Diversity and Morphology of Planktonic Species of the Order Dinophysales (Dinoflagellata) from the Tropical Mexican Pacific and the Gulf of Mexico
Abstract
1. Introduction
2. Materials and Methods
3. Results and Observations
- Division: Dinoflagellata (Bütschli) Fensome et al.
- Class: Dinophyceae Pascher
- Subclass: Dinophysiphysidae Möhn ex Fensome et al.
- Order: Dinophysales Kofoid
- Family: Dinophysaceae Bütschli
- Genus: Citharistes Stein
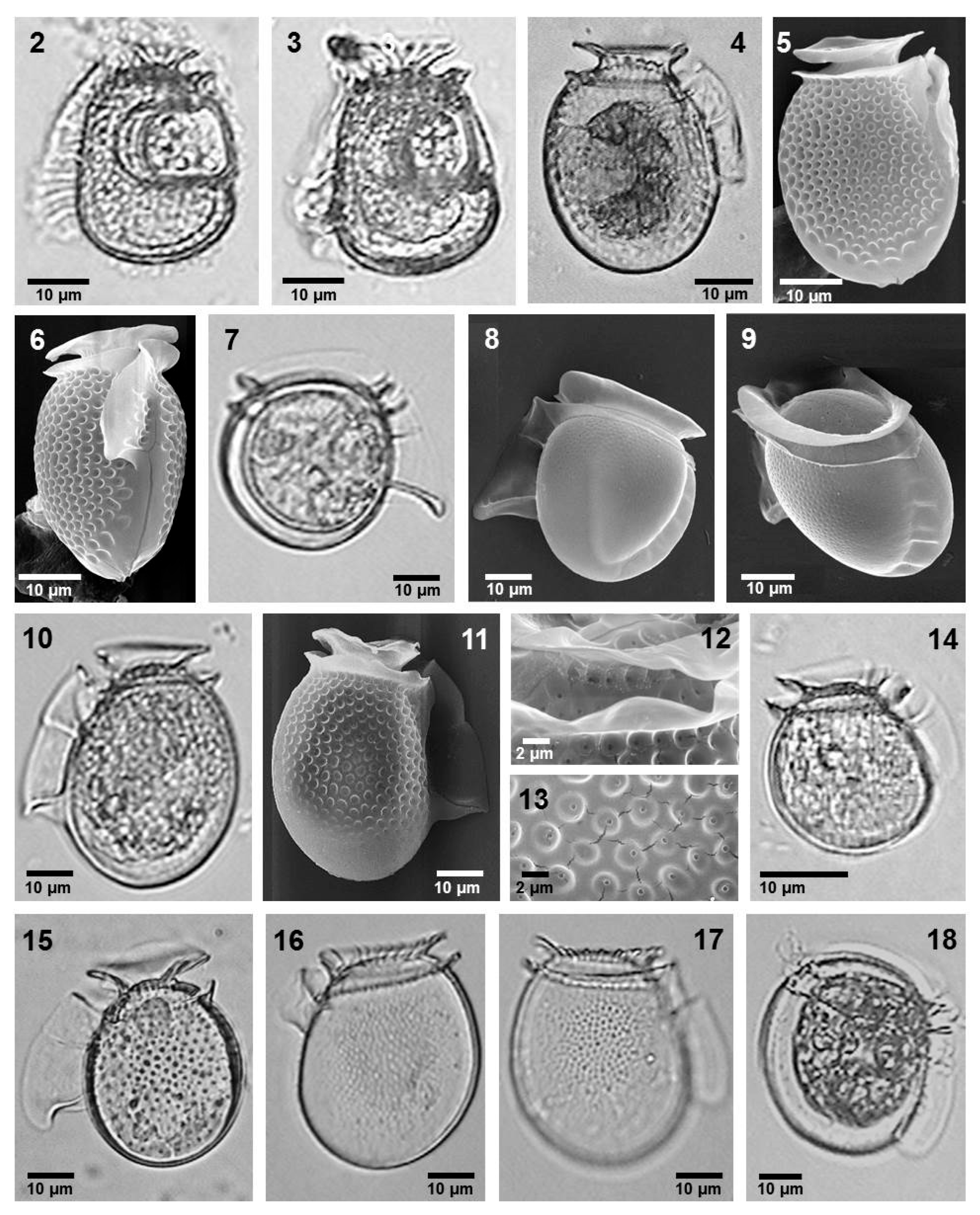
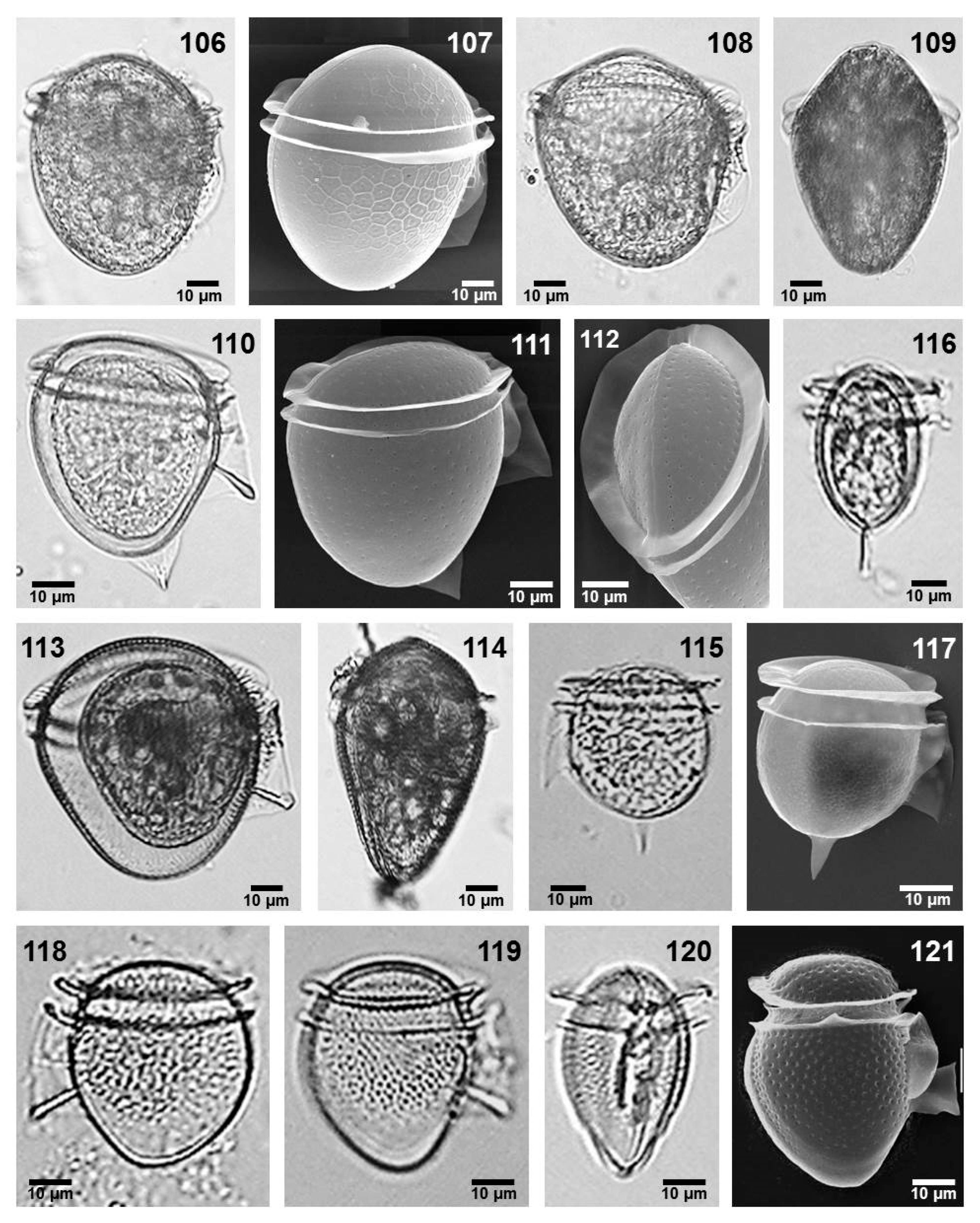
- Citharistes regius Stein (Figure 2, images 2 and 3)
- The theca has poroids with one pore. N = 18.
- Dimensions: L 40–55 µm, W 30–40 µm.
- Local distribution: western coast of the Baja California and central Mexican Pacific (localities n and bk).
- Dinophysis exigua Kofoid et Skogsberg (Figure 2, images 7–9)
- Dimensions: L 40–50 µm, W 37.5–45 µm.
- Local distribution: Gulf of California and central Mexican Pacific (localities n and t).
- Dinophysis fortii Pavillard (Figure 2, images 10–13)
- Dimensions: L 40–50 µm, W 32–40 µm.
- Local distribution: western coast of the Baja California, Gulf of California, central Mexican Pacific, and Gulf de Mexico (localities a, p, t, u, ab, ac, ae, ah, an, ao, as, bj, bk, and bn).
