A New Record of Antithamnion hubbsii (Ceramiales, Rhodophyta) from the Korean Coast: Invasive Species Interactions with Native and Non-Native Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Culture and Microscopy
2.2. Molecular Analysis
3. Results
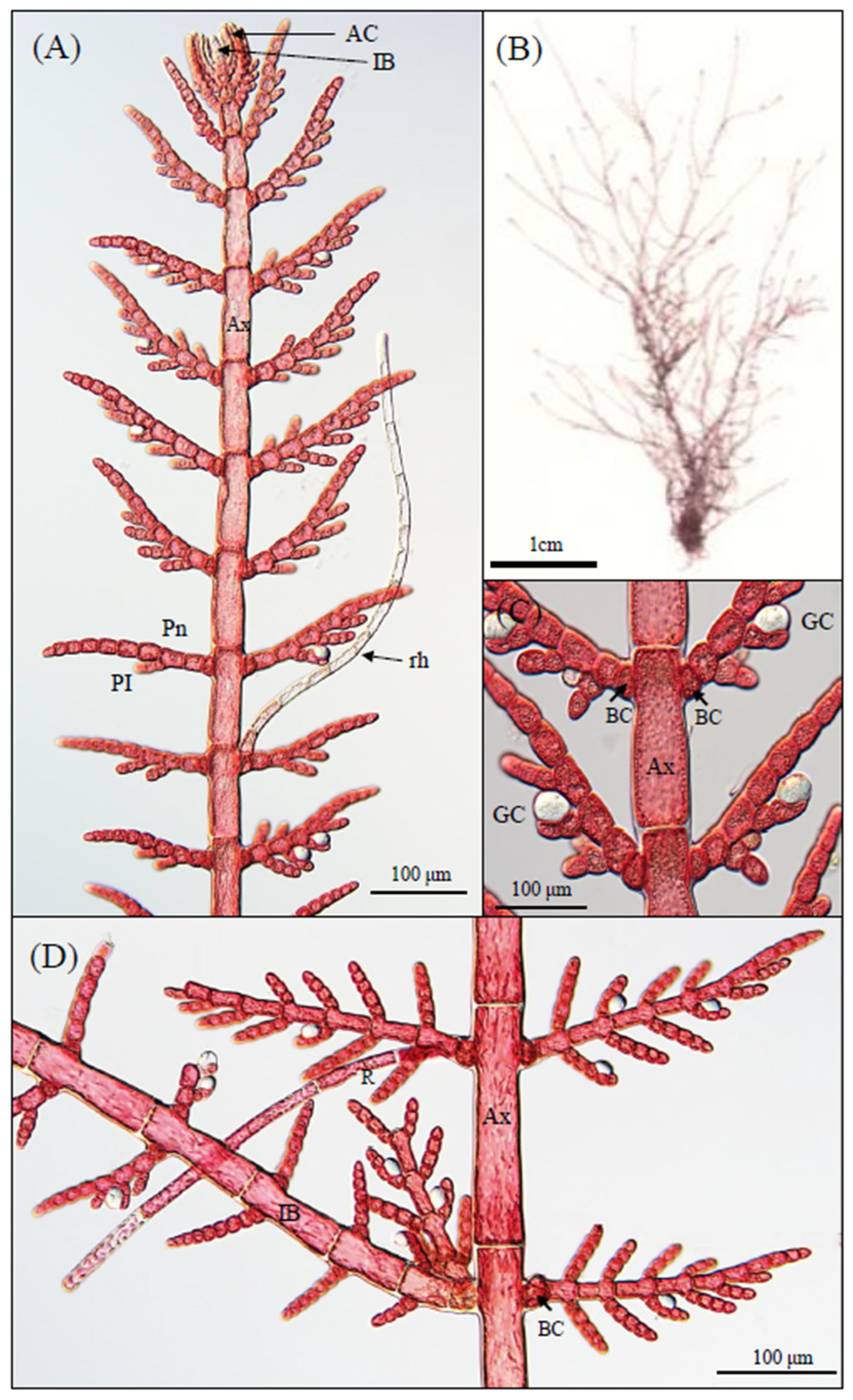
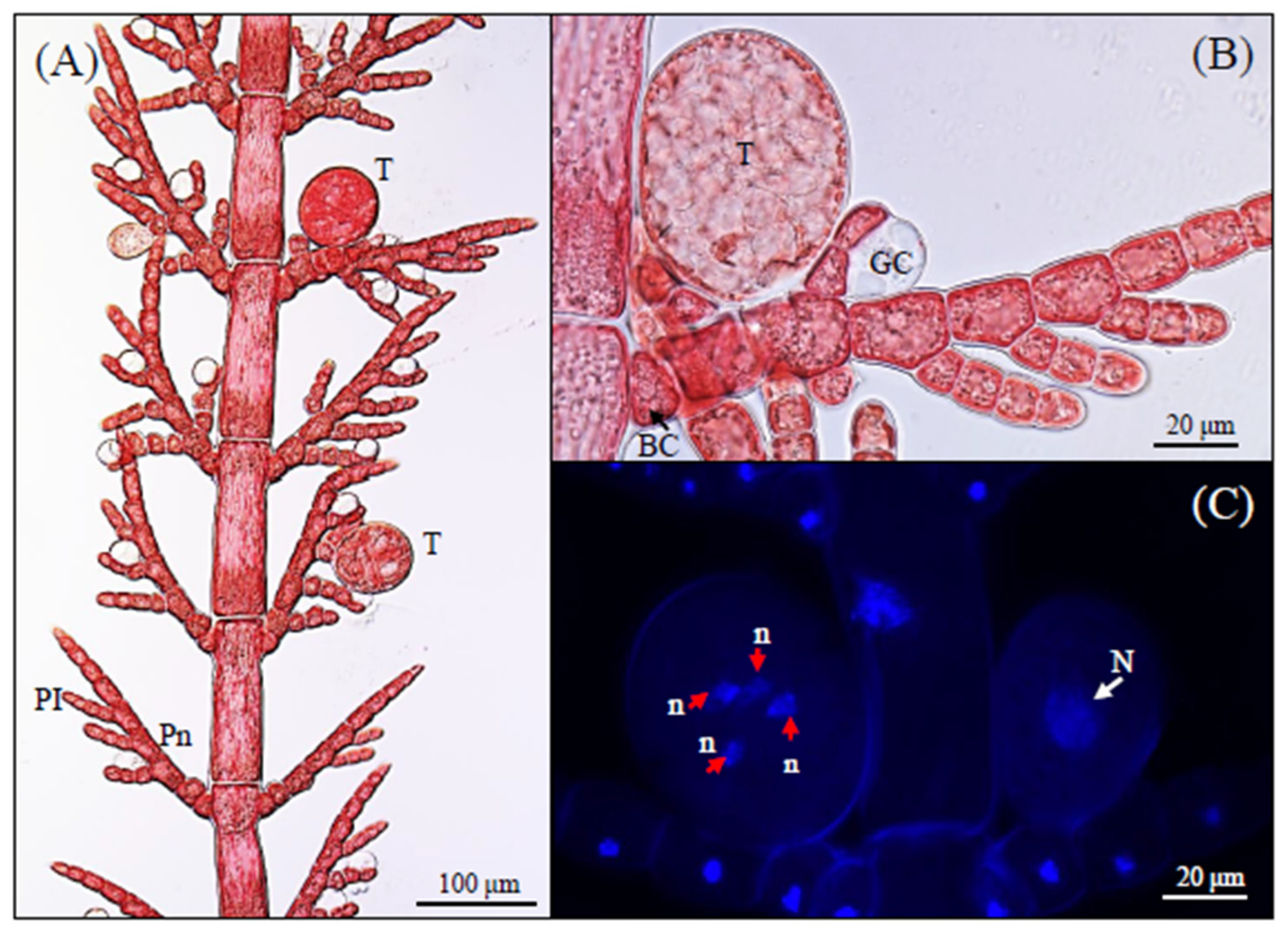
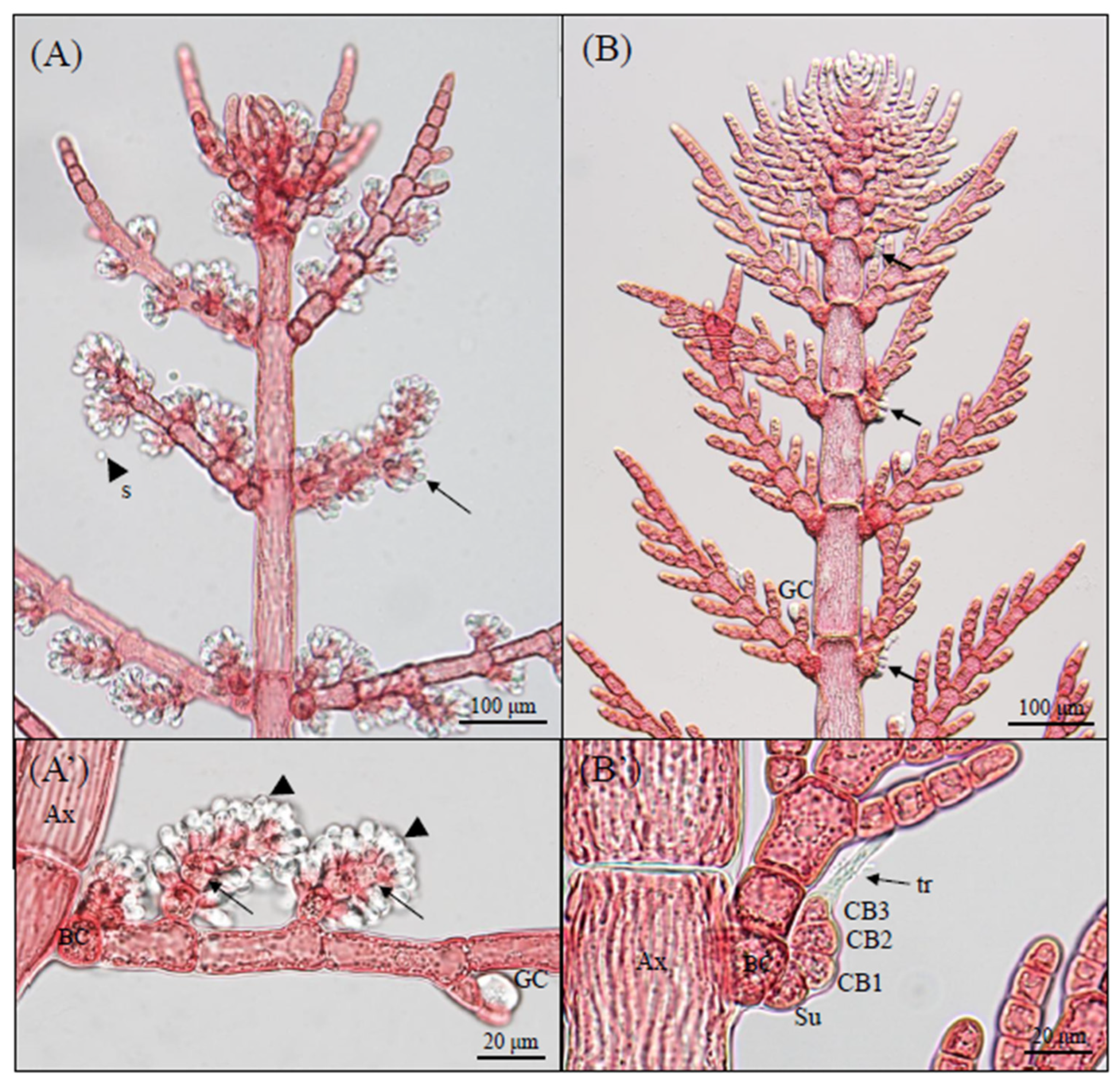
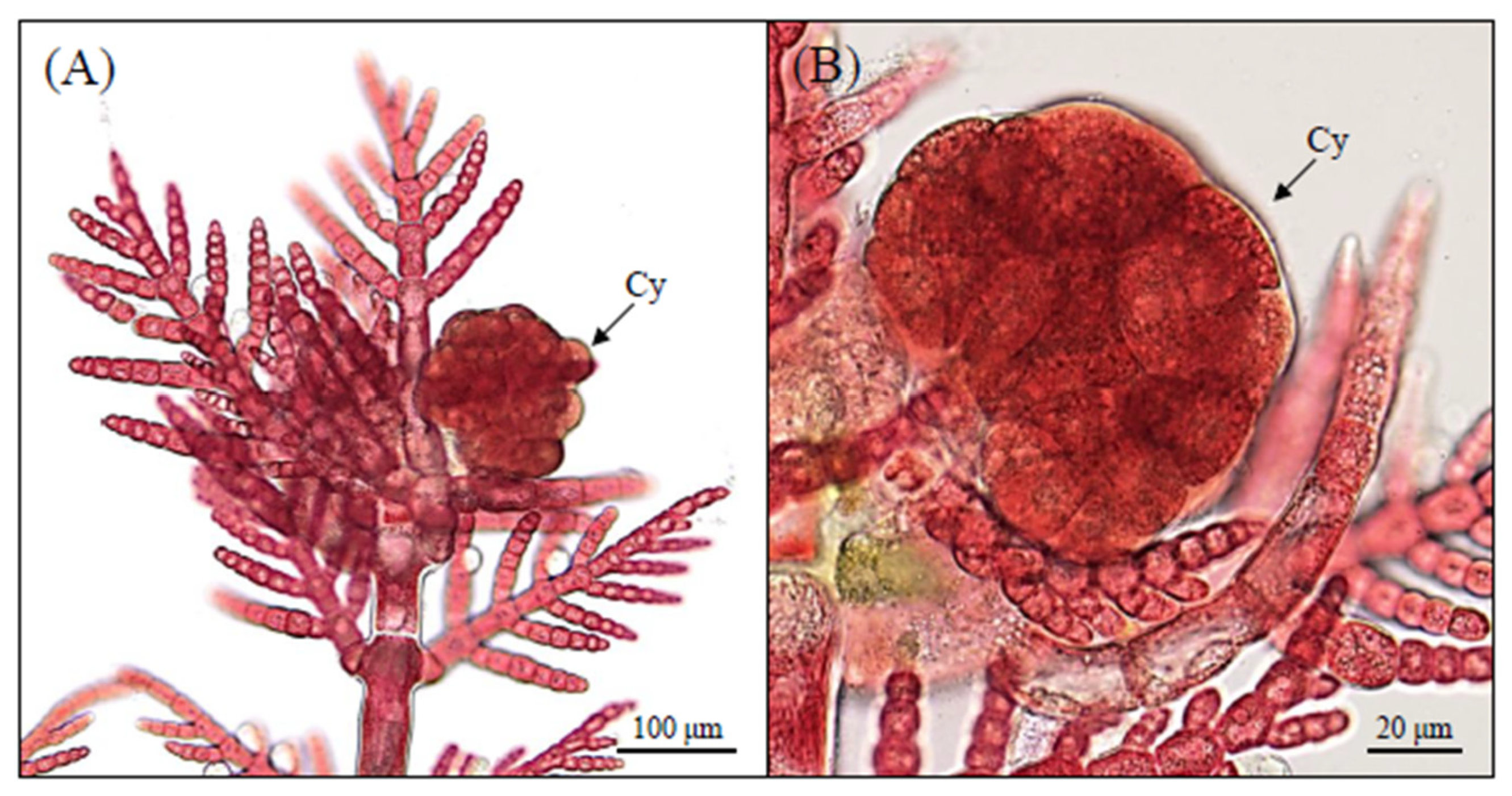
3.1. Morphological Observations
3.2. Phylogenetic Analyses
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rueness, J.; Heggøy, E.; Husa, V.; Sjøtun, K. First report of the Japanese red alga Antithamnion nipponicum on the west coast of Norway. Aquat. Invasions 2007, 2, 431–434. [Google Scholar] [CrossRef]
- Rueness, J. DNA barcoding of select freshwater and marine red algae (Rhodophyta). Cryptogam. Algol. 2010, 31, 377–386. [Google Scholar]
- Brooks, C.M.; Krumhansl, K. First record of the Asian Antithamnion sparsum Tokida, (Ceramiales, Rhodophyta) in Nova Scotia, Canada. BioInvasions Rec. 1932, 12, 745–752. [Google Scholar] [CrossRef]
- Cho, T.O.; Won, B.Y.; Fredericq, S. Antithamnion nipponicum (Ceramiaceae, Rhodophyta), incorrectly known as A. pectinatum in western Europe, is a recent introduction along the North Carolina and Pacific coasts of North America. Eur. J. Phycol. 2007, 40, 323–335. [Google Scholar] [CrossRef]
- Athanasiadis, A.; Tittley, I. Antithamnioid algae (Rhodophyta, Ceramiaceae) newly recorded from the Azores. Phycologia 1994, 33, 77–80. [Google Scholar] [CrossRef]
