A Novel Approach Towards RSM-Based Optimization of LED-Illuminated Mychonastes homosphaera Culture, Emphasizing Input Energy: An Industrial Perspective of Microalgae Cultivation
Abstract
1. Introduction
2. Methodology
2.1. Experimental Setup Design and Development
2.2. Microalgae Strain and Culture Conditions
Control of Kinetic Processes
2.3. Experimental Design
2.4. Biomass Productivity
2.5. Lipid Productivity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Input Parameters on Biomass Production
3.2. Effect of Input Parameters on Lipid Production
3.3. Modeling and Validation
3.4. Optimization
4. Industrial Perspective
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milano, J.; Ong, H.C.; Masjuki, H.; Chong, W.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; Zepka, L.Q.; Jacob-Lopes, E. Roadmap to net-zero carbon emissions in commercial microalgae-based products: Environmental sustainability and carbon offset costs. J. Appl. Phycol. 2022, 34, 1255–1268. [Google Scholar] [CrossRef]
- González-Balderas, R.; Felix, M.; Bengoechea, C.; Ledesma, M.O.; Guerrero, A.; Velasquez-Orta, S. Development of composites based on residual microalgae biomass cultivated in wastewater. Eur. Polym. J. 2021, 160, 110766. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N.J.R. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: Current state and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1088. [Google Scholar] [CrossRef]
- Khalili, A.; Najafpour, G.D.; Amini, G.; Samkhaniyani, F. Influence of nutrients and LED light intensities on biomass production of microalgae Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2015, 20, 284–290. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tyagi, V.; Jeyraj, A.; Selvaraj, L.; Rahim, N.; Tyagi, S. Recent advances in solar photovoltaic systems for emerging trends and advanced applications. Renew. Sustain. Energy Rev. 2016, 53, 859–884. [Google Scholar] [CrossRef]
- Shubbak, M.H. Advances in solar photovoltaics: Technology review and patent trends. Renew. Sustain. Energy Rev. 2019, 115, 109383. [Google Scholar] [CrossRef]
- Sirisuk, P.; Ra, C.-H.; Jeong, G.-T.; Kim, S.-K. Effects of wavelength mixing ratio and photoperiod on microalgal biomass and lipid production in a two-phase culture system using LED illumination. Bioresour. Technol. 2018, 253, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Fettah, N.; Derakhshandeh, M.; Tezcan Un, U.; Mahmoudi, L.J. Effect of light on growth of green microalgae Scenedesmus quadricauda: Influence of light intensity, light wavelength and photoperiods. Int. J. Energy Environ. Eng. 2022, 13, 703–712. [Google Scholar] [CrossRef]