- Dinophysis infundibulus Schiller (Figure 2, image 14)
- Dimensions: L 40 µm, W 35 µm.
- Local distribution: central Mexican Pacific (locality v).
- Dinophysis similis Kofoid et Skogsberg (Figure 2, images 16 and 17)
- Dimensions: L 50–55 µm, W 50 µm.
- Local distribution: Gulf of California, at 29 °C and 35 salinity (locality b).
- Dinophysis sourniae Balech (Figure 2, Image 18)
- Dimensions: L 49 µm, W 44 µm.
- Local distribution: central Mexican Pacific (locality e).
- Section: Hastata Pavillard
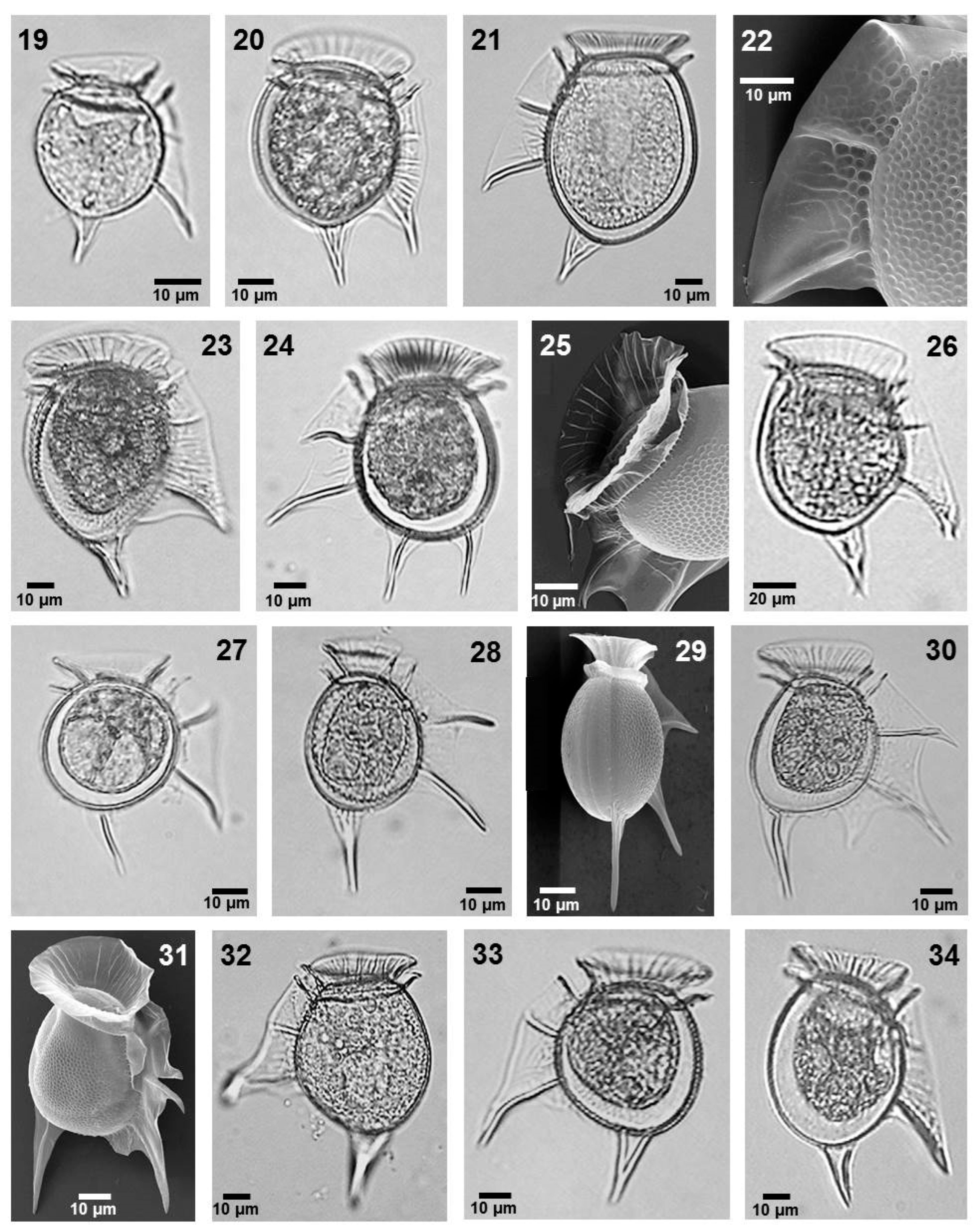
- Dinophysis balechii Norris et Berner (Figure 3, image 19)
- Reference: [27] (p. 1189, figs 3, 23, 34, 47, 62).
