Abstract
Clay minerals and other flocculants are used to mitigate the effects of some species that produce harmful algal blooms due to their physical and chemical characteristics. In this study, we applied calcium bentonite clay (Bca) and zeolite (Ze) to flocculate and remove cells of the dinoflagellate Gymnodinium catenatum (Graham), a producer of paralyzing toxins. The flocculants were characterized by scanning electron microscopy (SEM) in combination with an energy-dispersive X-ray spectroscopy (EDS) microanalysis system. During experiments, Bca and Ze were suspended in distilled water, deionized water, and seawater at concentrations of 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, and 4.0 gL−1. The percentage of removal efficiency (RE%) of biomass indicators of G. catenatum was calculated. The cell number and concentration of chlorophyll a and peridinin were analyzed using high-performance liquid chromatography (HPLC-UV and HPLC-DAD). The external effects on cells of G. catenatum were recorded. As a result, the maximum RE% of Bca was 79% with respect to the total number of cells, chlorophyll a was 69% and peridinin of 73%. The RE% of Ze was less than 40%. In the matrix of sedimented Bca, malformation of cells was observed, inhibiting their swimming, as well as death and rupture of cells with temporary cyst formation after 72 h. We conclude that Bca, suspended in deionized and distilled water, was more efficient in flocculating cells of G. catenatum.
1. Introduction
There is a scientific consensus that the public health, recreation, tourism, fishery, aquaculture, and ecosystem impacts from harmful algal blooms (HABs) have all increased over the past several decades. The extent to which climate change is intensifying these HABs is not fully clear, but there has been a wealth of research on this topic this century alone [1].
HABs display range expansion and increased frequency in coastal areas since the 1980s in response to both climatic and non-climatic drivers, such as increased riverine nutrient run-off. The observed trends in HABs are attributed partly to the effects of ocean warming, marine heatwaves, oxygen loss, eutrophication, and pollution. HABs have had negative impacts on food security, tourism, the local economy, and human health [1]. These trends can be partly attributed to improved monitoring and coastal eutrophication [2,3,4,5,6] affecting fisheries and aquaculture activities and tourism [4]. This implies significant economic losses [7,8], in some cases, harming human health when the dominant species is toxic [9,10,11].
Different HAB mitigation strategies have been implemented for decades, using chemicals [12,13,14,15], and recently, nonmetallic clay minerals [16,17,18,19]. The use of clays is one of the few HAB mitigation methods applied in the field; however, low flocculation efficiency has always been the main drawback associated with natural clays [19]. Some clays have shown high efficiency in mitigating HAB, declining economic losses in the aquaculture industry [19,20,21]. The use of large amounts of clay may affect the substrate, causing alteration in filter-feeder organisms, suffocation, and burial of clams and other organisms [22,23,24].
The harmful microalgae G. catenatum is a unique unarmoured dinoflagellate that produces paralytic toxins [25]. Global distribution of G. catenatum has increased in some regions over recent decades, and the species is currently known to be distributed worldwide across various coastal ecosystems [25,26]. This species is common along the coasts of the Mexican Pacific and Gulf of California and is responsible for paralytic shellfish poisoning (PSP) in human cases (37 intoxicated and three deaths) and recurring sanitary closure for the extraction and marketing of mollusks [25,27,28,29,30,31]. Its presence in the southeastern Gulf of Mexico has also been recently documented [32,33]. The HABs of this species have generated notable economic losses in both fisheries and aquaculture. In the Gulf of California, G. catenatum has been related to mass mortality events in fish, shrimp, seabirds, sea turtles, and marine mammals and has also been associated with this dinoflagellate [25,27,28,30,31,34].
In Mexico, the HABs produced by this species result in millions of dollars in losses due to the bans implemented on the extraction and marketing of marine products. In some cases, they cause mass mortality of farmed organisms, thus affecting the trade of high-value marine species such as shrimp (Penaeus spp.), geoduck clam (Panopea spp.), scallop shells (Atrina spp.), oysters (Crassostrea spp.), and other bivalve mollusks, impacting the country’s domestic and export markets. Other economic impacts stem from investment in monitoring harmful species and routine marine toxin analysis, as well as direct impacts on medical and hospital care for intoxicated individuals. There are also indirect effects on the tourism industry [25,28,29,30,31]. An example is the case of the HAB in the northern Gulf of California in 2015, which caused eight cases of PSP in humans, an epizootic, and mass mortality of marine organisms, such as more than 6000 seabirds (Gavia immer, Pelecanus occidentalis, and Phalacrocorax spp.) and 150 marine mammals (Delphinus spp. and Zalophus californianus). This HAB is considered one of the events with the greatest economic and environmental impact in the country, as it generated sanitary closures for months and the confiscation and destruction of contaminated shellfish. HABs of G. catenatum have been recurring in the last decade in the northern Gulf of California and other regions of the Mexican Pacific [25,28,29,30,31].
Hence, mitigation strategies need to be investigated. Sengco et al. (2001) [17], and other authors [14,15,19,35] suggest testing different types of flocculants in different HAB-forming species to assess their impact on organisms and the efficiency of removal by flocculation. Based on the frequent presence of this species and its impacts in the Mexican Pacific, in this study, we evaluate the potential use of two flocculants for the mitigation of G. catenatum under laboratory conditions.
2. Materials and Methods
2.1. Cultivation
The dinoflagellate G. catenatum (strain # 52-strain collection of the CIBNOR) was cultivated in culture medium f/2 modified static (1 L batch culture, n = 3), with added selenium (10−8 of H2SeO3) and the reduction of copper (10−8M of CuSO4) [36]. The light intensity was 19.5–23.5 µmol m2s−1 at a constant temperature of 22 °C. Growth was monitored daily by cell counting in triplicate with a light microscope (Leica, Wetzlar, Germany, DMLS-020-518-500) and a Sedgewick Rafter chamber. A cell growth curve was obtained:
and progressive summation of the accumulated growth was calculated.
µ2 = [ln (X2/X1)/ln2]/(t2 – t1)
(∑µ2)
In the middle of the exponential growth, cells were harvested for removal experiments under the same environmental conditions as those used for the G. catenatum culture; the cell densities recorded in the exponential phase of each batch culture were inoculated into the experimental tubes without adding new fresh f/2 medium.