- Eldiehy, K.S.; Daimary, N.; Borah, D.; Mandal, M.; Deka, D. Biodiesel Production from Chlorella homosphaera by Two-Step Catalytic Conversion Using Waste Radish Leaves as a Source for Heterogeneous Catalyst. Appl. Biochem. Biotechnol. 2023, 195, 4347–4367. [Google Scholar] [CrossRef]
- Eldiehy, K.S.; Bardhan, P.; Borah, D.; Rather, M.A.; Chutia, H.; Bhagya Raj, G.V.; Mandal, M.; Deka, D. Optimization of nutrient composition for enhanced microalgal biomass and macromolecules using RSM: An integrated approach towards improving microalgal biodiesel feasibility. J. Appl. Phycol. 2022, 34, 2869–2882. [Google Scholar] [CrossRef]
- Lu, R.; Hong, B.; Wang, Y.; Cui, X.; Liu, C.; Liu, Y.; Wu, X.; Ruan, R.; Zhang, Q. Microalgal biofilm cultivation on lignocellulosic based bio-carriers: Effects of material physical characteristics on microalgal biomass production and composition. Chem. Eng. J. 2025, 510, 161656. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Borah, D.; Eldiehy, K.S.; Hatiboruah, D.; Mandal, M.; Deka, D. An Integrated Approach for Simultaneous Monitoring and Data Acquisition on the Culture of Green Microalga Chlorella homosphaera Using Different LED Illumination. BioEnergy Res. 2023, 16, 601–610. [Google Scholar] [CrossRef]
- Sandani, W.P.; Premaratne, M.; Ariyadasa, T.U.; Premachandra, J.K. Novel strategy for microalgae cell disruption and wet lipid extraction by employing electro-Fenton process with sacrificial steel anode. Bioresour. Technol. 2022, 343, 126110. [Google Scholar] [CrossRef] [PubMed]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Biduski, B.; Treichel, H.; Colla, L.M. Microalgae growth with a high concentration of emerging pollutants and phytotoxicity evaluation of cultivation wastewater. J. Water Process Eng. 2022, 46, 102616. [Google Scholar] [CrossRef]
- da Costa, A.C.A.; Leite, S.G.F. Metals biosorption by sodium alginate immobilized Chlorella homosphaera cells. Biotechnol. Lett. 1991, 13, 559–562. [Google Scholar] [CrossRef]
- de Freitas, B.C.B.; Brächer, E.H.; de Morais, E.G.; Atala, D.I.P.; de Morais, M.G.; Costa, J.A.V. Cultivation of different microalgae with pentose as carbon source and the effects on the carbohydrate content. Environ. Technol. 2019, 40, 1062–1070. [Google Scholar] [CrossRef]
- Costa, S.S.; Peres, B.P.; Machado, B.R.; Costa, J.A.V.; Santos, L.O. Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour. Technol. 2020, 315, 123880. [Google Scholar] [CrossRef]