- Dinophysis conjuncta Parra-Toriz, Esqueda-Lara et Hernández-Becerril (Figure 3, image 20)
- Reference: [27] (p. 1189, figs 10, 27, 72, 83).
- Dinophysis hastata Stein (Figure 3, images 21 and 22)
- Reference: [27] (p. 1189, figs 4, 5, 24, 35, 36, 48, 55, 63).
- Local distribution in this paper: Gulf of Mexico (localities an, ao, ap, as, ay, bb, bs).
- Dinophysis monacantha Kofoid et Skogsberg (Figure 3, image 23)
- Reference: [27] (p. 1189, figs 11 and 28).
- Dinophysis nias Karsten (Figure 3, images 24 and 25)
- Reference: [27] (p.1198, figs 18, 19, 32, 45, 54, 70).
- Dinophysis phalacromoides (Jörgensen) Gómez, López-García et Moreira (Figure 3, image 26)
- Reference: [27] (p. 1195, fig. 12).
- Dinophysis pusilla Jörgensen (Figure 3, image 27)
- Reference: [27] (p. 1196, figs 13, 14, 29, 41, 52, 58, 67).
- Dinophysis schuettii Murray et Whitting (Figure 3, images 28 and 29)
- Reference: [27] (p. 1197, figs 15, 16, 30, 42, 53, 68).
- Local distribution in this paper: Gulf of Mexico (localities ah, ak, ay, bc, and bd).
- Dinophysis swezyae Kofoid et Skogsberg (Figure 3, images 30 and 31)
- Reference: [27] (p. 1198, figs 20, 21, 33, 46, 61, 71).
- Dinophysis uracantha Stein (Figure 3, image 32)
- Reference: [27] p. 1190, figs 6, 37, 38, 49, 56, 64.
- Local distribution in this paper: Gulf of Mexico (localities af, ao, be, and bf).
- Reference: [27] (p. 1191, figs 7, 8, 39, 50, 65).
- Dinophysis uracanthoides (Jörgensen) Gómez, López-García et Moreira (Figure 3, image 34)
- Reference: [27] (p. 1191, figs 9, 26, 40, 51, 57, 66).
- Section: Caudata Kofoid et Skogsberg
- Dinophysis caudata Saville-Kent (Figure 4, images 35–39)
- Dimensions: L 60–100 µm, W 47–95 µm.
- Local distribution: western coast of the Baja California, Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities a, b, c, m, n, o, r, s, t, u, ab, ac, ad, af, ah, ai, aj, ak, al, am, ao, ap, aq, ar, as, at, au, aw, ax, ay, az, bb, bc, bd, bk, bn, bo, bq, br, and bs).
- Remarks: potentially toxic species, producing Okadaic acid and Dinophysistoxins [15].
- Dimensions: L 65 µm, W 35–38 µm.
- Local distribution: central Mexican Pacific (locality n).
- Dinophysis tripos Gourret (Figure 4, images 42–46)
- The theca ornamentation is of one pore per poroid. N = 40.
- Dimensions: L 100–110 µm, W 50 µm.
- Local distribution: western coast of the Baja California and Gulf of California (localities a and u).
- Remarks: potentially toxic species, producing Pectenotoxins and Dinophysistoxins; nevertheless, its toxic potential for human intoxications is low [15].
- Genus: Metaphalacroma Tai et Skogsberg
- Metaphalacroma skogsbergi Tai (Figure 4, images 47 and 48)

- The sagittal suture is serrated, except the ventral margin of the epitheca and sulcal lists area. N = 6.
- Dimensions: L 50–55 μm, W 45–55 μm.
- Local distribution: Gulf of California (localities a, b, and t).
- Genus: Pseudophalacroma Jörgensen
- Pseudophalacroma nasutum (Stein) Jörgensen (Figure 4, images 49 and 50)
- The theca is ornamented with one pore in every poroid. N = 1.
- Dimensions: T 45 μm, W 39.5 μm.
- Local distribution: western coast of Baja California (locality u).
- Genus: Histioneis Stein
- Histioneis biremis Stein (Figure 5, images 51–56)
- Dimensions: L 31 µm, W 51 µm.
- Local distribution: Gulf of California (locality a).
- Histioneis costata Kofoid et Michener (Figure 5, images 57–61)
- Dimensions: L 76–82 µm, W 35 µm.
- Local distribution: Gulf of California and Gulf of Mexico (localities a and aa).
- Histioneis crateriforme Stein (Figure 5, images 67–71)
- Dimensions: L 34 µm, W 34 µm.