- Athanasiadis, A. Typification of Antithamnion nipponicum Yamada et Inagaki (Antithamnieae, Ceramioideae, Ceramiaceae, Ceramiales, Rhodophyta). Bot. Mar. 2009, 52, 256–261. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Maggs, C.A.; Le Gall, L.; Verbruggen, H. Phylogenetic analysis of the red algal tribe Ceramieae reveals multiple morphological homoplasies but defines new genera. Cryptogam. Algol. 2023, 44, 101–134. [Google Scholar]
- Carlton, J.T.; Geller, J.B. Ecological roulette: The global transport of nonindigenous marine organisms. Science 1993, 261, 78–82. [Google Scholar] [CrossRef]
- Klochkova, T.A.; Kang, S.-H.; Cho, G.Y.; Pueschel, C.M.; West, J.A.; Kim, G.H. Biology of a terrestrial green alga, Chlorococcum sp. (Chlorococcales, Chlorophyta), collected from the Miruksazi stupa in Korea. Phycologia 2006, 45, 349–358. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Burger, G.; West, J.A.; King, R.J. A mitochondrial marker for red algal intraspecific relationships. Mol. Ecol. 1999, 8, 1443–1447. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Kim, J.H.; Kim, M.S. DNA barcoding of the genus Grateloupia (Rhodophyta) from Korea: An assessment of DNA barcoding markers for species identification. Diversity 2020, 12, 3. [Google Scholar]
- Yoon, H.S.; Hackett, J.D.; Bhattacharya, D. A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 11724–11729. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4: Tree Figure Drawing Tool. Institute of Evolutionary Biology, University of Edinburgh. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 6 May 2025).