- Margarites, A.C.; Volpato, N.; Araújo, E.; Cardoso, L.G.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Spirulina platensis is more efficient than Chlorella homosphaera in carbohydrate productivity. Environ. Technol. 2017, 38, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Li, S.; Song, C.; Li, M.; Chen, Y.; Lei, Z.; Zhang, Z. Effect of different nitrogen ratio on the performance of CO2 absorption and microalgae conversion (CAMC) hybrid system. Bioresour. Technol. 2020, 306, 123126. [Google Scholar] [CrossRef]
- Peng, H.; De-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, X. Comparison about the three central composite designs with simulation. In Proceedings of the International Conference on Advanced Computer Control, Singapore, 22–24 January 2009; pp. 163–167. [Google Scholar]
- Hosseini, N.S.; Shang, H.; Scott, J.A. Optimization of microalgae-sourced lipids production for biodiesel in a top-lit gas-lift bioreactor using response surface methodology. Energy 2018, 146, 47–56. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Bialevich, V.; Zachleder, V.; Bišová, K. The effect of variable light source and light intensity on the growth of three algal species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Wu, L.F.; Chen, P.C.; Huang, A.P.; Lee, C.M. The feasibility of biodiesel production by microalgae using industrial wastewater. Bioresour. Technol. 2012, 113, 14–18. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Borella, L.; Diotto, D.; Barbera, E.; Fiorimonte, D.; Sforza, E.; Trivellin, N. Application of flashing blue-red LED to boost microalgae biomass productivity and energy efficiency in continuous photobioreactors. Energy 2022, 259, 125087. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef]
- Wong, Y.; Ho, Y.; Ho, K.; Lai, Y.; Tsang, P.; Chow, K.; Yau, Y.; Choi, M.; Hosseini, R. Effects of Light Intensity, Illumination Cycles on Microalgae Haematococcus Pluvialis for Production of Astaxanthin. J. Mar. Biol. Aquac. 2016, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Yustinadiar, N.; Manurung, R.; Suantika, G. Enhanced biomass productivity of microalgae Nannochloropsis sp. in an airlift photobioreactor using low-frequency flashing light with blue LED. Bioresour. Bioprocess. 2020, 7, 43. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Z.; Wen, W.; Yan, J. Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J. Appl. Phycol. 2013, 25, 129–137. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Eldiehy, K.S.H.; Das, V.; Borah, D.; Mandal, M.; Deka, D. Strategic approaches to enhance lipid accumulation in microalgae for bioprospecting. In Algal Biorefinery; Dalai, A., Goud, V., Nanda, S., Borugadda, V., Eds.; Routledge: London, UK, 2021; pp. 50–84. [Google Scholar]
