Agro-Physiological Performance of Iceberg Lettuce (Lactuca sativa L.) Cultivated on Substrates Amended with the Invasive Algae Caulerpa prolifera from the Mar Menor
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
- (1)
- 0% AW: 5 L of coconut fiber
- (2)
- 50% AW: Mixture of 2.5 L of algae + 2.5 L of coconut fiber
- (3)
- 75% AW: Mixture of 3.75 L of algae + 1.25 L of coconut fiber
- (4)
- 100% AW: 5 L of algae.
2.2. Mineral Concentration
2.3. Total Phenolic Compounds and Antioxidant Activity
2.4. Lipid Peroxidation
2.5. Extraction and Quantification of Total Soluble Sugars
2.6. Polyamine Analysis
2.7. Statistical Analysis
3. Results
3.1. Physical Characterization of Iceberg Lettuce
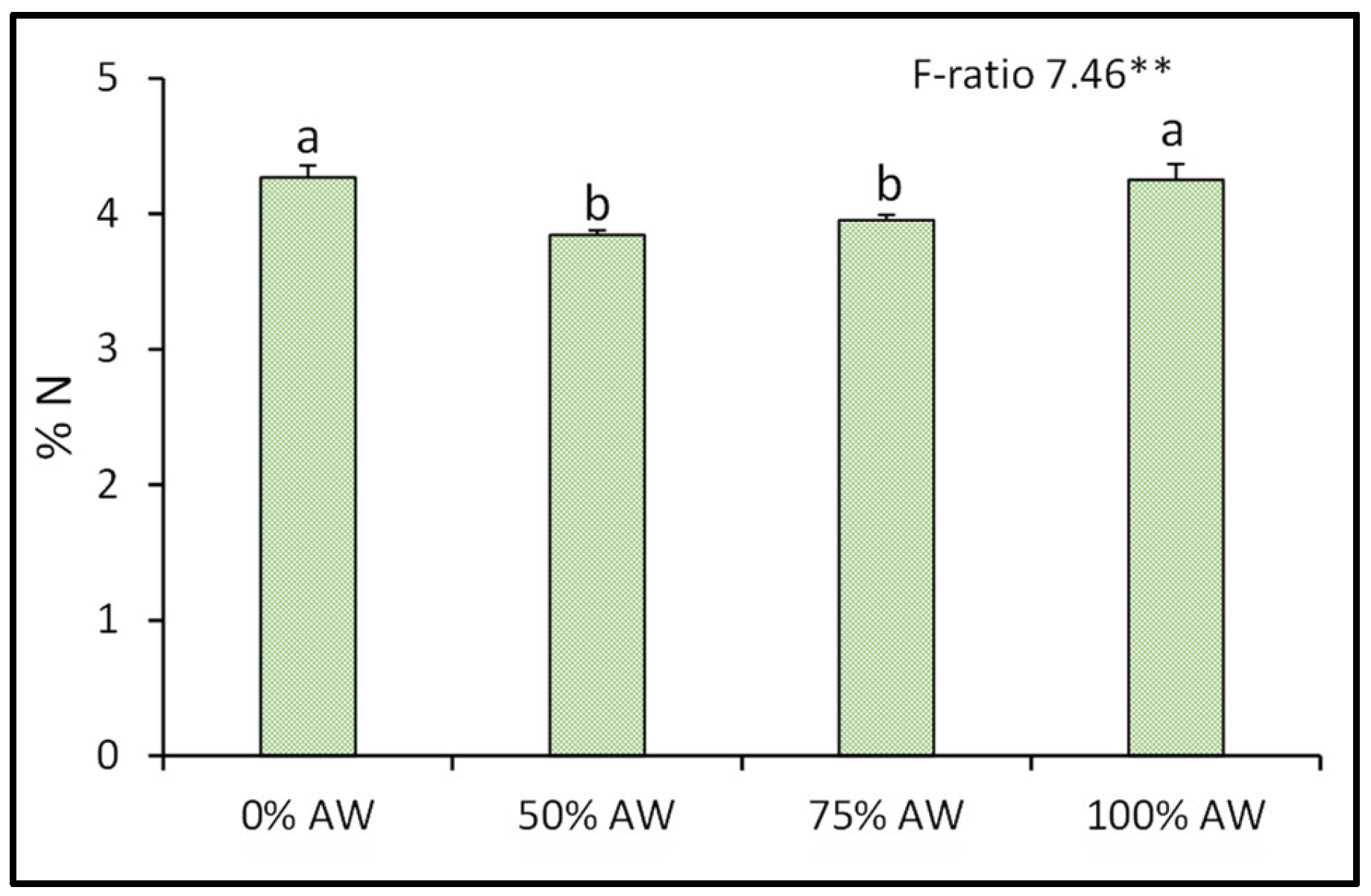
3.2. Mineral Composition
3.3. Total Phenolic Compounds, Antioxidant Activity (ABTS+*) and Lipid Peroxidation
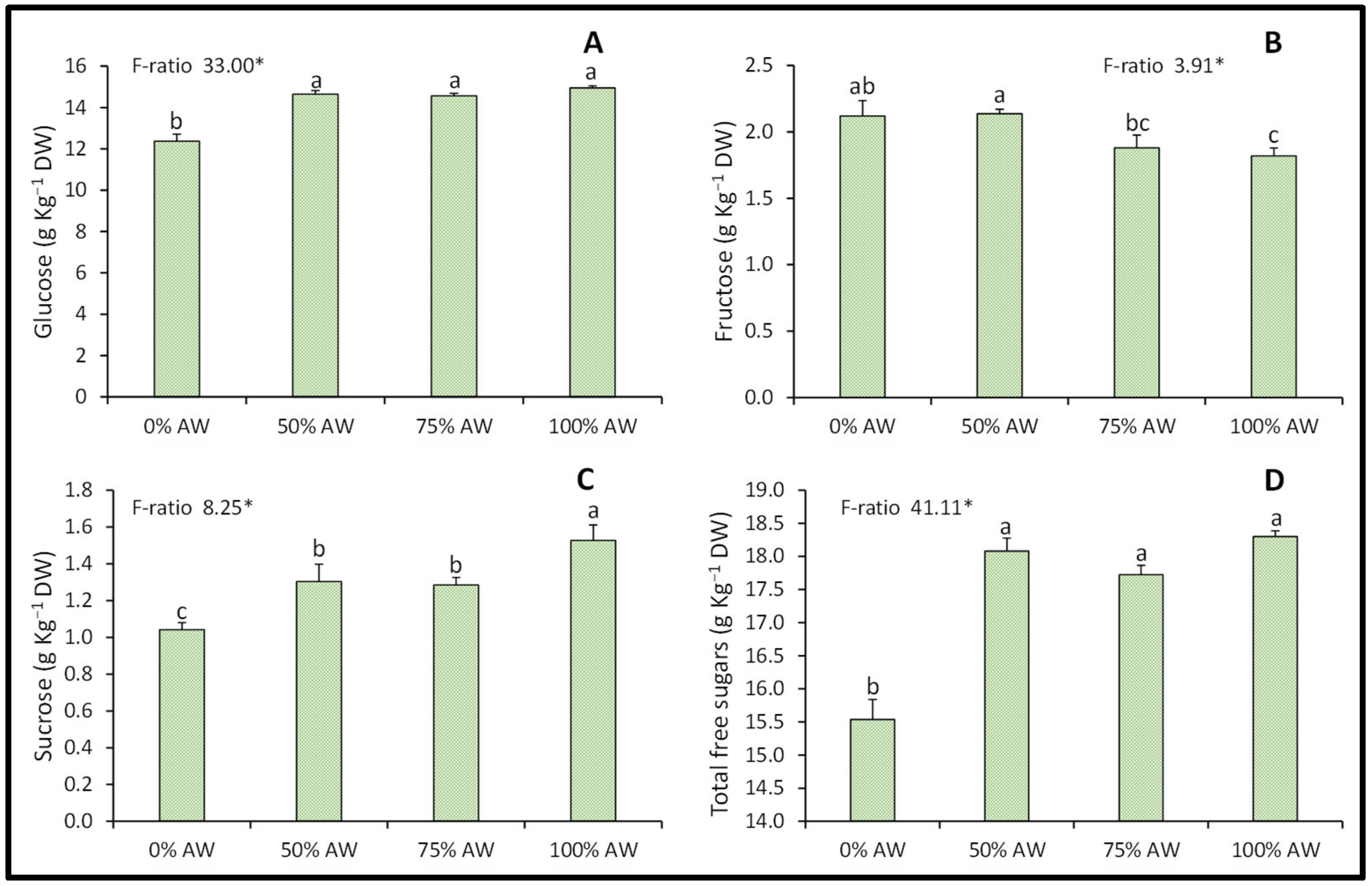
3.4. Total Soluble Sugars