2.2. Preparation of Flocculants
Calcium bentonite (Bca) clay (size: 9.64 µm) and zeolite (Ze) (size: 11.91 µm) were provided by Fosfatos Tricalcicos S.A. de C.V., San Luis Potosi, Mexico. Three treatments were applied to each type of clay: (1) suspended in distilled water (DW), (2) in deionized water (IW), and (3) seawater (SW) in 25 mL Erlenmeyer flasks. Dilutions for the two flocculants were 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, and 4.0 gL−1, as recommended by Sengco et al. [16,17]. To hydrate and activate the flocculants, let them stand for ~24 h before application. Subsequently, each dilution mixture was stirred before being applied.
2.3. X-Ray Microanalysis of Flocculants by the Scanning Electron Microscope
A sample of 2.5 µg Bca clay and Ze was characterized using a scanning electron microscope (Hitachi® S300N, Tokyo, Japan) in combination with an energy-dispersive X-ray spectroscopy (EDS) microanalysis system (Oxford Instruments Inc. INCAx-sight, Abingdon, UK). Low-background sample holders and a voltage of 15 KV were used.
2.4. Experimental Design
A total of 28 sterile glass tubes of 12 mL capacity were inoculated, of which 7 were considered controls and 21 were used in the experiment, with three replicates for each concentration of flocculants and their own water treatment. The cell densities recorded in the exponential phase of each batch culture were inoculated into the experimental tubes without adding new fresh f/2 medium. Controls consist of different dilutions of flocculants suspended in DW, IW, and SW, containing 10 mL of G. catenatum culture cells (3.5 × 103−4.5 × 103 cells mL−1) and 1 mL of water treatment without clay. In the experiment tubes, the flocculants were applied with an Eppendorf pipette every 30 min, and after applying the solution slowly, the flocculants were allowed to interact with G. catenatum cells for 60 min. After this time, the extraction was carried out, starting with the lowest concentration and replicates. From each tube, 8 mL of the aqueous phase was extracted and placed in glass tubes (12 mL capacity). The content was gently homogenized (Vortex-Genie VWR Scientific), and 1 mL was withdrawn immediately for cell count, which was placed in a 1.5 mL Eppendorf vial and fixed with a dilute solution of Lugol-acetate. The counts were made with a LEICA DMLS optical microscope and a Sedgewick Rafter chamber. The remaining 7 mL of each experiment tube was used to determine chlorophyll a (Chl a) and peridinin.
2.5. Percentage of Removal Efficiency
For each treatment, the flocculants and dilution rate were calculated from the percent removal efficiency (% RE) of cells, following the equation proposed by Sengco (2001a, b) [16,17]:
where NiCel is the control cell counts, and Nf Cel is the cell count treatments. The same equation was applied to the Chl a and peridinin values.
2.6. Microscope Observation
The floc–cell adhesions were observed (qualitative) with an optical microscope (LEICA DMLS). Observations were made with the optical microscope (LEICA DMLS) of floc–cell adhesion to photograph the flocculant particles in the cell wall, external effects on the cells, and formation of cysts.
2.7. Photosynthetic Pigments
Seven mL of the homogenate was concentrated on glass fiber filters GF/D (2.7 µm Whatman, Maidstone, UK). The material retained on the filters was placed in glass tubes with HPLC-grade 100% acetone and incubated for 24 h at −20 °C. Afterward, it was centrifuged at 4000× g (Beckman Coulter, Brea, CA, USA) for 15 min at 5 °C. The supernatant was filtered through a glass fiber filter of 0.7 µm (Whatman, Maidstone, UK), recovered in Eppendorf vials, and refrigerated at −20 °C. The acetone extract of 20 µL was also analyzed using high-performance liquid chromatography (HPLC-UV and HPLC-DAD; Hewlett Packard Model HP1100). The pigment composition was analyzed according to the methods in Vidussi et al. (1996) [37], with some modifications. Quantification was based on absorbance at 440 nm and the factor value of each pigment response (peak area/concentration of pigment) as described by Mantoura & Repeta (1997) [38]. For the identification of the characteristic pigments, we compared the retention time of the sample peaks with those of the pure standards and the adsorption spectra of the problem sample with those of the generated library of the standards certified (precision ˂ 1%). The pigment quantification was performed by constructing a calibration curve (R2 = from 0.910 tol) that included the concentrations for each standard (20, 30, 40, 50, 60, and 100 ng) (International Agency for 14C Determinations, Hørsholm, Denmark).
2.8. Statistical Analysis
A one-way ANOVA test was performed to identify significant differences for each treatment and type of flocculant. The normal distribution of the variables was verified with the normality test (Kolmogorov–Smirnov), and to observe significant differences, the homogeneity of variance test (Levene’s) was used. A p value < 0.05 was considered statistically significant for each treatment. Statistica 6.0 software was used for all analyses.
3. Results
3.1. Cell Growth
In the growth curve, it can be observed that the exponential phase began after the third day of inoculation (Figure 1). The maximum daily growth rate was on the third day, beginning its decline from day 9, so the time of harvest for experimental incubation was between the fifth and sixth day of incubation (3.5 × 103–5.5 × 103 cel mL−1) (Figure 1).
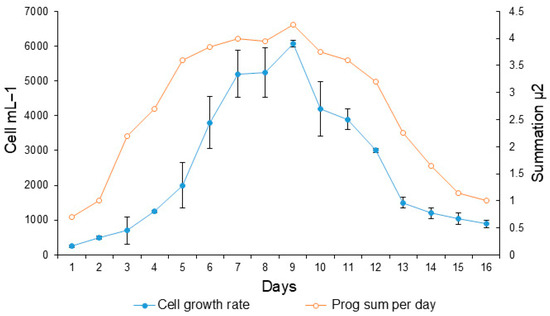
Figure 1.
Growth curve of the dinoflagellate Gymnodinium catenatum in the culture medium f/2 enriched with selenium. Shaded circles indicate cell growth (cell mL−1). Open circles indicate daily progressive cell growth as a cumulative growth rate. The bars indicated the standard deviations.
3.2. X-Ray Microanalysis of Flocculants by the Scanning Electron Microscope
The percentage of chemical elements (from highest to lowest) in Bca and Ze was calculated based on the X-ray spectral microanalysis by the scanning electron microscope. The calcium bentonite (Bca) detected the O, S, Al, Ca, Fe, Na, Mg, K, T, and S, and the zeolite (Ze) detected the Si, Al, Ca, K, Sr, Fe, P, Mg, and Zn (Figure 2A,B).
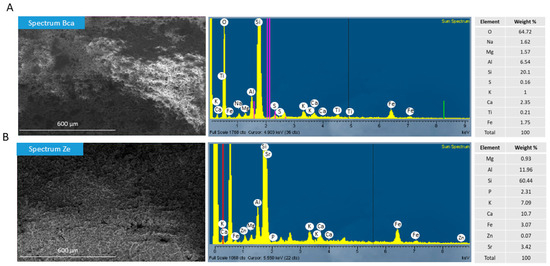
Figure 2.
