white Pieris rapae, 3th instar larvae | LC50 | 9.4% extract | 2.8–113 mg/L | [18] | |
| Epilachna paenulata | Complete feeding rejection with ethanol extract of A. annua | 1.5 mg/cm2 | 36 μg/cm2 | [19] | |
| Codling moth, Cydia pomonella | Feeding deterrent at p < 0.05, extract | 1 g/L | 0.2 mg/L | [20] | |
| Plant | Sorghum, Sorghum bicolor L. | Germination | No effect up to 9.3 μg/mL | No effect up to 56 μg/Petri dish | [21] |
| Soybean, Glycine max | Growth inhibition, 25% | 0.73% dry leaf material in soil | 3.3 mg/kg soil | [22] | |
| Potato, Solanum tuberosum | Root growth inhibition, 100% | material in soil | 32.5 kg/ha | [23] | |
| Barley, Hordeum vulgare | Germination inhibition, 22% | 100 mg/L | 600 μg/Petri dish | [24] | |
| Redroot pigweed, Amaranthus retroflexus L. dish | Growth inhibition | 9.3 mg/L | 56 μg/Petri | [21] | |
| Freshwater algae, Pseudokirchneriella subcapitata | Relative growth rate, EC50 | 0.24 mg/L | [25] | ||
| Duckweed, Lemna minor | Relative growth rate, EC50 | 0.19 mg/L | [25] | ||
| Microalgae Scenedesmus obliquus | Growth inhibition | 12–20 mg/L | [12] | ||
| Microalgae Microcystis aeruginosa | Growth inhibition | 8–12 mg/L | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ning, W.; Wang, W.; Hu, Y.; Yang, A. Effects of Artesunate on the Growth and Chlorophyll Fluorescence of the Cyanobacterium Microcystis aeruginosa. Phycology 2025, 5, 63. https://doi.org/10.3390/phycology5040063
Wang H, Ning W, Wang W, Hu Y, Yang A. Effects of Artesunate on the Growth and Chlorophyll Fluorescence of the Cyanobacterium Microcystis aeruginosa. Phycology. 2025; 5(4):63. https://doi.org/10.3390/phycology5040063
Chicago/Turabian StyleWang, Huan, Wenyu Ning, Wenxia Wang, Yue Hu, and Aoao Yang. 2025. "Effects of Artesunate on the Growth and Chlorophyll Fluorescence of the Cyanobacterium Microcystis aeruginosa" Phycology 5, no. 4: 63. https://doi.org/10.3390/phycology5040063
APA StyleWang, H., Ning, W., Wang, W., Hu, Y., & Yang, A. (2025). Effects of Artesunate on the Growth and Chlorophyll Fluorescence of the Cyanobacterium Microcystis aeruginosa. Phycology, 5(4), 63. https://doi.org/10.3390/phycology5040063