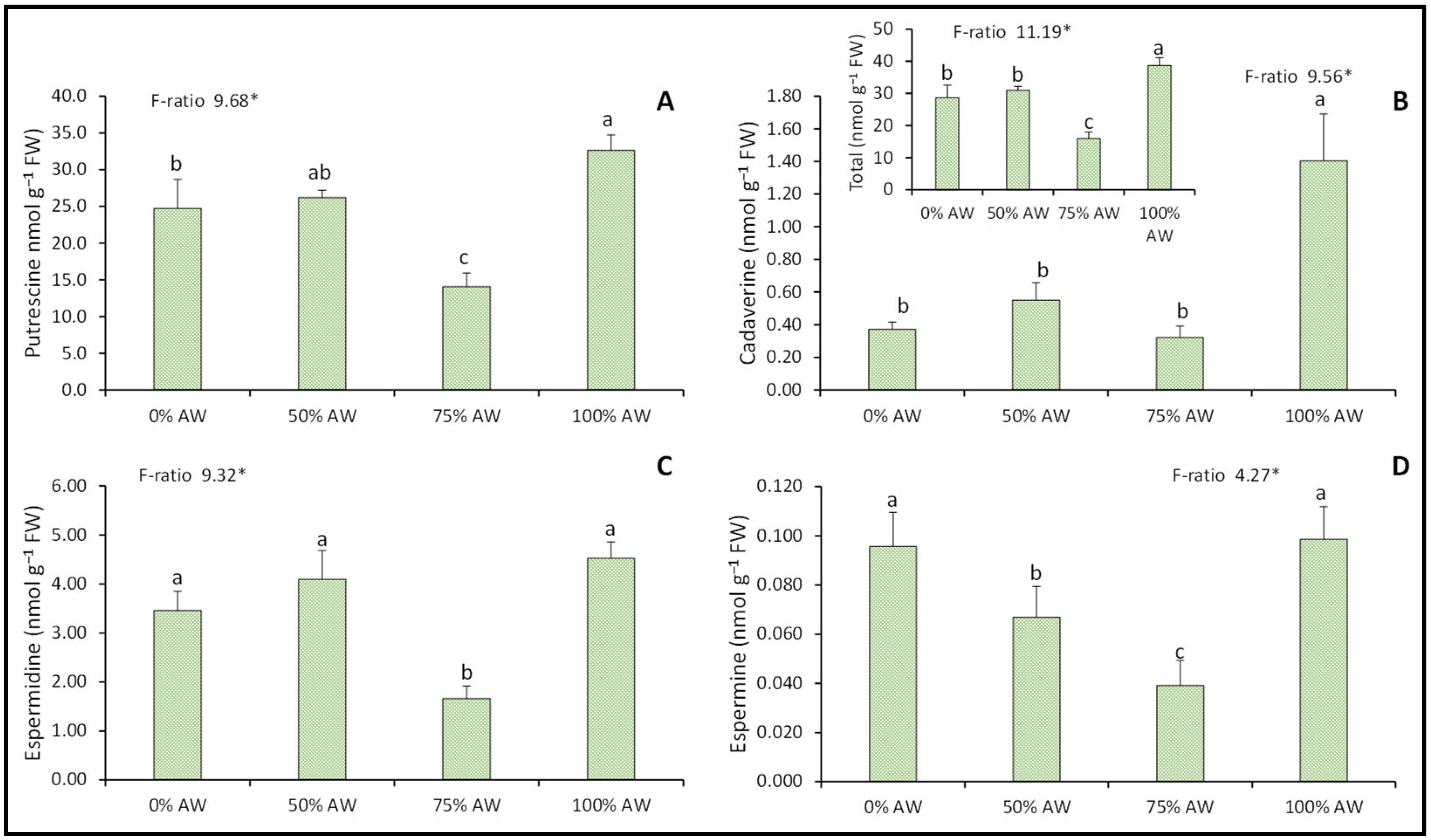
3.5. Polyamines
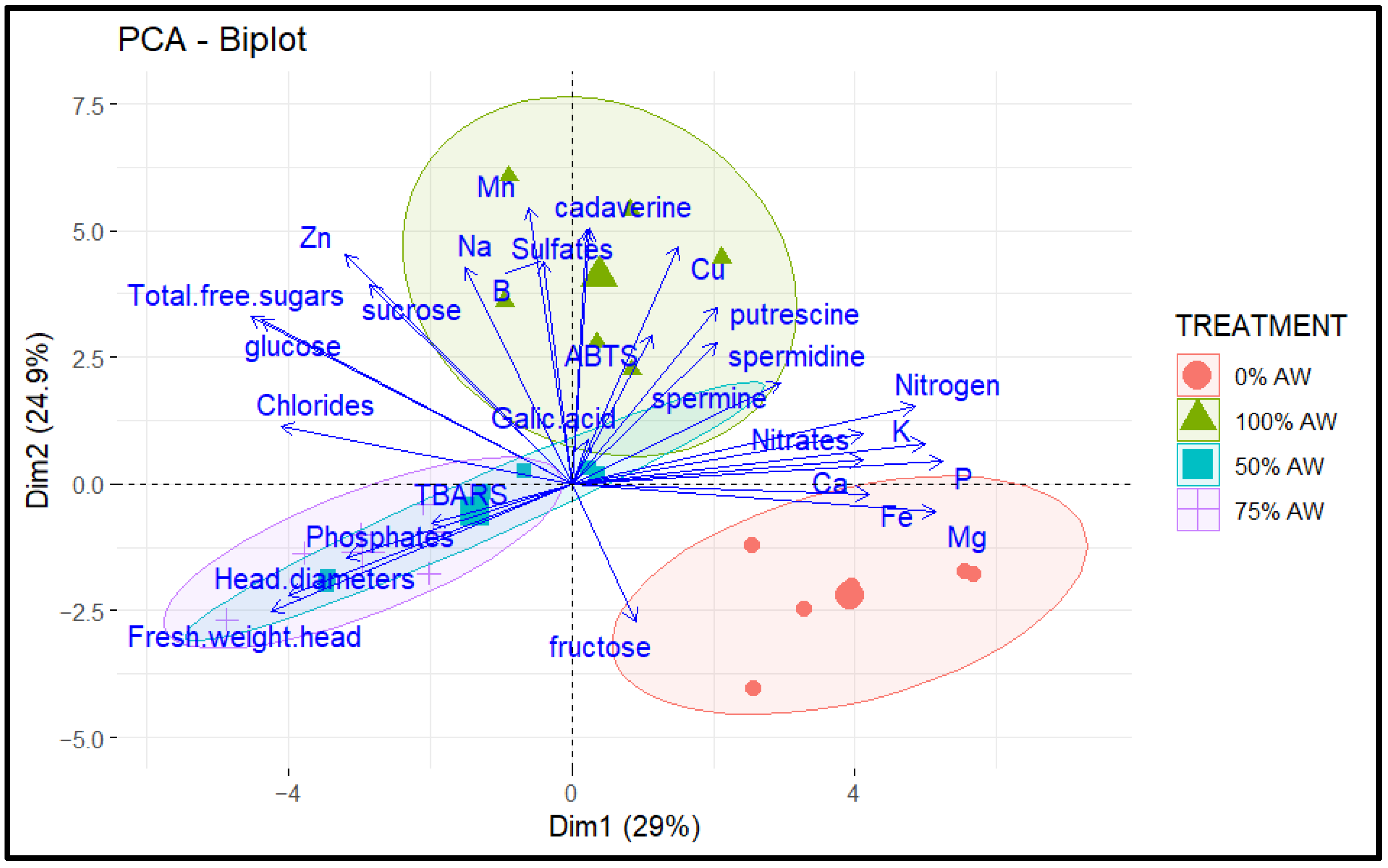
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Physical Characterization of Iceberg Lettuce
4.2. Mineral Composition
4.3. Total Phenolic Compounds, Antioxidant Activity (ABTS+*) and Lipid Peroxidation
4.4. Total Soluble Sugars
4.5. Polyamines
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Ruzafa, A.; Morkune, R.; Marcos, C.; Pérez-Ruzafa, I.M.; Razinkovas-Baziukas, A. Can an oligotrophic coastal lagoon support high biological productivity? Sources and pathways of primary production. Mar. Environ. Res. 2020, 153, 104824. [Google Scholar] [CrossRef] [PubMed]
- Ammaturo, C.; Pacheco, D.; Cotas, J.; Formisano, L.; Ciriello, M.; Pereira, L.; Bahcevandziev, K. Use of Chlorella vulgaris and Ulva lactuca as biostimulant on lettuce. Appl. Sci. 2023, 13, 9046. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Panda, D.; Pramanik, K.; Nayak, B.R. Use of seaweed extracts as plant growth regulators for sustainable agriculture. Int. J. Bio-Resour. Stress Manag. 2012, 3, 404–411. [Google Scholar]
- Espinosa-Antón, A.A.; Zamora-Natera, J.F.; Zarazúa-Villaseñor, P.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Águila Alcántara, E.; Torres-Morán, M.I.; Velasco-Ramírez, A.P.; Hernández-Herrera, R.M. Application of seaweed generates changes in the substrate and stimulates the growth of tomato plants. Plants 2023, 12, 1520. [Google Scholar] [CrossRef]
- Ouahabi, S.; Daoudi, N.; Chebaibi, M.; Moujahid, M.E.; Idrissi, M. A comparative study of the phytochemical composition, antioxidant properties, and in vitro anti-diabetic efficacy of different extracts of Caulerpa prolifera. Mar. Drugs 2025, 21, 372. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 7 May 2024).
- da Cruz Ferreira, R.L.; de Mello Prado, R.; de Souza Junior, J.P.; Gratão, P.L.; Tezotto, T.; Cruz, F.J.R. Oxidative stress, nutritional disorders, and gas exchange in lettuce plants subjected to two selenium sources. J. Soil Sci. Plant Nutr. 2020, 20, 1215–1228. [Google Scholar] [CrossRef]
- Shatilov, M.; Razin, A.; Ivanova, M. Analysis of the world lettuce market. IOP Conf. Ser. Earth Environ. Sci. 2019, 395, 012053. [Google Scholar] [CrossRef]
- del Amor, F.M.; Gomez-Lopez, M.D. Agronomical response and water use efficiency of sweet pepper plants grown in different greenhouse substrates. HortScience 2009, 44, 810–814. [Google Scholar] [CrossRef]
- Porras, M.E.; Lorenzo, P.; Medrano, E.; Sánchez-González, M.J.; Otálora-Alcón, G.; Piñero, M.C.; del Amor, F.M.; Sánchez-Guerrero, M.C. Photosynthetic acclimation to elevated CO2 in sweet pepper under Mediterranean greenhouse conditions: Influence of nitrogen source and salinity. Funct. Plant Biol. 2017, 44, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Effects of selenium on chlorophylls, gas exchange, antioxidant activity and amino acids in lettuce under aquaponics. Horticulturae 2022, 8, 30. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Aluminium stress affects nitrogen fixation and assimilation in soybean. Plant Growth Regul. 2006, 48, 271–281. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Cuartero, J.; Bolarín, M.C.; Pérez-Alfocea, F. Sucrolytic activities during fruit development in Lycopersicon genotypes differing in salinity tolerance. Physiol. Plant. 2003, 118, 38–46. [Google Scholar] [CrossRef]
- Rodriguez, S.C.; López, B.; Chaves, A.R. Effect of different treatments on the evolution of polyamines during refrigerated storage of eggplants. J. Agric. Food Chem. 2001, 49, 4700–4705. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 26 May 2025).