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Dawson, E.Y. Studies of intertidal algae at Pacific Grove, California. Bull. Calif. Acad. Sci. 1925, 24, 31–59. [Google Scholar]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algol. 2010, 31, 435–449. [Google Scholar]
- Nägeli, C. Die neueren Algensysteme und Versuch zur Begründung eines eigenen Systems der Algen und Florideen. Neue Denkschr. Der Allg. Schweiz. Ges. Für Die Gesammten Nat. 1847, 9, 1–275. [Google Scholar]
- Payo, D.A.; Leliaert, F.; Verbruggen, H.; D’Hondt, S.; Calumpong, H.P.; De Clerck, O. Extensive cryptic species diversity and fine-scale endemism in the marine red alga Portieria in the Philippines. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122660. [Google Scholar] [CrossRef] [PubMed]
- Leliaert, F.; Verbruggen, H.; Vanormelingen, P.; Steen, F.; López-Bautista, J.M.; Zuccarello, G.C.; De Clerck, O. DNA-based species delimitation in algae. Eur. J. Phycol. 2014, 49, 179–196. [Google Scholar] [CrossRef]
- Steen, H.; Scrosati, R. Intraspecific competition in Fucus serratus and Fucus evanescens (Phaeophyceae: Fucales) germlings: Effects of settlement density, nutrient supply, and temperature. Mar. Biol. 2004, 144, 61–70. [Google Scholar]
- Suzuki, M.; Terada, R. DNA-based floristic survey of red algae (Rhodophyta) growing in the mesophotic coral ecosystems (MCEs) offshore of Tanegashima Island, northern Ryukyu Archipelago, Japan. PLoS ONE 2025, 20, e0316067. [Google Scholar] [CrossRef]
- Olenin, S.; Alemany, F.; Cardoso, A.C.; Gollasch, S.; Goulletquer, P.; Lehtiniemi, M.; McCollin, T.; Minchin, D.; Miossec, L.; Occhipinti-Ambrogi, A.; et al. Marine Strategy Framework Directive–Task Group 2 Report: Non-Indigenous Species; Office for Official Publications of the European Communities: Luxembourg, 2011. [Google Scholar]