- Local distribution: central Mexican Pacific (locality p).
- Histioneis depressa Schiller (Figure 5, images 62 and 63)

- Dimensions: L 56 µm, W 30 µm.
- Local distribution: central Mexican Pacific (locality v).
- Histioneis garrettii Kofoid (Figure 5, images 65 and 66)
- Dimensions: L 88 µm, W 39 µm.
- Local distribution: central Mexican Pacific, at 28.9 °C and 32 salinity (locality r).
- Histioneis mitchellana Murray et Whitting (Figure 5, image 64)
- Local distribution: central Mexican Pacific (locality v).
- Dimensions: L 77 µm, W 57 µm.
- Genus: Ornithocercus Stein
- Synonym: Ornithocercus galea (Pouchet) Abé
- Dimensions: L 125–154 μm, W 81–115 μm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities b, d, e, f, g, n, and au).
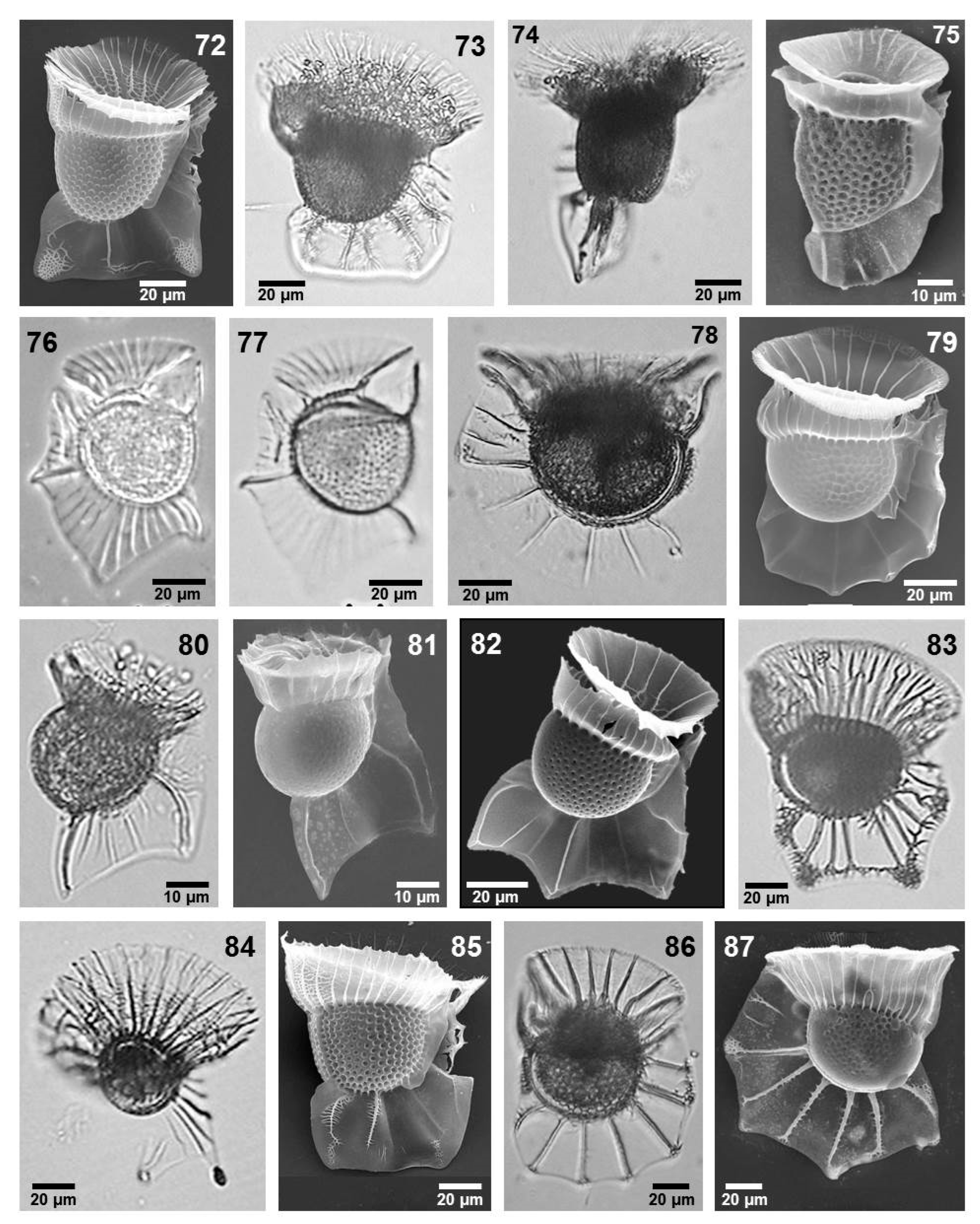
- Ornithocercus cristatus Matzenauer (Figure 6, image 75)
- Dimensions: L 70 μm, W 47 μm.