Microanalysis of two flocculants by SEM in combination with an energy-dispersive X-ray spectroscopy (EDS). (A) Percentage of chemical elements in calcium bentonite (Bca) and (B) percentage of chemical elements in Zeolite (Ze). The red, violet, and green lines represent the Kα and Lα energy levels, as a relative reference to the X-ray intensity for each element analyzed.
3.3. Calcium Bentonite
The number of G. catenatum cells varied significantly among treatments. Comparing the different dilution concentrations, significant differences were in IW and SW (0.25, 0.5, 1.0, and 4.0 gL−1) (p < 0.05), but not with DW (Figure 3). The highest number of cells was removed from 2530 and 2143 cells mL−1 with dilutions of 0.5 and 4.0 gL−1 IW-Bca, respectively (Figure 3). The Bca-SW was the flocculant with fewer cells removed.
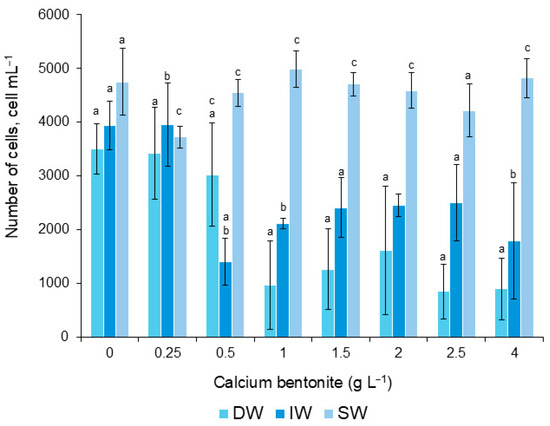
Figure 3.
Number of cells of G. catenatum surviving during application of calcium bentonite, diluted in distilled water (DW), deionized (IW), and seawater (SW). Bars (0) are the control treatment. Significant differences between dilutions are indicated with letters a, b, and c (α = 0.05).
The chlorophyll a content differed between the three treatments and a few of the dilutions (Figure 4A). In DW, chlorophyll a at 0.5 gL−1 dilution was significantly different from the rest. For IW, differences were significant in the dilutions 0.25, 0.5, 2.0, and 4.0 gL−1, but were not significant in SW (Figure 4A). For peridinin, differences were significant between the treatment and dilutions, mainly with IW (Figure 4B).
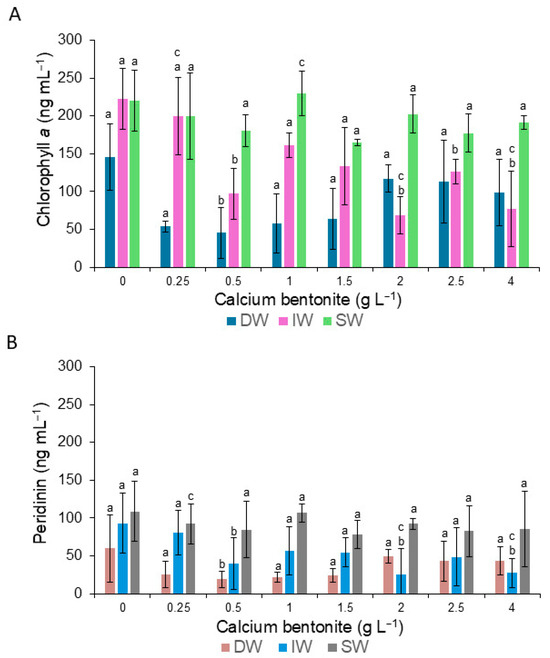
Figure 4.
Variation in the content of chlorophyll a (A) and peridinin (B) of cells of G. catenatum during the application of calcium bentonite diluted in distilled water (DW), deionized water (IW), and seawater (SW). Bars (0) are the control treatment. Letter a signifies that there is no significant difference at a level of confidence of α = 0.05. Letters b and c indicate significant differences (α = 0.05), and data and error bars are shown as mean ± SD.
3.4. Zeolite
The number of G. catenatum cells mixed with zeolite did not vary significantly between treatments. Comparison between dilutions 1.0, 1.5, 2.5, and 4.0 gL−1 was significant, although the number of removed cells was low (Figure 5).
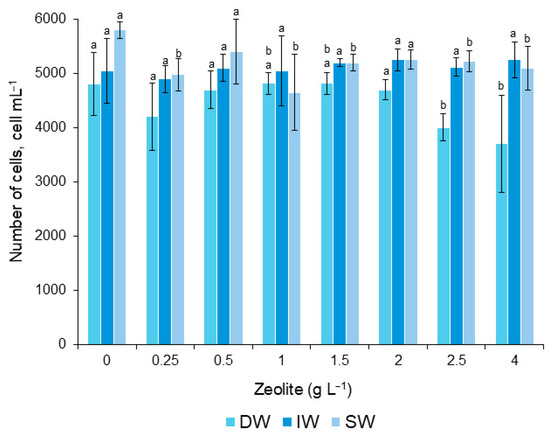
Figure 5.
Number of cells of the G. catenatum that survived during the application of the zeolite. Letter a means that there were no significant differences (α = 0.05). Letter b indicates significant differences (α = 0.05). Bars (0) are the control treatment. Data and error bars are shown as mean ± SD.
Regarding Chl a content, significant differences were found between treatments with distilled water and seawater at the dilution of 1.5 gL−1 (Figure 6A). Regarding peridinin, significant differences were found between treatments and dilutions, mainly with DW, when concentrations were 0.25, 1.5, 2.5, and 4.0 gL−1 (Figure 6B).
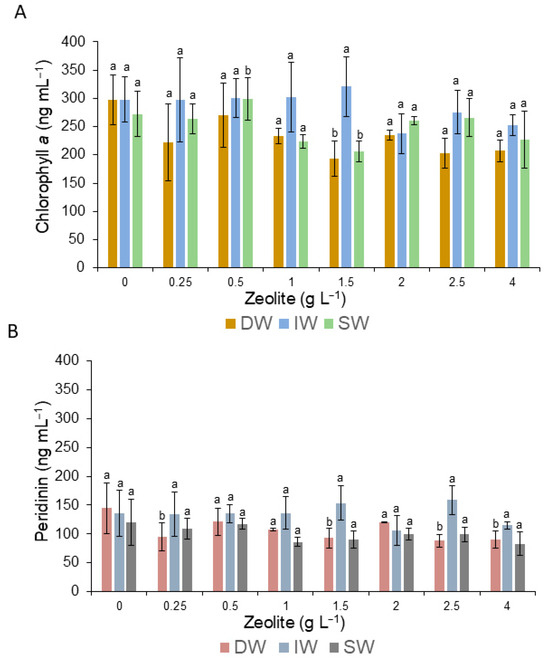
Figure 6.