- Markou, G. Effect of various colors of light-emitting diodes (LEDs) on the biomass composition of Arthrospira platensis cultivated in semi-continuous mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef]
- Jung, J.-H.; Sirisuk, P.; Ra, C.H.; Kim, J.-M.; Jeong, G.-T.; Kim, S.-K. Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem. 2019, 77, 93–99. [Google Scholar] [CrossRef]
- Kim, S.H.; Sunwoo, I.Y.; Hong, H.J.; Awah, C.C.; Jeong, G.-T.; Kim, S.-K. Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system. Bioprocess Biosyst. Eng. 2019, 42, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Contreras-Gómez, A.; Molina-Grima, E. Modeling shear-sensitive dinoflagellate microalgae growth in bubble column photobioreactors. Bioresour. Technol. 2017, 245, 250–257. [Google Scholar] [CrossRef]
- Morales, M.; Sánchez, L.; Revah, S. The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol. Lett. 2018, 365, fnx262. [Google Scholar] [CrossRef] [PubMed]
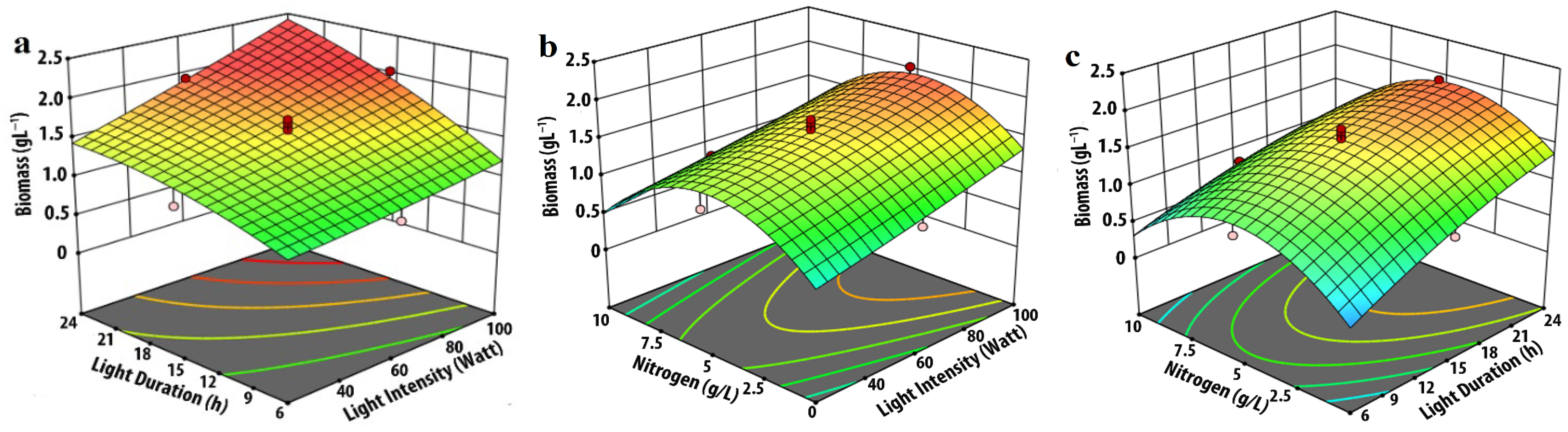
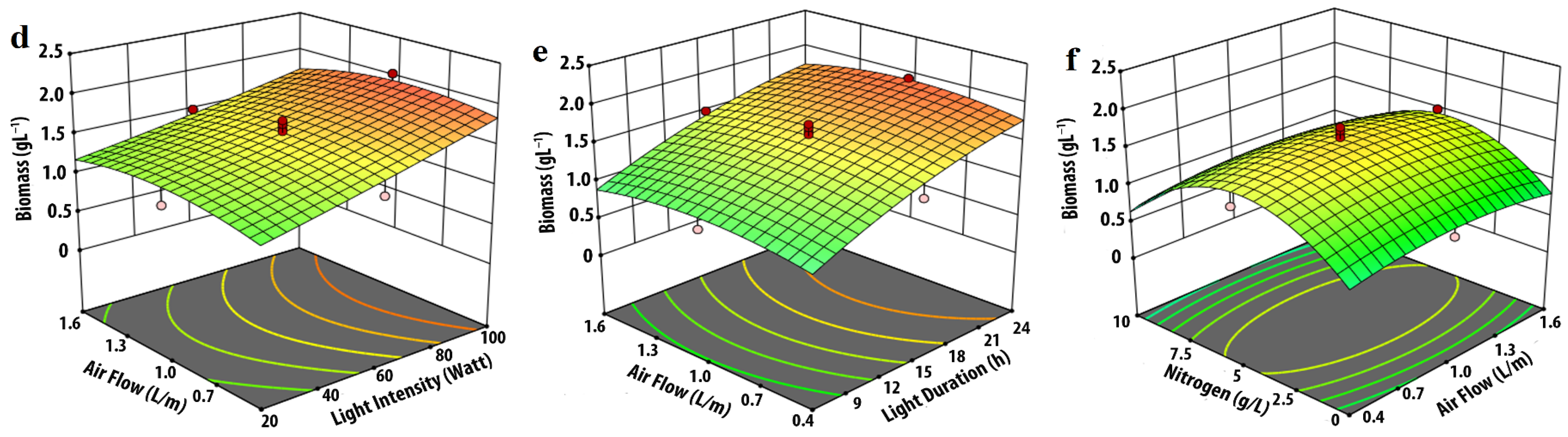
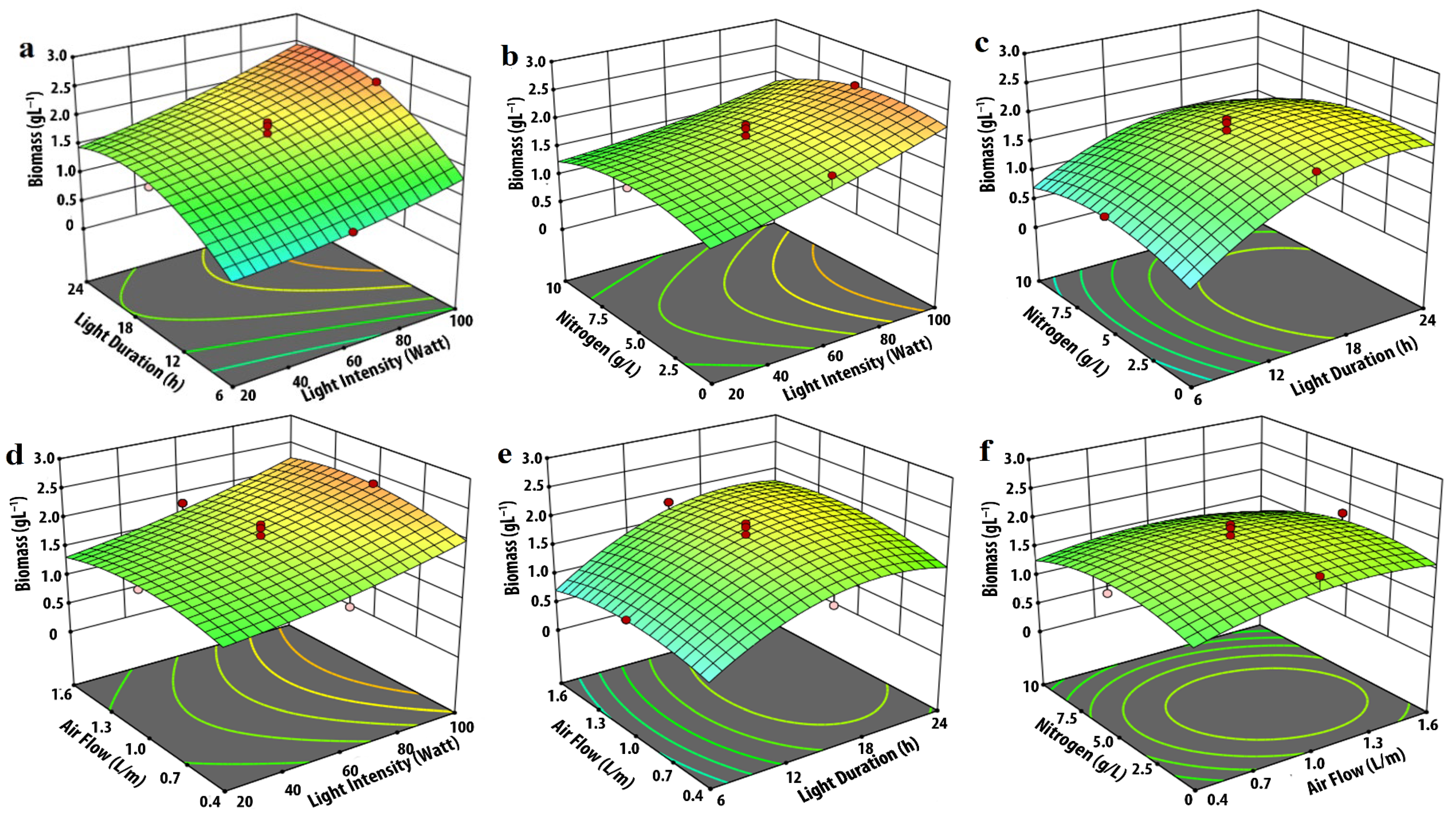
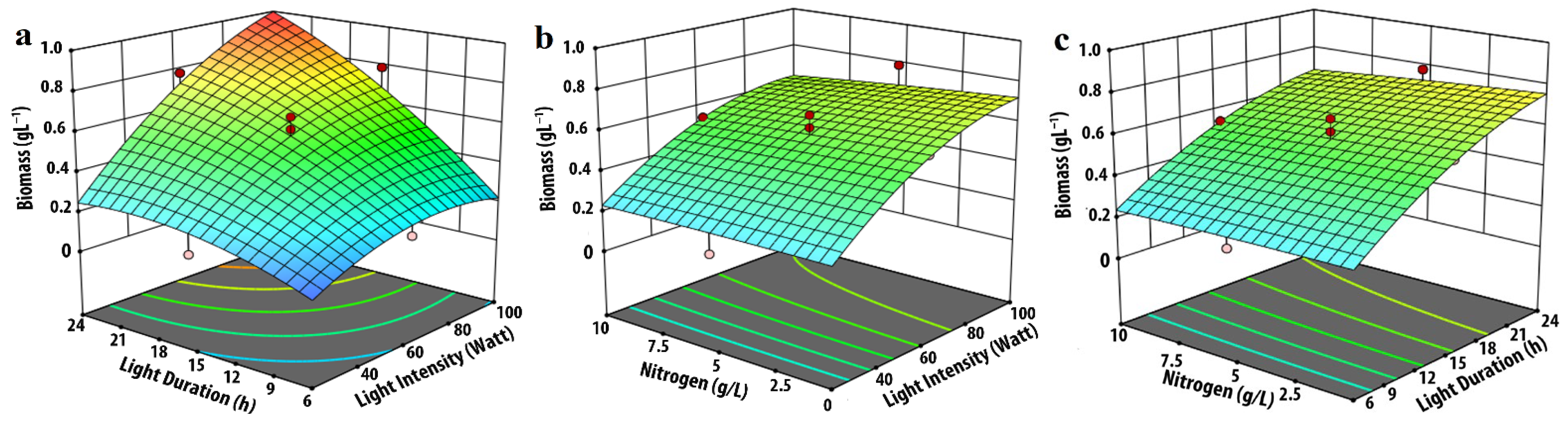

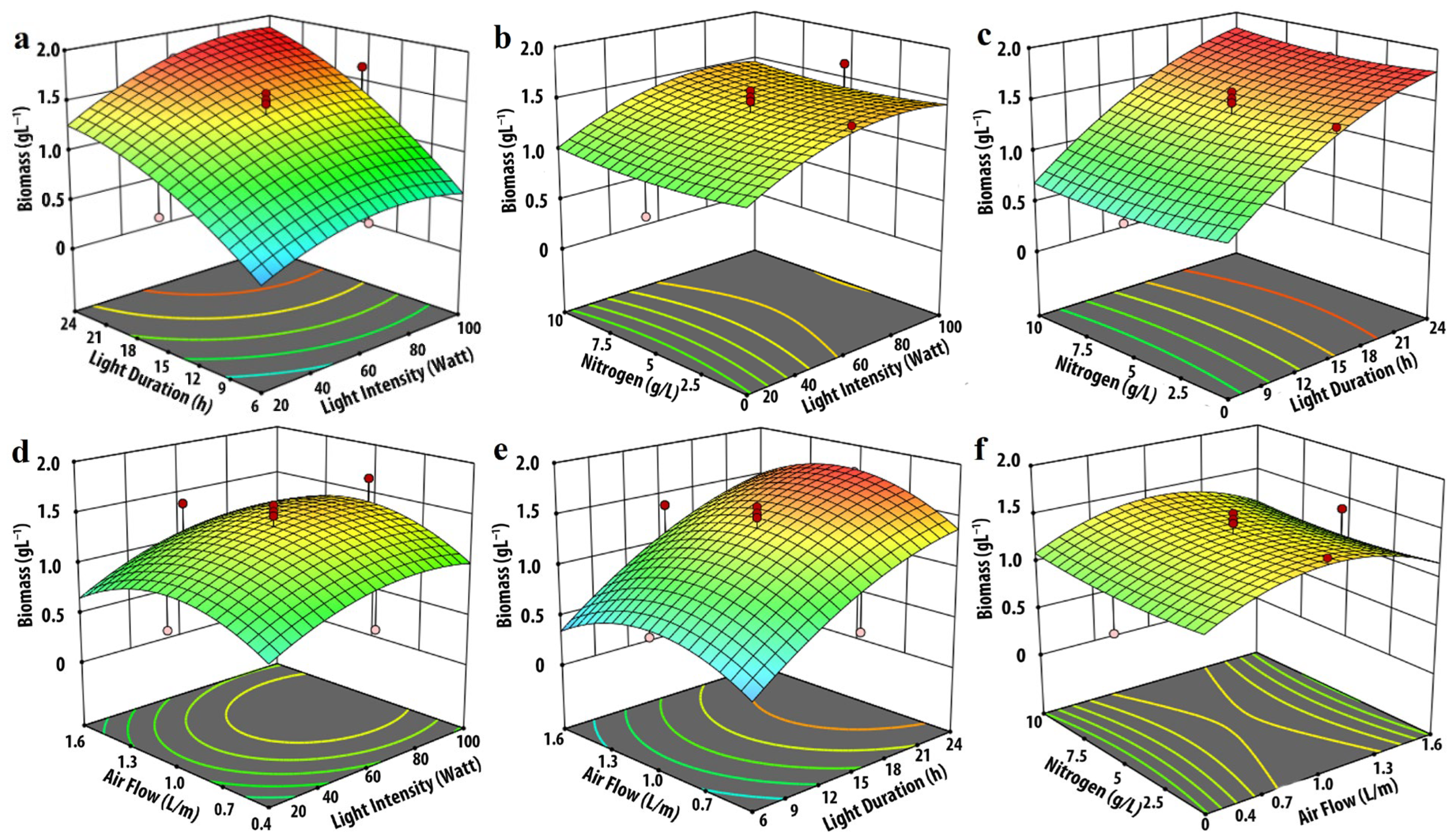

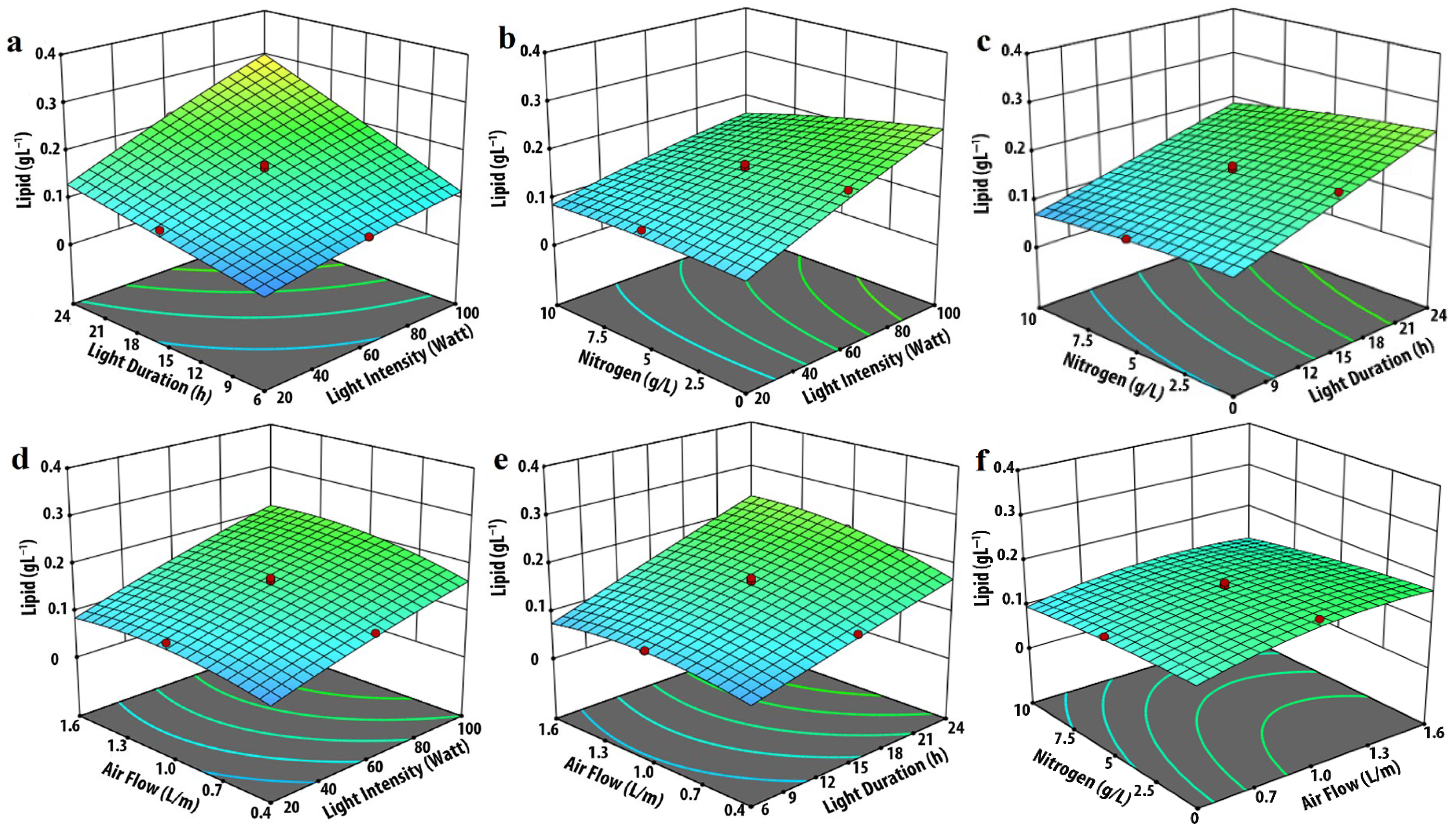
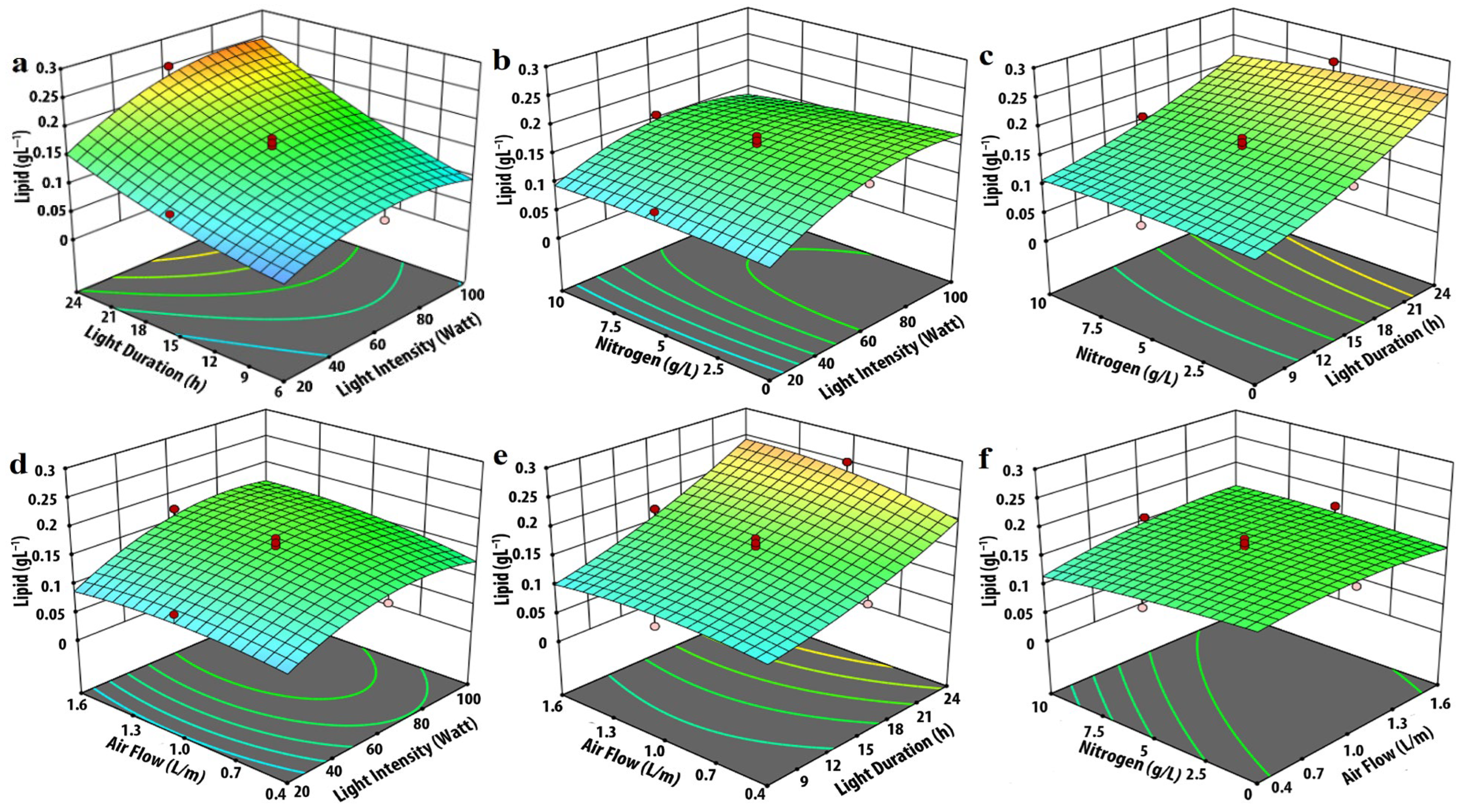

| Experimental Variables | Code | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Light duration (h) | A | 0 | 6 | 24 |
| Light intensity (%) | B | 0 | 20 | 100 |
| Airflow (L min−1) | C | 0 | 0.4 | 1.6 |
| Nitrogen content (g L−1) | D | 0 | 0.1 | 10.0 |
| Four Factor FCCCD Experimental Design | |||||
|---|---|---|---|---|---|
| Exp. No. | Intensity (%) | Duration (h) | Air (L min−1) | Nitrogen (g L−1) | Group |
| 1 | 20 | 6 | 0.4 | 0.1 | I |
| 2 | 20 | 6 | 0.4 | 10 | |
| 3 | 20 | 6 | 1.6 | 0.1 | |
| 4 | 20 | 6 | 1.6 | 10 | |
| 5 | 20 | 15 | 1 | 5.05 | II |
| 6 | 20 | 24 | 0.4 | 0.1 | III |
| 7 | 20 | 24 | 0.4 | 10 | |
| 8 | 20 | 24 | 1.6 | 0.1 | |
| 9 | 20 | 24 | 1.6 | 10 | |
| 10 | 60 | 6 | 1 | 5.05 | IV |
| 11 | 60 | 15 | 0.4 | 5.05 | V |
| 12 | 60 | 15 | 1 | 0.1 | |
| 13 | 60 | 15 | 1 | 5.05 | |
| 14 | 60 | 15 | 1 | 5.05 | |
| 15 | 60 | 15 | 1 | 5.05 | VI |
| 16 | 60 | 15 | 1 | 5.05 | |
| 17 | 60 | 15 | 1 | 5.05 | VII |
| 18 | 60 | 15 | 1 | 5.05 | |
| 19 | 60 | 15 | 1 | 10 | |
| 20 | 60 | 15 | 1.6 | 5.05 | |
| 21 | 60 | 24 | 1 | 5.05 | VIII |
| 22 | 100 | 6 | 0.4 | 0.1 | IX |
| 23 | 100 | 6 | 0.4 | 10 | |
| 24 | 100 | 6 | 1.6 | 0.1 | |
| 25 | 100 | 6 | 1.6 | 10 | |
| 26 | 100 | 15 | 1 | 5.05 | X |
| 27 | 100 | 24 | 0.4 | 0.1 | XI |
| 28 | 100 | 24 | 0.4 | 10 | |
| 29 | 100 | 24 | 1.6 | 0.1 | |
| 30 | 100 | 24 | 1.6 | 10 | |