- Williams, S.L.; Smith, J.E. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]

| Species | Location of Collection and/or Source of Culture; Collector or Depositor | Date | GeneBank Accession NO. |
|---|---|---|---|
| ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) gene | |||
| Antithamnion aglandum Kim et Lee | Jeoongdori, Wando, Republic of Korea; S.M. Boo, T.O. Cho & H.G. Choi | 29 January 1999 | AY594700 |
| Antithamnion cruciatum | Mulroy Bay, Co. Donegal, Ireland; C.A. Maggs | 16 February 1993 | JN089394 |
| Antithamnion defectum | Sitka, AK, USA | 21 June 2005 | GO252484 |
| Antithamnion defectum | La Jolla, CA, USA; C.A. Maggs | 27 July 1995 | JN089391 |
| Antithamnion defectum | Skellig Rocks, Co. Kerry, Ireland; C.A. Maggs | 1 June 1992 | JN089395 |
| Antithamnion hanovioides (Sonder) De Toni | Pennington Bay, Kangaroo Island, S. Australia; T.O. Cho & B.Y. Won | 7 September 1995 | AY591927 |
| Antithamnion hubbsii | Spain | 7 May 2019 | MK814610 |
| Antithamnion hubbsii Shim et Kim | Sungeut beach, Gangwon-do, Republic of Korea; E. Shim, S.Y. Kim & G.H. Kim | 6 January 2024 | PV453999 |
| Antithamnion kylinii | Canada | JN089393 | |
| Antithamnion nipponicum Yamada et Inagaki | Hachinohe, Aomori, Japan; M. Kamiya | 21 May 1995 | AY594699 |
| Antithamnion nipponicum NC-1 | In front of Duke University Marine Laboratory, Pivers I, Beaufort, Carteret Co., NC, USA; T.O. Cho & B.Y. Won | 29 October 2003 | AY591928 |