Variation in the content of chlorophyll a (A) and peridinin (B) of the surviving cells of G. catenatum during the application of zeolite. Bars (0) are the control treatment. Letter a means no significant differences (α = 0.05), and letter b means that there were significant differences (α = 0.05), and data and error bars are shown as mean ± SD.
3.5. Percentage Removal
The highest values of RE% relative to the number of cells were 78.7% and 72.6% with a dilution of 1.0 and 2.5 gL−1 of Bca in DW, respectively. Following in descending order, when diluted in water with 0.5 and 4.0 gL−1 of IW, the RE% was 56.0 and 59.0%, respectively. Dilutions less efficient (<15.0%) were with SW (Figure 7A). The zeolite with the three treatments showed a low removal process of <40.0% (Figure 7B).
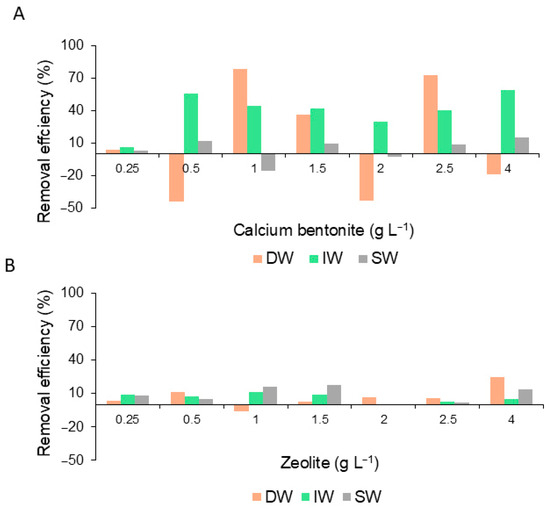
Figure 7.
Percentage of cell removal efficiency of G. catenatum during the application of calcium bentonite (A) and zeolite (B) diluted in distilled water (DW), deionized water (IW), and seawater (SW).
The RE% calculated for Chl a and peridinin was 69.3–73.2% and 68.0–68.7% when Bca was diluted in DW (0.5 gL−1) and IW (2.0 gL−1), respectively. In seawater, it was <40% (Figure 8A,B).
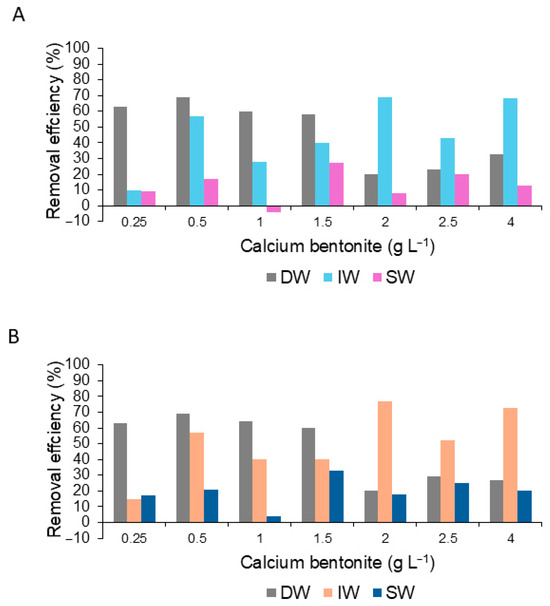
Figure 8.
Percent removal efficiency for the content of chlorophyll a (A) and peridinin (B) dinoflagellate G.catenatum during the application of the calcium bentonite diluted in distilled water (DW), deionized water (IW), and seawater (SW).
When zeolite was applied in the three treatments, the RE% was <40% in both Chl a and peridinin (Figure 9A,B).
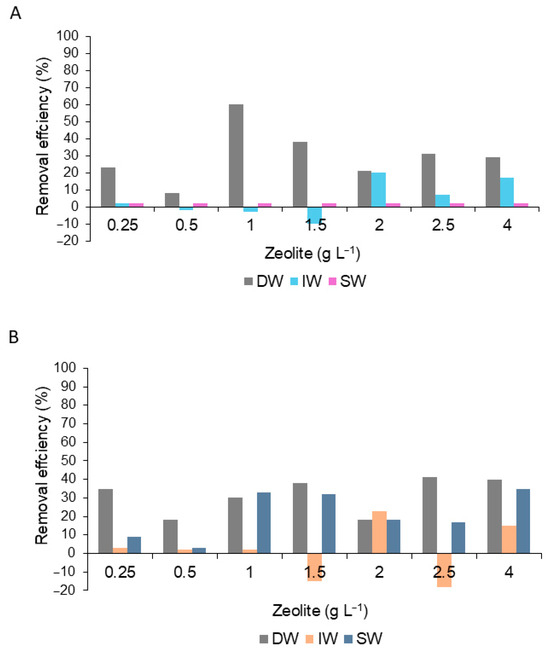
Figure 9.
Percent removal efficiency for the content of chlorophyll a (A) and peridinin (B) dinoflagellate G.catenatum during the application of the zeolite diluted in distilled water (DW), deionized water (IW), and seawater (SW).
3.6. External Effects on Cells of G. catenatum
The concentration of 0.5 gL−1 was arbitrarily chosen for both flocculants to observe (qualitative) under an optical microscope. Deformed cells were observed; the number of cells was highly variable in chain length, with a translucent green color, although some organisms showed swimming vitality between the zeolite matrix and a high number of temporary cysts after 72 h. However, in the Bca flocs, G. catenatum had dying cells, which were deformed, had fragmented chains, were broken, and eventually died. Altered motility, reduction in cell numbers per chain or only in isolated cells, color changes, structural deformation, cell rupture, and formation of transient cyst-like phases (temporary cysts) after exposure at 72 h were observed. (Figure 10).