| Biomass Production | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | Cool White LED | Pink LED | Blue LED | Red LED | ||||||||
| F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | ||||||
| Model | 14 | 30.86 | <0.0001 | Sig. | 53.28 | <0.0001 | Sig. | 13.66 | <0.0001 | Sig. | 52.95 | <0.0001 | Sig. |
| A-LI | 1 | 95.17 | <0.0001 | 117.77 | <0.0001 | 18.69 | 0.0006 | 250.37 | <0.0001 | ||||
| B-LD | 1 | 135.60 | <0.0001 | 213.48 | <0.0001 | 117.78 | <0.0001 | 283.07 | <0.0001 | ||||
| C-AF | 1 | 1.96 | 0.1815 | 0.0261 | 0.8739 | 0.3702 | 0.5520 | 0.2036 | 0.6583 | ||||
| D-Nitro | 1 | 7.73 | 0.0140 | 12.44 | 0.0030 | 0.0017 | 0.9681 | 2.52 | 0.1333 | ||||
| AB | 1 | 6.11 | 0.0259 | 26.89 | 0.0001 | 2.77 | 0.1170 | 104.83 | <0.0001 | ||||
| AC | 1 | 2.27 | 0.1526 | 2.10 | 0.1675 | 0.3754 | 0.5492 | 0.1045 | 0.7510 | ||||
| AD | 1 | 4.64 | 0.0479 | 1.05 | 0.3215 | 0.0065 | 0.9369 | 1.75 | 0.2059 | ||||
| BC | 1 | 3.35 | 0.0871 | 0.1473 | 0.7066 | 0.3446 | 0.5659 | 1.04 | 0.3239 | ||||
| BD | 1 | 12.44 | 0.0030 | 9.36 | 0.0079 | 0.2530 | 0.6223 | 0.9403 | 0.3476 | ||||
| CD | 1 | 3.30 | 0.0895 | 0.2212 | 0.6449 | 0.0139 | 0.9077 | 0.0192 | 0.8917 | ||||
| A2 | 1 | 0.5543 | 0.4681 | 0.0704 | 0.7943 | 2.05 | 0.1728 | 7.25 | 0.0167 | ||||
| B2 | 1 | 18.10 | 0.0007 | 2.28 | 0.1522 | 1.64 | 0.2196 | 4.01 | 0.0636 | ||||
| C2 | 1 | 4.14 | 0.0600 | 3.52 | 0.0803 | 5.59 | 0.0320 | 2.95 | 0.1062 | ||||
| D2 | 1 | 7.26 | 0.0167 | 81.17 | <0.0001 | 0.1866 | 0.6719 | 0.0123 | 0.9133 | ||||
| Lack of Fit | 10 | 1.51 | 0.3397 | N. Sig. | 3.16 | 0.1083 | N. Sig. | 2.10 | 0.2135 | N. Sig. | 1.21 | 0.4405 | N. Sig. |
| R2 | 0.9664 | 0.9803 | 0.9273 | 0.9802 | |||||||||
| Experimental Parameters | Biomass Production (g L−1) | Lipid Production (mg L−1) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Pink | Blue | Red | White | Pink | Blue | Red | |||||||||||||
| No | Int. | Dur. | Air | Nitro. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. |
| 1 | 20 | 6 | 0.4 | 0.1 | 0.058 | 0.132 | 0.042 | 0.058 | 0.146 | 0.144 | 0.029 | 0.008 | 26.0 | 40.0 | 18.0 | 19.0 | 52.0 | 64.0 | 9.0 | 18.0 |
| 2 | 20 | 6 | 0.4 | 10 | 0.642 | 0.543 | 0.068 | 0.021 | 0.134 | 0.090 | 0.031 | 0.027 | 51.0 | 49.0 | 22.0 | 41.0 | 62.0 | 59.0 | 18.0 | 18.0 |
| 3 | 20 | 6 | 1.6 | 0.1 | 0.118 | 0.115 | 0.094 | 0.079 | 0.126 | 0.089 | 0.03 | 0.005 | 35.0 | 27.0 | 38.0 | 25.0 | 45.0 | 23.0 | 7.0 | 1.0 |
| 4 | 20 | 6 | 1.6 | 10 | 0.201 | 0.220 | 0.088 | 0.098 | 0.102 | 0.010 | 0.042 | 0.023 | 56.0 | 53.0 | 26.0 | 28.0 | 50.0 | 62.0 | 34.0 | 49.0 |