- Local distribution: central Mexican Pacific (locality t).
- Ornithocercus francescae (Murray et Whitting) Balech (Figure 6, images 76 and 77)
- Dimensions: L 92 μm, W 64 μm.
- Local distribution: central Mexican Pacific (locality t).
- Ornithocercus heteroporus Kofoid (Figure 6, images 80 and 81)
- Dimensions: L 69–81 μm, W 40–55 μm.
- Local distribution: central Mexican Pacific (locality s).
- Ornithocercus magnificus Stein (Figure 6, image 82)
- Dimensions: L 47–55 μm, W 75–93 μm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities o, r, t, aa, ac, ad, ae, ao, au, and bg).
- Ornithocercus orbiculatus Kofoid et Michener (Figure 6, images 78 and 79)
- Dimensions: L 112–143 μm, W 102–112 μm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities o, r, and ac).
- Remarks: This species has been considered as a synonym of O. steinii; however, they can be separated according to the number of ribs in the anterior cingular list and the shape of the margin of the left sulcal list.
- Ornithocercus quadratus Schütt (Figure 6, image 83)
- Dimensions: L 85 μm, W 58 μm.
- Local distribution: Gulf of California (locality e).
- Ornithocercus splendidus Schütt (Figure 6, image 84)
- Dimensions: L 141 μm, W 43.6 μm.
- Local distribution: Gulf of California and Gulf of Mexico (localities i, k, and au).
- Ornithocercus steinii Schütt (Figure 6, image 86)
- Dimensions: L 137–150 μm, W 100 μm.
- Local distribution: Gulf of California, central Mexican Pacific and Gulf of Mexico (localities a, b, c, d, e, f, g, h, i, j, k, m, n, q, r, s, t, and ao).
- Ornithocercus thumii (Schmidt) Kofoid et Skogsberg (Figure 6, image 87)
- Dimensions: L 125–153 μm, W 89–106 μm.