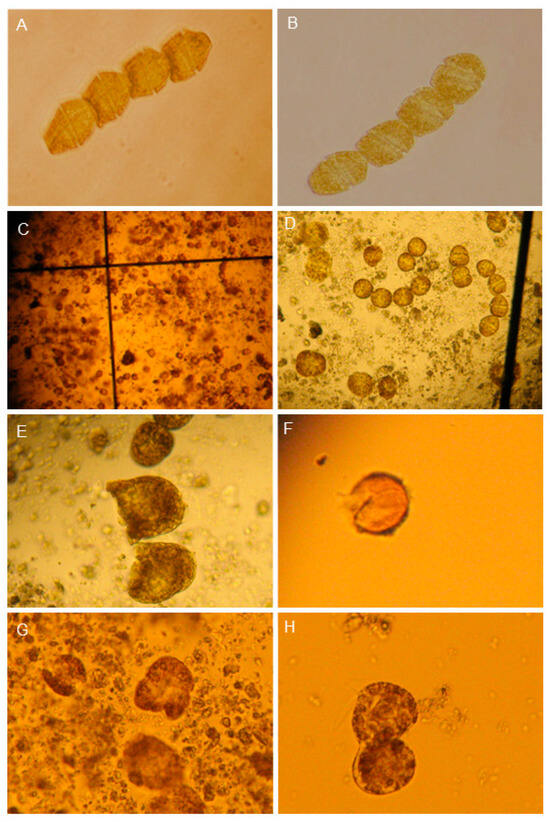
Figure 10.
Micrographs of G. catenatum during bentonite application diluted in distilled water. (A,B) Control showed normal chain cells (10×). (C,D) Single cells and chain-deformed cells (5×). (E) Dead cells (10× magnification). (F) Temporary cyst, open (10×). (G,H) Lysis cells are observed in a matrix, and flocculated cells and some calcium bentonite clay particles are attached to the cell wall (10×).
4. Discussion
This study showed that the calcium bentonite clay (Bca) was relatively more efficient in the flocculating cells of G. catenatum than zeolite. Significant differences between treatments showed that the aqueous medium, in which Bca was suspended, is important mainly in the flocculation process and removal of cell G. catenatum. Although differences were not significant among dilutions with respect to the number of cells, this was probably due to their large standard deviations of the counts. However, the RE% by cell number showed that DW diluted with Bca had the most significant number of cells flocculated (78.7–72.6%) at 2.5 to 1.0 gL−1, respectively. For Chl a, there were significant differences only between the control group and the dilution of 0.5 gL−1, with a maximum RE% of 68.7%. With respect to peridinin, no significant differences were found, although RE% was 68.0%, similar to Chl a. For the IW, the maximum RE% in terms of cell number ranged from 56.1 to 59.5% (0.5–4.0 gL−1, respectively). The estimates of Chl a and peridinin values at these same dilutions showed a removal percentage of 52.6% and 65.3% in Chl a, and 57.3% and 70.0% in peridinin. The RE% of Bca-SW was <14.0%. This suggests that Bca diluted with distilled and deionized water was the most efficient in removing G. catenatum cells. In the case of zeolite with its three water treatments, RE% with respect to cell number, Chl a, and peridinin was less than 30.0%
It has been suggested that when the flocculant particles adhere to the cell wall, their ionic equilibrium is disturbed, causing deformation, rupture, and death [19,39,40,41]. Sengco et al. (2001) [16,17] suggested that the surface charge of clays and other flocculants, determined by their mineralogy, determines its ability to remove certain algal species. According to these authors, the Bca has a structure of tetrahedra together form dimensional planar laminar networks. Its interlayers are located at Ca2+, Al2+, and Mg2+ (Figure 2). It is likely that these divalent ions altered the balance of the cell wall of G. catenatum during contact and adhesion to the cell wall, causing changes in its swimming, deformation, cell death, and rupture (Figure 10). Zeolite also contains these and other cations, but its molecular structure, by forming three-dimensional tetrahedral networks, forms a framework with channels and cavities that give it different properties for adhesion of organic particles [19,42]. In addition, the zeolites have a low swelling index [16,17], which probably determines their low RE in cell G. catenatum. Other recent methodologies, such as those described by Yu et al. (2017) [19] and Balaji Prasath et al. (2022) [15], determined different removal percentages of various harmful microalgae. The removal efficiency of coagulants–flocculants on harmful Gymnodinium species has also been evaluated [41,43,44,45,46] (Table 1).

Table 1.
Comparative removal efficiency (RE) of coagulants-flocculants on harmful Gymnodinium species.
Recently, AziziHariri et al. (2025) [47] used Karenia brevis as harmful microalgae to evaluate a new methodology that would allow flocculation and elimination of species that produce HABs in large areas. This study was based on the use of a surfactant foam that transports hydrogen peroxide-producing algaecides (soluble gallic acid or particulate calcium peroxide) and/or a flocculant such as polyaluminum chloride. These authors describe that the release of this compound at the surface of the water column produces high concentrations of algaecide in the surface layers, with dilution at greater depth in the water column to minimize the impact on non-target organisms, easily integrating into the foam and being transported uniformly to the water column, generating highly effective cellular flocculation. AziziHariri et al. [47] also describe that, compared to current mitigation methods, these may have advantages in scalability and coverage. Therefore, these types of foams may be a better practical way to remove HABs in large areas, since they remain localized on the water surface, attacking the bloom, and eventually dissipate without leaving residue.
The individual cell size from the Gulf of California strain is 58.85 ± 7.52 µm long and 38.44 ± 5.26 µm wide, with a cell chain of eight, which is 35.96 ± 5.39 µm long and 3.21 ± 36.09 µm wide [48,49,50]. This size leads to a large surface area of adhesion (~2262.0 µm2) relative to the size of flocculants applied in this work (9.64–11.91 µm), increasing the contact area mainly with Bca. According to Jackson and Lochmann (1993) [51], a collision between flocculant particles and phytoplankton cells increases the swimming ability of flagellates. Kiørboe et al. (1990) [52] suggest that the mobility of the cells can increase the rate of collisions between particles, which is irrelevant for diatoms and flagellates but may be relevant in this study. So it is probable that the swimming speed of G. catenatum (247 µm s−1) [53], and its relatively high surface area, increases the likelihood of contact with the Bca, according to Archambault et al. (2003) [54] suggest that flocculant contact with Heterocapsa triquetra cells causes them stress, decreasing their ability to swim. In this study, likely the adherence of the flocculant to the cell wall of G. catenatum reduced its swimming ability, increasing the size of flocs and the sedimentation of cells.