| 5 | 20 | 15 | 1 | 5.05 | 1.494 | 1.551 | 1.066 | 1.286 | 0.638 | 0.970 | 0.136 | 0.239 | 112.0 | 106.0 | 107.0 | 94.0 | 114.0 | 99.0 | 66.0 | 47.0 |
| 6 | 20 | 24 | 0.4 | 0.1 | 1.122 | 0.991 | 0.706 | 0.707 | 1.042 | 0.945 | 0.18 | 0.173 | 116.0 | 108.0 | 72.0 | 81.0 | 144.0 | 142.0 | 124.0 | 124.0 |
| 7 | 20 | 24 | 0.4 | 10 | 0.754 | 0.808 | 0.422 | 0.396 | 1.186 | 0.999 | 0.138 | 0.135 | 90.0 | 86.0 | 92.0 | 76.0 | 116.0 | 109.0 | 84.0 | 85.0 |
| 8 | 20 | 24 | 1.6 | 0.1 | 1.134 | 1.283 | 0.842 | 0.795 | 0.996 | 1.016 | 0.212 | 0.222 | 114.0 | 121.0 | 124.0 | 142.0 | 132.0 | 146.0 | 78.0 | 86.0 |
| 9 | 20 | 24 | 1.6 | 10 | 0.914 | 0.793 | 0.421 | 0.424 | 0.938 | 1.045 | 0.218 | 0.192 | 108.0 | 116.0 | 122.0 | 118.0 | 146.0 | 156.0 | 106.0 | 97.0 |
| 10 | 60 | 6 | 1 | 5.05 | 0.966 | 0.954 | 0.884 | 1.033 | 0.598 | 0.660 | 0.197 | 0.251 | 122.0 | 124.0 | 94.0 | 91.0 | 96.0 | 114.0 | 56.0 | 39.0 |
| 11 | 60 | 15 | 0.4 | 5.05 | 1.422 | 1.593 | 1.258 | 1.446 | 0.659 | 1.033 | 0.526 | 0.491 | 116.0 | 120.0 | 127.0 | 120.0 | 126.0 | 139.0 | 122.0 | 115.0 |
| 12 | 60 | 15 | 1 | 0.1 | 1.902 | 1.690 | 0.818 | 0.984 | 1.494 | 1.438 | 0.578 | 0.579 | 175.0 | 169.0 | 186.0 | 173.0 | 154.0 | 164.0 | 134.0 | 129.0 |
| 13 | 60 | 15 | 1 | 5.05 | 2.081 | 1.862 | 1.526 | 1.589 | 1.482 | 1.379 | 0.545 | 0.561 | 170.0 | 161.0 | 166.0 | 158.0 | 168.0 | 160.0 | 92.0 | 102.0 |
| 14 | 60 | 15 | 1 | 5.05 | 1.854 | 1.862 | 1.742 | 1.589 | 1.483 | 1.379 | 0.514 | 0.561 | 166.0 | 161.0 | 154.0 | 158.0 | 148.0 | 160.0 | 108.0 | 102.0 |
| 15 | 60 | 15 | 1 | 5.05 | 1.894 | 1.862 | 1.722 | 1.589 | 1.526 | 1.379 | 0.656 | 0.561 | 155.0 | 161.0 | 150.0 | 158.0 | 162.0 | 160.0 | 98.0 | 102.0 |
| 16 | 60 | 15 | 1 | 5.05 | 2.02 | 1.862 | 1.698 | 1.589 | 1.587 | 1.379 | 0.594 | 0.561 | 160.0 | 161.0 | 172.0 | 158.0 | 176.0 | 160.0 | 94.0 | 102.0 |
| 17 | 60 | 15 | 1 | 5.05 | 1.718 | 1.862 | 1.642 | 1.589 | 1.318 | 1.379 | 0.51 | 0.561 | 160.0 | 161.0 | 148.0 | 158.0 | 166.0 | 160.0 | 108.0 | 102.0 |
| 18 | 60 | 15 | 1 | 5.05 | 1.742 | 1.862 | 1.606 | 1.589 | 1.146 | 1.379 | 0.542 | 0.561 | 162.0 | 161.0 | 166.0 | 158.0 | 158.0 | 160.0 | 110.0 | 102.0 |
| 19 | 60 | 15 | 1 | 10 | 1.208 | 1.469 | 0.808 | 0.773 | 1.288 | 1.434 | 0.536 | 0.535 | 146.0 | 150.0 | 113.0 | 131.0 | 158.0 | 148.0 | 120.0 | 118.0 |
| 20 | 60 | 15 | 1.6 | 5.05 | 1.826 | 1.704 | 1.494 | 1.436 | 1.379 | 1.095 | 0.469 | 0.504 | 140.0 | 134.0 | 144.0 | 156.0 | 172.0 | 159.0 | 100.0 | 100.0 |
| 21 | 60 | 24 | 1 | 5.05 | 1.817 | 1.878 | 1.926 | 1.907 | 1.728 | 1.756 | 0.777 | 0.723 | 210.0 | 206.0 | 215.0 | 223.0 | 250.0 | 232.0 | 126.0 | 136.0 |