- Local distribution: Gulf of California, central Mexican Pacific and Gulf of Mexico (localities d, f, g, h, j, k, l, q, r, s, and ae).
- Family: Oxyphysaceae Sournia
- Genus: Phalacroma Stein
- Section: Rotundatum Kofoid et Skogsberg
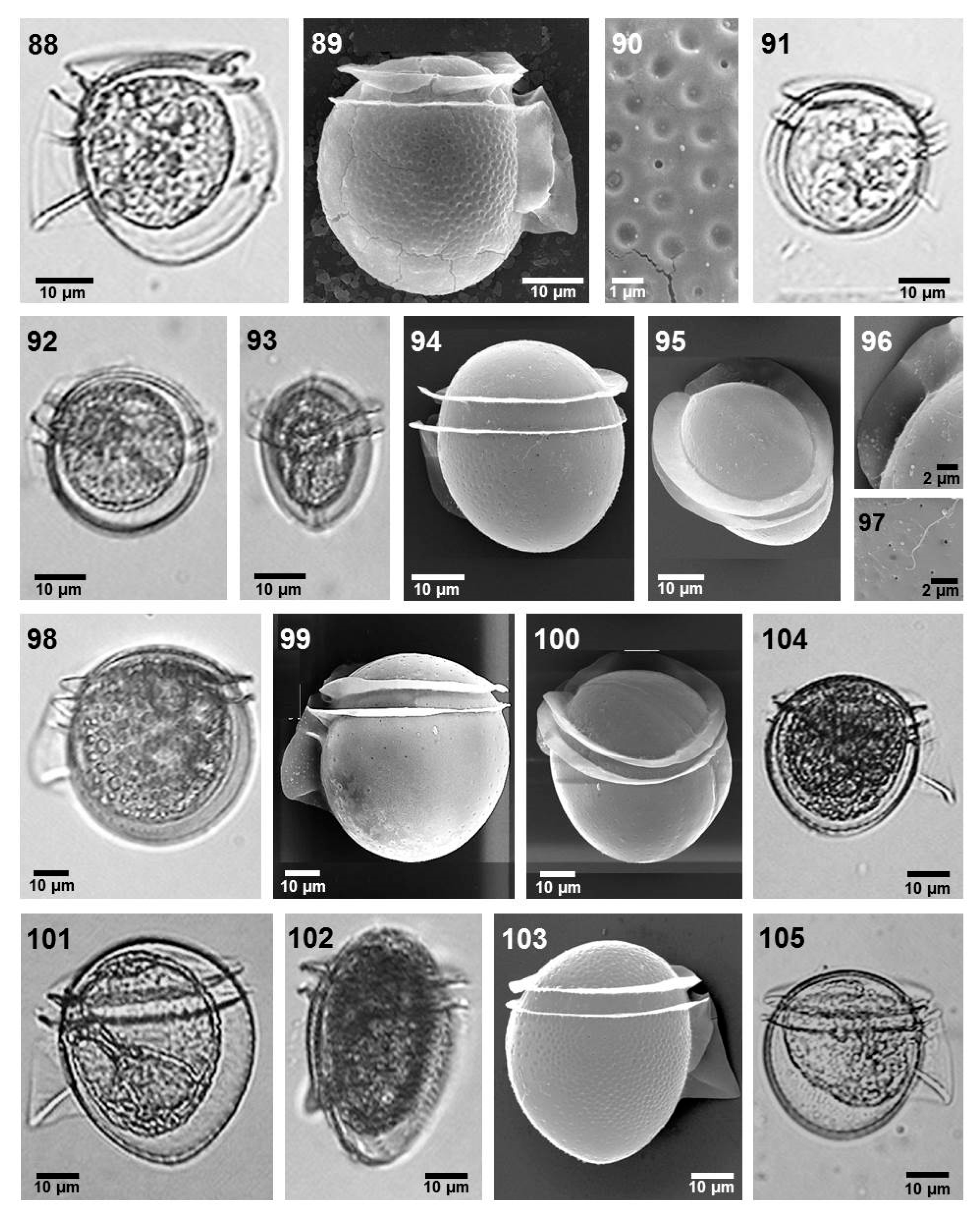
- Phalacroma laeve (Claparède et Lachman) Díaz-Ramos (Figure 7, images 92–97)
- Dimensions: L 67–75 µm, W 53–57.5 µm.
- Local distribution: Gulf of Mexico (locality ab, ah, aj, ak, as, and bo).
- Remarks: See the remarks under Phalacroma ovum Schütt.
- Phalacroma ornamentatum Esqueda-Lara et Hernández-Becerril (Figure 10, images 147–149)
- Dimensions: L 54–57 µm, W 64–67 µm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities a, aa, ah, ai, aj, aw, ax, ay, bb, bd, dj, and bn).
- Phalacroma parvulum (Schütt) Jörgensen (Figure 7, images 104 and 105)
- Dimensions: L 50–62 µm, W 50–61 µm.
- Local distribution: central Mexican Pacific (localities n, o, and r).
- Phalacroma rotundatum Claparéde et Lachmann (Figure 7, images 88–90)
- Dimensions: L 39–46 µm, W 35–45 µm.
- Local distribution: Gulf of California, central Mexican Pacific and Gulf of Mexico (localities a, b, j, t, ac, af, ag, am, an, ao, and bo).
- Phalacroma paulsenii Schiller (Figure 8, images 115–117)
- Dimensions: L 38–50 µm, W 33–46.5 µm.
- Local distribution: Gulf of California (at 28–29 °C and 34–35 salinity) (locality a and c).
- Phalacroma rudgei Murray et Whitting (Figure 7, images 98–100)
- Dimensions: L 30–31 µm, W 30–34 µm.
- Local distribution: Gulf of California at 30 °C and 34 salinity, Gulf of Mexico (localities a, l, m, and ab).
- Section: Argus Kofoid et Skogsberg
- Phalacroma apicatum Kofoid et Skogsberg (Figure 8, images 108 and 109)
- Dimensions: L 100 µm, W 80 µm.