During the microscopic review of the sediment floc behavior, Bca and zeolite flocculant particles were observed adhering to the cell walls, inducing the deaths and deformations of cells, broken and without cell contents, with some cells undergoing division, altered motility, reduction in cell numbers per chain or only isolated cells, color changes, structural deformation, and causing the formation of something transient and cyst-like (Figure 10). After 72 h, temporary cysts were observed, which is consistent with those reported by Bae et al. (1998) [55] and Sengco et al. (2001) [16,17] after adding clays and clay-prolonged contact cells. In this study, the deformation, death, and lysis of G. catenatum were probably caused by the aluminum content (Figure 2) and the Ca++ cations. In this respect, it has been suggested that the mechanism that causes death is yet unknown; however, it has been proposed that cell lysis is induced by the presence of flocculants of prosthetic groups such as aluminum. Shirota (1989) [20] suggests that when aluminum increases, flocculants can cause weakness and cell lysis.
Some studies that have experimented with different types of clays mentioned that it is important to assess the negative effects after applying clays, mainly in the benthic biota [16,17,23,40,54]. Our study demonstrates that both clays, with variation in flocculation and sedimentation efficiency, caused death and cell rupture, affecting the cytoplasmic contents, and their toxins (PSTs) are likely incorporated into the aqueous medium; this has also been observed for other toxic species when clays are applied [16,17,40,56], increasing the risk of pollution mainly in filter bodies-feeders like bivalves [18,23,41].
Lu et al. (2015) [57] evaluated the effects of modified flocculant on the concentration, composition, and conversion of PST in cultures of the dinoflagellate Alexandrium tamarense. They reported that the content of these toxins in the bottom water and sediments decreased after the addition of a modified flocculant. These types of flocculants could also provide specific polymerization and catalytic interaction sites for chlorophyll a or algal neurotoxins and gonyautoxins (GTXs) such as PST in the modified clay–algae–sediment matrix. The biodegradation rate, or selectively catalyzed molecular degradation, of PST reached up to 94% in a sediment environment treated with modified clay. The high toxicity of GTX-1 and GTX-4 decreased, transforming into decarbamoyl gonyautoxins and GTX-2, with lower toxicity, during treatment with modified clay [19]. Cuellar-Martínez et al. (2016) [46] evaluated the efficiency of several coagulating or flocculating agents in eliminating cells and toxins from G. catenatum in laboratory experiments. These authors used various combinations to measure their combined performance. In some cases, they determined an efficiency of 95–99% and described a combination of calcium oxide and chitosan that effectively eliminated cells but failed to eliminate extracellular toxins from this dinoflagellate. In further work, it will be interesting to investigate other flocculants for greater efficiency in the removal of the toxic dinoflagellate, as well as evaluate the fate of their toxins and cyst formation. Also, the evaluation of the effect on filter-feeder organisms and the impact of sediment flocculants on the seabed warrants further research.
5. Conclusions
The addition of benthic calcite suspended in distilled water to G. catenatum cultures showed greater cell removal efficiency. The aqueous medium in which the flocculants are suspended is important for the flocculation and cell removal processes of G. catenatum. It was observed that Bca added in suspension in distilled water at concentrations of 2.5 and 1.0 gL−1 removed 78.7 and 72.6%, respectively. Our results demonstrate relatively high removal rates of G. catenatum. Further investigation into the pathways of paralyzing toxins when exposed to the environment after cell rupture, as well as into the monitoring of the cysts formed, is needed. Furthermore, studies are required to verify that laboratory results are reproducible under natural conditions. This study provides significant information for conducting and improving studies in the marine environment.
The flocculation percentages were like those of other studies, considering a relatively high percentage of cell removal. The experiments in this study were conducted in short periods, so it is necessary to conduct and plan long-term studies to guarantee the use of these flocculants in the management of HABs. It is important to evaluate the adsorption and transformation of dissolved paralytic toxins generated by dinoflagellate cell rupture and assess the toxin adsorption percentages, thus reducing the incorporation of dissolved paralytic toxins into the food chain by aquatic organisms. It is also suggested that the evaluation and combination of other flocculants is recommended, particularly modified clays, which have proven to be one of the most widely used recent methods for HAB mitigation.
Author Contributions
All authors contributed significantly to this study. Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft preparation, visualization, review, editing: F.E.H.-S.; conceptualization, methodology, formal analysis, investigation, supervision, funding acquisition, writing—original draft preparation, review, editing: E.J.N.-V.; software, formal analysis, investigation, data curation, writing, visualization, review, editing: L.J.F.-H.; methodology, investigation, writing—original draft preparation, review: J.G.-Z.; methodology, software, visualization, writing, review: A.A.C.-V.; conceptualization, methodology, resources, investigation, writing—original draft preparation, review: J.J.B.-G.; visualization, writing—review and editing: D.O.C.-O.; conceptualization, resources, funding acquisition, methodology, supervision, software, formal analysis, investigation, data curation, writing—original draft preparation, visualization: D.J.L.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The research projects PC 0.12 and PC 0.11 were funded by the Centro de Investigaciones Biológicas del Noroeste (CIBNOR) and Red Temática de Florecimientos Algales Nocivos, Consejo Nacional de Ciencia y Tecnología (2015–2017 projects, RedFAN-CONACYT, currently Secretaría de Ciencia, Humanidades, Tecnología e Innovación; SECIHTI), and PRONAII-CONAHCYT (Project 319104) “Atención de la problemática asociada florecimientos algales nocivos en Baja California: integración del conocimiento a necesidades socioambientales y económicas”. This is the first research work of CIBNOR as part of the Laboratorio Nacional-Ficotox of SECIHTI (Project LN-2025-I-63: Consolidación del LNC-FICOTOX para la atención de la problemática en salud pública y ambiental asociada a florecimientos algales en México).