- Górka, B.; Lipok, J.; Wieczorek, P.P. Biologically active organic compounds, especially plant promoters, in algae extracts and their potential application in plant cultivation. In Marine Algae Extracts: Processes, Products, and Applications; Wiley-VCH: Weinheim, Germany, 2015; pp. 659–680. [Google Scholar] [CrossRef]
- Dasgan, H.; Temtek, T. Impact of biofertilizers on plant growth, physiological and quality traits of lettuce (Lactuca sativa L. var. longifolia) grown under salinity stress. Agronomy 2022, 12, 2035. [Google Scholar] [CrossRef]
- Arun, M.; Kumar, R.; Nori, S.; Sreedevi, B.; Padmavathi, G.; Revathi, P.; Pathak, N.; Srinivas, D.; Sundaram, R. Biostimulant properties of marine bioactive extracts in plants: Toward sustainable crop production in rice. In Marine Ecosystems—Biodiversity, Ecosystem Services and Human Impacts; IntechOpen: London, UK, 2023. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Kappel, N.; Boros, I.F.; Ravelombola, F.S.; Sipos, L. EC sensitivity of hydroponically-grown lettuce (Lactuca sativa L.) types in terms of nitrate accumulation. Agriculture 2021, 11, 315. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Unraveling the nutritional and bioactive constituents in baby-leaf lettuce for challenging climate conditions. Food Chem. 2022, 384, 132506. [Google Scholar] [CrossRef]
- Piñero, M.C.; Pérez-Jiménez, M.; López-Marín, J.; del Amor, F.M. Changes in the salinity tolerance of sweet pepper plants as affected by nitrogen form and high CO2 concentration. J. Plant Physiol. 2016, 200, 18–27. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Scalschi, L.; Vicedo, B.; Marcos-Barbero, E.L.; Morcuende, R.; Camañes, G. Putrescine: A key metabolite involved in plant development, tolerance and resistance responses to stress. Int. J. Mol. Sci. 2022, 23, 2971. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties. Int. J. Food Prop. 2005, 8, 521–528. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Favela-González, K.; Hernández-Almanza, A.; de la Fuente-Salcido, N. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, L.; Chojnacka, K.W. Co-composting of algae and effect of the compost on germination and growth of Lepidium sativum. Pol. J. Environ. Stud. 2016, 25, 1107–1115. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Nidumolu, L.C.M.; Lorilla, K.M.; Chakravarty, I.; Uhde-Stone, C. Soybean root transcriptomics: Insights into sucrose signaling at the crossroads of nutrient deficiency and biotic stress responses. Plants 2023, 12, 2117. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, T.; Yang, Q.; Guo, W. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef]
- Piñero, M.C.; Porras, M.E.; López-Marín, J.; Sánchez-Guerrero, M.C.; Medrano, E.; Lorenzo, P.; del Amor, F.M. Differential nitrogen nutrition modifies polyamines and the amino acid profile of sweet pepper under salinity stress. Front. Plant Sci. 2019, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kanungo, M. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 1982, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, H.R.; Wang, L.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Effects of different types of polyamine on growth, physiological and biochemical nature of lettuce under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012010. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Bai, G.; Li, P.; Yan, J. Polyamine pathways interconnect with GABA metabolic processes to mediate the low-temperature response in plants. Front. Plant Sci. 2022, 13, 1035414. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Algal Waste | Coconut Fiber |