| Antithamnion nipponicum | Maengbang, Samcheok, Republic of Korea; E.C. Yang & S.M. Boo | 2016 | AY295174 |
| Antithamnion nipponicum CA | Halfmoon Bay, CA, USA; T.O. Cho & B.Y. Won | AY591930 | |
| Antithamnion nipponicum | Aragami, Funakoshi, Yamada, Japan; Masahiro Suzuki | 29 August 2014 | LC821146 |
| Antithamnion pectinatum (Montagne) Brauner | Lee Bay, Stewart Island, New Zealand; W.A. Nelson | 3 October 2004 | DQ023481 |
| Antithamnion pectinatum | Kenton On Sea, South Africa; Faith Mshiywa | 24 November 2017 | OR939842 |
| Antithamnion sp. | Mallacoota, VIC, Australia; H. Verbruggen & K. Dixon | 2019 | MK125356 |
| Antithamnion sp. | Ewing Bank, Offshore Louisiana, USA; J. Richards | 29 May 2011 | KY994130 |
| Antithamnion sparsum Tokida | Nova Sotia, Canada | 2021 | OP600459 |
| Antithamnion sparsum | Daechon, Republic of Korea; H.G. Cho | 23 April 1992 | JN089392 |
| Antithamnion ternifolia (Hooker et Harvey) Lyle | Williamston, Australia; J. West | 28 May 2002 | AY591926 |
| Amoenothamnion planktonicum | WestAP, Australia | OR359632 | |
| Antithamnionella miharai | Youngeumjun, Sokcho, Republic of Korea | 9 May 2006 | GQ252485 |
| Antithamnionella spirographidis | Monterey Bay, CA, USA; T.O. Cho | 11 December 1999 | AY591923 |
| Photosystem I P700 apoprotein (psaA) | |||
| Aglaothamnion callophyllidicola | Kamo Bay, Oki Island, Japan; E.C. Yang & S.M. Boo | 7 May 2003 | DQ787601 |
| Aglaothamnion hookeri | Castilo San Cristobal, Canary, Spain | 24 April 2004 | EU195020 |
| Antithamnion hubbsii | MK814610 | ||