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This study is dedicated to the memory of David J. López-Cortes (1951–2019), an esteemed Mexican marine ecologist who initiated and promoted the project, but unfortunately did not have the time to see it published. This study received support from Centro de Investigaciones Biológicas del Noroeste (CIBNOR) and Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT; currently SECIHTI). We thank Rubén Guzmán García of the company Fosfatos Tricalsicos S.A. de C.V. of San Luis Potosi, Mexico, for providing calcium bentonite and zeolite, Saul Chavez Lopez for their technical information, and Alfonsina Romo-Curiel (University of Texas at Austin) for English editing. Leyberth Fernandez-Herrera received a postdoctoral fellowship (SECIHTI, project 319865 and postdoctoral residence for Mexico 2022 (3) CVU 337101). E.J.N.-V also thanks Raíz de Fondo A.C. (La Paz) for providing a pleasant space to finish writing and editing this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gobler, C. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Smayda, T. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42 Pt 2, 1137–1153. [Google Scholar] [CrossRef]
- Anderson, D.; Glibert, P.; Burkholder, J. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Anderson, D.; Cembella, A.; Hallegraeff, G. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Anderson, D.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef]
- Kudela, R.; Gobler, C. Harmful dinoflagellate blooms by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 2012, 14, 71–86. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, L. Algal Bloom and Its Economic Impact; EUR 27905 EN; EU Publications: Luxembourg, 2016. [Google Scholar]
- Anderson, D. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Mang. 2009, 52, 342–347. [Google Scholar]
- Trainer, V.; Bates, S.; Lundholm, N.; Thessen, A.; Adams, N.; Cochlan, W. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Young, R.; Sharpe, R.; Barciela, R.; Nichols, G.; Davidson, K.; Berdalet, E.; Lora, E.; Fleming, L. Marine harmful algal blooms and human health: A systematic scoping review. Harmful Algae 2020, 98, 101901. [Google Scholar] [CrossRef] [PubMed]
- Rounsefell, G.; Evans, J. Large-Scale Experimental Test of Copper Sulfate as a Control for the Florida Red Tide; US Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1958. [Google Scholar]
- Jeong, H.; Kim, H.; Kim, K.; Park, K.H.; Kim, S.T.; Du Yoo, Y.; Song, J.Y.; Kim, J.S.; Seong, K.A.; Yih, W.H.; et al. NaOCl produced by electrolysis of natural seawater as a potential method to control marine red-tide dinoflagellates. Phycologia 2002, 41, 643–656. [Google Scholar]
- Gallardo-Rodríguez, J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.; Hamilton, D.; Lin, H.; Zhen, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Sengco, R. The Aggregation of Clay Minerals and Marine Microalgal Cells: Physicochemical Theory and Implications for Controlling Harmful Algal Blooms. Ph.D. Thesis, Long Island University, New York, NY, USA, 2001. [Google Scholar]
- Sengco, R.; Li, A.; Tugend, K.; Kulis, D.; Anderson, D. Removal of red- and brown- tide cells using clay flocculation. I. Laboratory culture experiments with Gymnodinium breve and Aureococcus anophagefferens. Mar. Ecol. Prog. Ser. 2001, 210, 41–53. [Google Scholar] [CrossRef]
- Sengco, R. Prevention and control of Karenia brevis blooms. Harmful Algae 2009, 8, 623–628. [Google Scholar] [CrossRef]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Shirota, A. Red Tide problem and counter measures (1). Int. J. Aquat. Fish Technol. 1989, 1, 195–293. [Google Scholar]
- Sengco, R.; Anderson, D. Controlling Harmful Algal Blooms through clay flocculation. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef]
- Lewis, M.; Dantin, D.; Walker, C.; Kurt, J.; Green, R. Toxicity of clay flocculation of the toxic dinoflagellate, Karenia brevis, to estuarine invertebrates and fish. Harmful Algae 2003, 2, 235–246. [Google Scholar] [CrossRef]
- Archambault, M.; Bricelj, M.; Grant, J.; Anderson, D. Effects of suspended and sedimented clays on juvenile hard clams, Mercenaria mercenaria, within the context of harmful algal mitigation. Mar. Biol. 2004, 144, 553–565. [Google Scholar]
- Orizar, I.; Rivera, P.; San Diego-McGlone, M.; Azanza, R. Harmful Algal Bloom (HAB) mitigation using ball clay: Effect non-target organisms. J. Environ. Sci. Manag. 2013, 16, 36–43. [Google Scholar]
- Band-Schmidt, C.; Durán-Riveroll, L.; Bustillos-Guzmán, J.; Leyva-Valencia, I.; López-Cortés, D.; Núñez-Vázquez, E.; Hernández-Sandoval, F.; Ramírez-Rodríguez, D. Paralytic toxin producing dinoflagellates in Latin America, ecology and physiology. Front. Mar. Sci. 2019, 6, 42. [Google Scholar] [CrossRef]
- Cembella, A.; Band-Schmidt, C. Gymnodinium catenatum. Harmful algal species fact sheets. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 605–611. [Google Scholar]
- Mee, D.L.; Espinosa, M.; Diaz, G. Paralytic Shellfish Poisoning with a Gymnodinium catenatum Red Tide on the Pacific Coast of México. Mar. Environ. Res. 1986, 19, 77–92. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Bustillos-Guzmán, J.; López-Cortés, D.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.; Hernández-Sandoval, F. Ecological and physiological studies of Gymnodinium catenatum in the Mexican Pacific: A review. Mar. Drugs 2010, 8, 1935–1961. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.; Band-Schmidt, C.; Bustillos-Guzmán, J.; López-Cortés, D.; Cordero-Tapia, A.; Heredia-Tapia, A.; García-Mendoza, E.; Ruíz-de la Torre, M.; Medina-Elizalde, J. Impactos de los FAN en la Salud Pública y Animal (Silvestres y de Cultivo) en el Golfo de California. In Florecimientos Algales Nocivos en México; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortiz, A., Núñez-Vazquez, E., Eds.; CICESE: Ensenada, Mexico, 2016; ISBN 978-607-95688-5-6. [Google Scholar]
- García-Mendoza, E.; Medina, J.; Rivas, D.; Ruiz, M.C.; Bustillos-Guzmán, J.; Núñez-Vázquez, E.J. Paralytic shellfish toxins cause seabirds and marine mammal’s massive mortalities in the upper gulf of California. In Proceedings of the 17th International Conference on Harmful Algae, Florianopolis, Brazil, 9–14 October 2016; p. 83. [Google Scholar]