|---|---|---|
| pH | 6.5 ± 0.01 | 6.3 ± 0.1 |
| CE (µS/cm) | 17,400 ± 1200 | 4100 ± 50 |
| Organic matter (%) | 73.9 ± 0.1 | 80% ± 0.1 |
| %N | 2.51 ± 0.54 | Trace levels |
| Concentration (mg/kg DW) | ||
| Cl− | 5324.01 ± 257.95 | Trace levels |
| NO3− | 165.48 ± 15.85 | Trace levels |
| PO43− | 317.65 ± 169.13 | Trace levels |
| SO42− | 4315.69 ± 9.11 | Trace levels |
| Na | 6.87 ± 0.33 | Trace levels |
| K | 2.17 ± 0.18 | Trace levels |
| Ca | 51.40 ± 0.66 | Trace levels |
| Mg | 7.10 ± 0.19 | Trace levels |
| P | 0.91 ± 0.02 | Trace levels |
| Fe | 3278.43 ± 504.89 | Trace levels |
| Cu | 8.53 ± 0.19 | Trace levels |
| Mn | 324.93 ± 36.92 | Trace levels |
| Zn | 146.78 ± 21.38 | Trace levels |
| B | 133.59 ± 0.58 | Trace levels |
| Treatment | Cl− | NO3− | PO43− | SO42− |
|---|---|---|---|---|
| g kg−1 DW | ||||
| 0% AW | 5.8 ± 0.4 b | 32.3 ± 2.0 a | 9.6 ± 1.22 a | 4.1 ± 0.2 b |
| 50% AW | 6.8 ± 0.1 a | 23.8 ± 1.1 bc | 9.6 ± 2.45 a | 4.1 ± 0.2 b |
| 75% AW | 7.3 ± 0.3 a | 21.9 ± 1.9 c | 10.0 ± 1.39 a | 4.6 ± 0.2 b |
| 100% AW | 6.8 ± 0.2 a | 28.7 ± 2.6 ab | 9.6 ± 0.99 a | 5.7 ± 0.1 a |
| F-ratio | 5.1 * | 5.3 ** | 1.5 ns | 18.8 *** |
| Treatment | K | Ca | Mg | P | Fe | Cu | Mn | Zn | B |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DW | mg kg−1 DW | ||||||||
| 0% AW | 49.2 a | 3.6 a | 2.3 a | 8.1 a | 103.0 a | 4.5 b | 4.8 d | 59.0 c | 16.0 b |
| 50% AW | 41.0 bc | 3.3 ab | 2.0 bc | 7.5 a | 95.2 ab | 4.1 b | 11.5 c | 144.0 b | 17.3 ab |
| 75% AW | 37.8 c | 2.9 b | 1.8 c | 6.7 b | 88.1 b | 4.1 b | 17.6 b | 160.7 b | 16.6 b |
| 100% AW | 44.8 ab | 3.3 ab | 2.0 b | 7.5 a | 96.1 ab | 6.4 a | 74.2 a | 220.1 a | 18.7 a |
| F-ratio | 9.0 ** | 4.5 * | 9.7 ** | 7.1 ** | 2.6 ns | 32.3 *** | 588.1 *** | 107.1 *** | 4.3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñero, M.C.; Collado-González, J.; Otálora, G.; González, Y.; del Amor, F.M. Agro-Physiological Performance of Iceberg Lettuce (Lactuca sativa L.) Cultivated on Substrates Amended with the Invasive Algae Caulerpa prolifera from the Mar Menor. Phycology 2025, 5, 60. https://doi.org/10.3390/phycology5040060
Piñero MC, Collado-González J, Otálora G, González Y, del Amor FM. Agro-Physiological Performance of Iceberg Lettuce (Lactuca sativa L.) Cultivated on Substrates Amended with the Invasive Algae Caulerpa prolifera from the Mar Menor. Phycology. 2025; 5(4):60. https://doi.org/10.3390/phycology5040060
Chicago/Turabian StylePiñero, María Carmen, Jacinta Collado-González, Ginés Otálora, Yamara González, and Francisco M. del Amor. 2025. "Agro-Physiological Performance of Iceberg Lettuce (Lactuca sativa L.) Cultivated on Substrates Amended with the Invasive Algae Caulerpa prolifera from the Mar Menor" Phycology 5, no. 4: 60. https://doi.org/10.3390/phycology5040060
APA StylePiñero, M. C., Collado-González, J., Otálora, G., González, Y., & del Amor, F. M. (2025). Agro-Physiological Performance of Iceberg Lettuce (Lactuca sativa L.) Cultivated on Substrates Amended with the Invasive Algae Caulerpa prolifera from the Mar Menor. Phycology, 5(4), 60. https://doi.org/10.3390/phycology5040060