| 22 | 100 | 6 | 0.4 | 0.1 | 0.807 | 0.756 | 0.466 | 0.419 | 0.417 | 0.329 | 0.157 | 0.198 | 125.0 | 108.0 | 104.0 | 109.0 | 106.0 | 89.0 | 84.0 | 79.0 |
| 23 | 100 | 6 | 0.4 | 10 | 0.788 | 0.804 | 0.312 | 0.367 | 0.352 | 0.292 | 0.16 | 0.140 | 80.0 | 74.0 | 66.0 | 48.0 | 62.0 | 47.0 | 46.0 | 46.0 |
| 24 | 100 | 6 | 1.6 | 0.1 | 0.882 | 0.993 | 0.338 | 0.372 | 0.258 | 0.405 | 0.175 | 0.167 | 100.0 | 106.0 | 124.0 | 140.0 | 78.0 | 83.0 | 30.0 | 37.0 |
| 25 | 100 | 6 | 1.6 | 10 | 0.776 | 0.735 | 0.304 | 0.260 | 0.228 | 0.344 | 0.094 | 0.116 | 91.0 | 90.0 | 68.0 | 61.0 | 90.0 | 85.0 | 68.0 | 54.0 |
| 26 | 100 | 15 | 1 | 5.05 | 2.336 | 2.329 | 2.024 | 1.934 | 1.648 | 1.407 | 0.787 | 0.685 | 171.0 | 174.0 | 192.0 | 210.0 | 141.0 | 155.0 | 88.0 | 100.0 |
| 27 | 100 | 24 | 0.4 | 0.1 | 1.886 | 2.031 | 1.844 | 1.841 | 1.434 | 1.485 | 0.966 | 0.973 | 204.0 | 208.0 | 284.0 | 282.0 | 246.0 | 233.0 | 232.0 | 225.0 |
| 28 | 100 | 24 | 0.4 | 10 | 1.654 | 1.485 | 1.43 | 1.401 | 1.502 | 1.556 | 0.807 | 0.857 | 144.0 | 143.0 | 179.0 | 194.0 | 146.0 | 161.0 | 160.0 | 153.0 |
| 29 | 100 | 24 | 1.6 | 0.1 | 2.65 | 2.577 | 1.742 | 1.745 | 1.626 | 1.688 | 0.984 | 1.002 | 239.0 | 232.0 | 384.0 | 367.0 | 276.0 | 271.0 | 180.0 | 165.0 |
| 30 | 100 | 24 | 1.6 | 10 | 1.634 | 1.725 | 1.138 | 1.245 | 1.772 | 1.734 | 0.884 | 0.894 | 196.0 | 184.0 | 262.0 | 261.0 | 256.0 | 243.0 | 144.0 | 142.0 |
| Lipid Production | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | Cool White | Pink | Blue | Red | ||||||||
| F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | F-Value | p-Value Prob > F | ||||||
| Model | 14 | 67.49 | <0.0001 | Sig. | 47.42 | <0.0001 | Sig. | 26.51 | <0.0001 | Sig. | 33.28 | <0.0001 | Sig. |
| A-LI | 1 | 280.76 | <0.0001 | 220.53 | <0.0001 | 57.64 | <0.0001 | 93.58 | <0.0001 | ||||
| B-LD | 1 | 367.99 | <0.0001 | 279.95 | <0.0001 | 226.72 | <0.0001 | 284.32 | <0.0001 | ||||
| C-AF | 1 | 10.99 | 0.0047 | 21.85 | 0.0003 | 6.76 | 0.0201 | 6.37 | 0.0234 | ||||
| D-Nitro | 1 | 20.15 | 0.0004 | 29.95 | <0.0001 | 4.27 | 0.0565 | 3.51 | 0.0806 | ||||
| AB | 1 | 12.36 | 0.0031 | 44.44 | <0.0001 | 14.92 | 0.0015 | 11.06 | 0.0046 | ||||
| AC | 1 | 1.42 | 0.2524 | 2.24 | 0.1553 | 4.42 | 0.0528 | 3.33 | 0.0880 | ||||
| AD | 1 | 22.41 | 0.0003 | 25.04 | 0.0002 | 5.21 | 0.0375 | 7.16 | 0.0172 | ||||
| BC | 1 | 8.13 | 0.0121 | 10.96 | 0.0048 | 6.97 | 0.0186 | 2.25 | 0.1542 | ||||
| BD | 1 | 12.36 | 0.0031 | 2.62 | 0.1266 | 3.04 | 0.1015 | 10.01 | 0.0064 | ||||
| CD | 1 | 3.65 | 0.0754 | 1.22 | 0.2872 | 6.66 | 0.0209 | 16.12 | 0.0011 | ||||
| A2 | 1 | 13.04 | 0.0026 | 0.3602 | 0.5573 | 10.03 | 0.0064 | 13.73 | 0.0021 | ||||