- Medina-Elizalde, J.; García-Mendoza, E.; Turner, A.D.; Sánchez-Bravo, Y.A.; Murillo-Martínez, R. Transformation and Depuration of Paralytic Shellfish Toxins in the Geoduck Clam Panopea globosa from the Northern Gulf of California. Front. Mar. Sci. 2018, 5, 335. [Google Scholar] [CrossRef]
- Poot-Delgado, C.; Yuri, B.; Okolodkov, Y.; Aké-Castillo, J.; Rendón-Von Osten, J. Annual cycle of phytoplankton with emphasis on potentially harmful species in oyster beds of Terminos Lagoon, southeastern Gulf of Mexico. Reprod. BioMed. Online 2015, 50, 465–477. [Google Scholar]
- Poot-Delgado, C.; Okolodkov, Y.; Rendón-von Osten, J. Spatio-temporal Variation of Harmful Planktonic Microalgae and Cyanobacteria Along the Central Coast of Campeche, Southeastern Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2022, 108, 15–23. [Google Scholar] [CrossRef]
- Alonso-Rodríguez, R.; Páez-Osuna, F. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: A review with special reference to the situation in the Gulf of California. Aquaculture 2003, 219, 317–336. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yu, Z. An eco-environmental assessment of harmful algal bloom mitigation using modified clay. Harmful Algae. 2021, 107, 102067. [Google Scholar] [CrossRef]
- Anderson, D.; Kulis, D.; Binder, B. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: Cyst yield in batch cultures. J. Phycol. 1984, 20, 418–425. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Bustillos-Guzmán, J.; Cailleau, C.; Marty, J. Rapid HPLC method for determination of phytoplankton chemotaxinomic pigments: Separation of chlorophyll a from divinyl-chlorophyll a and zeaxanthin from lutein. J. Plank. Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Montoura, R.; Repeta, D. Calibration methods for HPLC. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S., Mantoura, R., Wright, S., Eds.; UNESCO: Paris, France, 1997; pp. 407–428. [Google Scholar]
- Avnimelech, Y.; Troeger, B.; Reed, L. Mutual flocculation of algae and clay: Evidence and implication. Science 1982, 216, 63–65. [Google Scholar] [CrossRef]
- Pierce, R.; Henry, M.; Higham, C.; Blum, P.; Sengco, M.; Anderson, D. Removal of harmful algal cells (Karenia brevis) and toxins from seawater culture by clay flocculation. Harmful Algae 2004, 3, 141–148. [Google Scholar] [CrossRef]
- Padilla, L.; San Diego-McGlone, M.; Azanza, R. Exploring the potential of clay in mitigating Pyrodinium bahamense var. compressum and other harmful algal species in the Philippines. J. Appl. Phycol. 2010, 22, 761–768. [Google Scholar] [CrossRef]
- García-Gutiérrez, M. Glosario de Términos Geológicos; Consejo de Recursos Minerales: Pachuca, Mexico, 2003; p. 781. [Google Scholar]
- Rivera, P.; Azanza, R.; Diego-McGlone, M.; Sellner, K. Notable physiological and morphological effects of Ball clay addition on bloom forming organisms Pyrodinium bahamense, Gymnodinium catenatum and Alexandrium catanella. In Proceedings of the 16th International Conference on Harmful Algae (ICHA 16 NZ), Wellington, New Zealand, 27–31 October 2014. [Google Scholar]
- Rivera, P.; Azanza, R. Mitigating Toxic Algal Blooms: Removal and effects of ball clay addition on the Paralytic Shellfish Toxin (PST) producing dinoflagellates. In Proceedings of the 13th National Symposium in Marine Science (PAMS 13), General Santos City, Philippines, 22–24 October 2015. [Google Scholar]
- Fausto-Sotelo, E.; Alonso-Rodríguez, R. Control de Florecimientos Algales Nocivos con aplicación en la camaronicultura. In Florecimientos Algales Nocivos en México; García-Mendoza, E., Quijano-Scheggia, S., Olivos-Ortiz, A., Núñez-Vázquez, E., Eds.; CICESE: Ensenada, México, 2016. [Google Scholar]
- Cuéllar-Martínez, T.; Alonso-Rodríguez, R.; Voltolina, D.; Morquecho, M. Effectiveness of coagulants-flocculants for removing cells and toxins of Gymnodinium catenatum. Aquaculture 2016, 452, 188–193. [Google Scholar] [CrossRef]
- AziziHariri, P.; Hossain, I.; Burni, F.; Raghavan, S.; Lovko, V.; McLean, T.; Vijay, T. A Simple Method to Clear Harmful Algal Blooms: Sprayable Foams with Algaecides and Flocculants. ACS EST Water 2025, 5, 2547–2555. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Morquecho, L.; Lechuga-Devéze, C.; Anderson, D. Effects of growth medium, temperature, salinity and seawater source on the growth of Gymnodinium catenatum (Dinophyceae) from Bahía Concepción, Gulf of California. J. Plankton Res. 2004, 26, 1459–1470. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Bustillos-Guzmán, J.; Morquecho, L.; Gárate-Lizárraga, I.; Alonso-Rodríguez, R.; Reyes-Salinas, A.; Erler, K.; Luckas, B. Variations of PSP toxin profiles during different growth phases in Gymnodinium catenatum (Dinophyceae) strains isolated from three locations in the Gulf of California, Mexico. J. Phycol. 2006, 42, 757–768. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Rojas-Posadas, D.; Morquecho, L.; Hernández-Saavedra, N. Heterogeneity of LSU rDNA sequences and morphology of Gymnodinium catenatum strains in Bahía Concepción, Gulf of California, Mexico. J. Plankton Res. 2008, 30, 755–763. [Google Scholar] [CrossRef]
- Jackson, G.; Lochmann, S. Modeling coagulation of algae in marine ecosystems. In Environmental Particles: Environmental, Analytical, and Physical Chemistry; Buffle, J., Leeuwen, H.P., Eds.; Lewis Publisher: Boca Raton, FL, USA, 1993; pp. 387–414. [Google Scholar]
- Kiørboe, T.; Andersen, K.; Dam, H. Coagulation efficiency and aggregate formation in marine phytoplankton. Mar. Biol. 1990, 107, 235–245. [Google Scholar] [CrossRef]
- Fraga, S.; Gallager, S.; Anderson, D. Chain-forming Dinoflagellates: An adaptation to Red Tide. In Red Tides: Biology, Environmental Science, and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier Science Publishing Co. Inc.: New York, NY, USA, 1989; pp. 281–284. [Google Scholar]
- Archambault, M.; Grant, J.; Bricelj, V. Removal efficience of the dinoflagellate Heterocapsa triquetra by phosphatic clay, and implications for the mitigation of harmful algal blooms. Mar. Ecol. Prog. Ser. 2003, 253, 97–109. [Google Scholar] [CrossRef][Green Version]
- Bae, H.; Choi, H.; Lee, W.; Yoon, S. Control of the red tide by yellow loess dispersion. In Proceedings of the Korea-China Joint Symposium on Harmful Algal Blooms, Pusan, Republic of Korea, 5-7 December 1997; pp. 53–60. [Google Scholar]
- Sengco, R.; Hagström, J.; Graneli, E.; Anderson, D. Removal of Prymnesium parvum (Haptophyceae) and its toxins using clay minerals. Harmful Algae 2005, 4, 261–274. [Google Scholar] [PubMed]
- Lu, G.; Song, X.; Yu, Z.; Cao, X.; Yuan, Y. Environmental effects of modified clay flocculation on Alexandrium tamarense and paralytic shellfish poisoning toxins (PSTs). Chemosphere 2015, 127, 188–194. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).