| Antithamnion hubbsii Shim et Kim | Sungeut beach, Gangwon-do, Republic of Korea; E. Shim, S.Y. Kim & G.H. Kim | 6 January 2024 | PV454032 |
| Antithamnion nipponicum | - | 2019 | AY295136 |
| Antithamnionella sp. | Choshi, Chiba, Japan; E.C. Yang & S.M. Boo | 27 July 2002 | DQ787600 |
| Antithamnionella ternifolia | - | 2019 | MK814608 |
| Campylaephora kondoi | Hakampo, Taean, Republic of Korea; E.C. Yang & S.M. Boo | 2003 | AY295138 |
| Corallina pilulifera | Nagasaki, Choshi, Chiba, Japan; E.C. Yang & S.M. Boo | 1 August 2004 | DQ787594 |
| Species | Gland Cells | Indeterminae Laterals | Pinnae | Branching Plane | Tetrasporangia |
|---|---|---|---|---|---|
| New collection, Antithamnion hubbsii Shim & Kim, 2024 | Borne on pinnules, adaxial, covering 2 cells | Originate from basal cells of whorl-branches (paired or irregularly produced) | Pinnate in both diploid (tetrasporophyte) and haploid (gametophyte). Some diploid individuals may appear partially pectinate due to rhizoid development. | Distichous | 1-cell pedicel |
| Antithamnion hubbsii Dawson, 1963 | Borne on pinnules, adaxial, covering 2 cells | Paired | Pinnate | Distichous | NA |
| Antithamnion nipponicum Yamada et Inagaki | Borne on pinnules, adaxial, covering 2 cells | Indeterminate axes often replace whorl branches | Pinnate | Distichous | 1-cell pedicel |
| Antithamnion cruciatum Nägeli, 1847 | On pinnules, covering 2 cells | Paired w/determinate lateral | Branched decussate | Decussate | 1-cell pedicel or sessile, tetrahedral |
| Antithamnion defectum Kylin, 1925 | On pinnules, covering 2 cells | Unpaired | Branched, pectinate | Distichous | Pedicellate, undefined pedicel length |