| B2 | 1 | 0.5703 | 0.4618 | 0.0129 | 0.9111 | 1.44 | 0.2484 | 3.53 | 0.0799 | ||||
| C2 | 1 | 36.21 | <0.0001 | 3.85 | 0.0685 | 1.22 | 0.2873 | 0.5372 | 0.4749 | ||||
| D2 | 1 | 0.0507 | 0.8249 | 0.3602 | 0.5573 | 0.1859 | 0.6725 | 7.96 | 0.0129 | ||||
| Lack of Fit | 10 | 3.97 | 0.0706 | N. Sig. | 3.66 | 0.0825 | N. Sig. | 4.14 | 0.0651 | N. Sig. | 3.12 | 0.1108 | N. Sig. |
| R2 | 0.9844 | 0.9779 | 0.9612 | 0.9688 | |||||||||
| LED | Optimized Conditions | Biomass Productivity (mg L−1 day−1) | Lipid Productivity (mg L−1 day−1) | Lipid Content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity (%) | Duration (h) | Airflow (L min−1) | Nitrogen (g L−1) | Pred. | Exp. | N.C. | Pred. | Exp. | N.C. | Pred. | Exp. | N.C. | |
| White | 100.00 | 22.86 | 1.31 | 1.60 | 528.44 | 512.0 ± 12.23 | 383.33 ± 22.12 | 47.80 | 45.86 ±1.73 | 30.53 ±3.20 | 9.05 | 8.96 ± 0.14 | 7.96 ± 0.51 |
| Pink | 100.00 | 24 | 1.44 | 1.02 | 404.80 | 401.33 ± 10.48 | 384.33 ± 8.26 | 71.23 | 69.80 ± 1.1 | 38.40 ±0.69 | 17.60 | 17.39 ± 0.27 | 9.99 ± 0.17 |
| Blue | 97.94 | 24 | 1.49 | 0.1 | 354.83 | 342.66 ± 3.53 | 339.33 ± 12.36 | 55.14 | 54.20 ±1.01 | 34.40 ±1.22 | 15.54 | 15.82 ± 0.39 | 10.14 ± 0.09 |
| Red | 100.00 | 24 | 0.40 | 0.1 | 194.34 | 189.60 ± 1.36 | 166.33 ± 6.12 | 45.75 | 43.30 ±1.59 | 26.40 ±1.90 | 23.54 | 22.84 ± 0.71 | 15.87 ± 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borah, D.; Eldiehy, K.S.H.; AL-Hothaly, K.A.; Deka, D. A Novel Approach Towards RSM-Based Optimization of LED-Illuminated Mychonastes homosphaera Culture, Emphasizing Input Energy: An Industrial Perspective of Microalgae Cultivation. Phycology 2025, 5, 62. https://doi.org/10.3390/phycology5040062
Borah D, Eldiehy KSH, AL-Hothaly KA, Deka D. A Novel Approach Towards RSM-Based Optimization of LED-Illuminated Mychonastes homosphaera Culture, Emphasizing Input Energy: An Industrial Perspective of Microalgae Cultivation. Phycology. 2025; 5(4):62. https://doi.org/10.3390/phycology5040062
Chicago/Turabian StyleBorah, Doljit, Khalifa S. H. Eldiehy, Khalid A. AL-Hothaly, and Dhanapati Deka. 2025. "A Novel Approach Towards RSM-Based Optimization of LED-Illuminated Mychonastes homosphaera Culture, Emphasizing Input Energy: An Industrial Perspective of Microalgae Cultivation" Phycology 5, no. 4: 62. https://doi.org/10.3390/phycology5040062
APA StyleBorah, D., Eldiehy, K. S. H., AL-Hothaly, K. A., & Deka, D. (2025). A Novel Approach Towards RSM-Based Optimization of LED-Illuminated Mychonastes homosphaera Culture, Emphasizing Input Energy: An Industrial Perspective of Microalgae Cultivation. Phycology, 5(4), 62. https://doi.org/10.3390/phycology5040062