| Antithamnion densum Howe, 1914 | On pinnules, covering 2 cells | Paired w/determinate lateral | Adaxially pectinate | Distichous | 1-cell pedicel |
| Antithamnion sparsum Tokida, 1932 | On pinnules, covering 2 cells | Unpaired | Adaxially pectinate | Distichous | 1-cell pedicel |
| Antithamnionella spirographidis Wollaston, 1968 | On pinnules, partly covering 1 cell | Unpaired | Simple | Distichous | Sessile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, E.; Kim, S.Y.; Kim, C.S.; Kim, G.H. A New Record of Antithamnion hubbsii (Ceramiales, Rhodophyta) from the Korean Coast: Invasive Species Interactions with Native and Non-Native Communities. Phycology 2025, 5, 55. https://doi.org/10.3390/phycology5040055
Shim E, Kim SY, Kim CS, Kim GH. A New Record of Antithamnion hubbsii (Ceramiales, Rhodophyta) from the Korean Coast: Invasive Species Interactions with Native and Non-Native Communities. Phycology. 2025; 5(4):55. https://doi.org/10.3390/phycology5040055
Chicago/Turabian StyleShim, Eunyoung, Soo Yeon Kim, Chan Song Kim, and Gwang Hoon Kim. 2025. "A New Record of Antithamnion hubbsii (Ceramiales, Rhodophyta) from the Korean Coast: Invasive Species Interactions with Native and Non-Native Communities" Phycology 5, no. 4: 55. https://doi.org/10.3390/phycology5040055
APA StyleShim, E., Kim, S. Y., Kim, C. S., & Kim, G. H. (2025). A New Record of Antithamnion hubbsii (Ceramiales, Rhodophyta) from the Korean Coast: Invasive Species Interactions with Native and Non-Native Communities. Phycology, 5(4), 55. https://doi.org/10.3390/phycology5040055