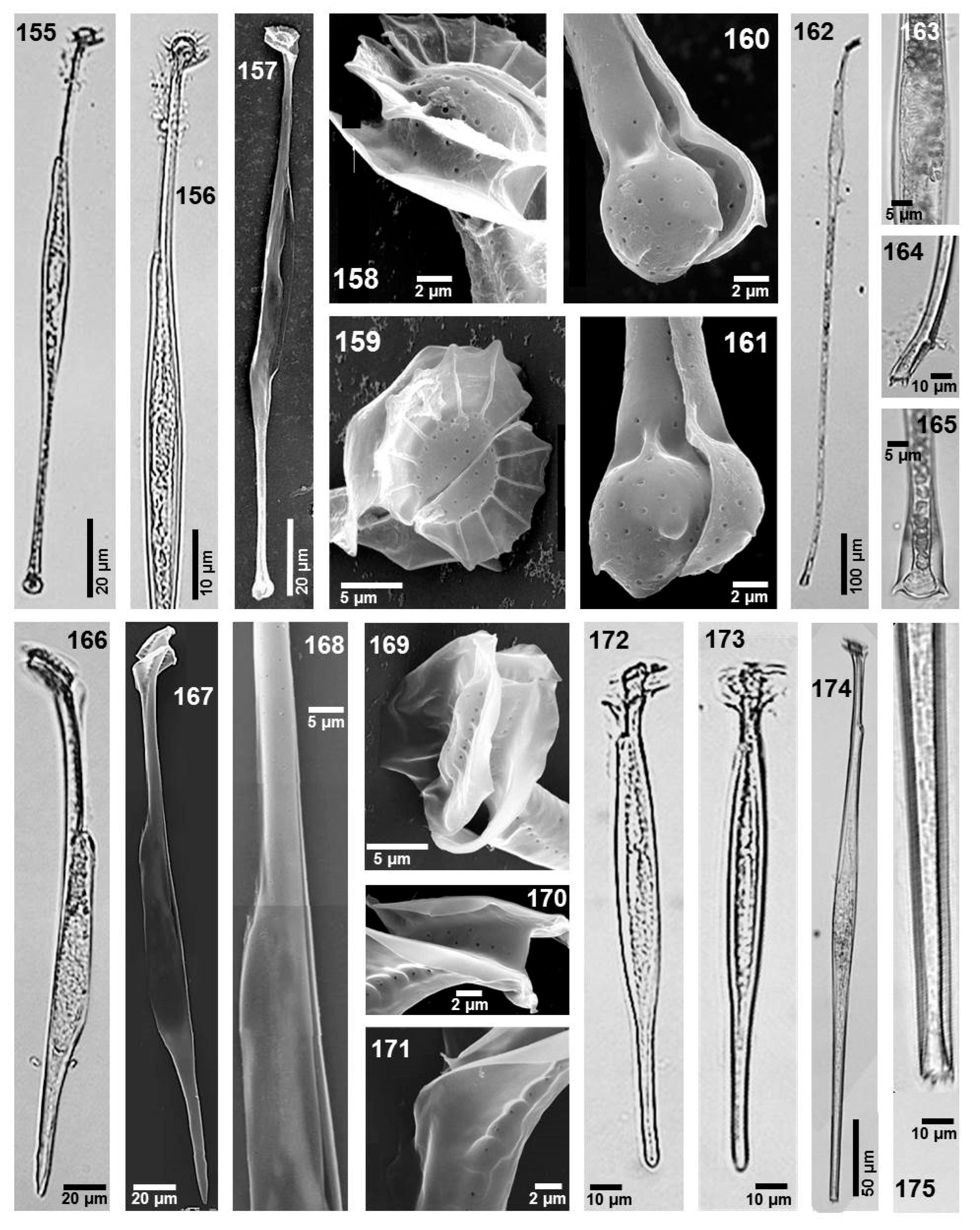
- Local distribution: Gulf of California and central Mexican Pacific (localities d, e, m, o, r, and s).
- Phalacroma argus Stein (Figure 8, images 106 and 107)
- Dimensions: L 80–97.5 µm, W 67.5–80 µm.
- Local distribution: Gulf of California and central Mexican Pacific (localities d, e, m, o, r, and s).
- Phalacroma ovum Schütt (Figure 10, images 145 and 146)
- Synonyms: Dinophysis amygdala Balech, Dinophysis amandula (Balech) Sournia
- Dimensions: L 55–72.5 µm, W 48–70 µm.
- Local distribution: Gulf of California and Gulf of Mexico (locality a, m, and af).
- Phalacroma scrobiculatum (Balech) Díaz-Ramos et Estrella (Figure 8, images 118–121)
- Dimensions: L 36–49 µm, W 35–45 µm.
- Local distribution: Gulf of California, at 30 °C and 35 salinity, and central Mexican Pacific at 28 °C and 35 salinity, and Gulf of Mexico (locality a, b, j, k, l, aa, af, ah, ai, aj, al, ao, ap, aq, ar, as, at, aw, ax, ay, az, bb, bf, bj. bk, bl, and bn).
- Section: Cuneus Kofoid et Skogsberg
- Phalacroma cuneus Schütt (Figure 9, images 122–125)
- Dimensions: LT 80–100 µm, W 62.5–87.5 µm.
- Local distribution: Gulf of California and central Mexican Pacific (localities k, m, n, q, r, s, and t).
- Section: Rapa Kofoid et Skogsberg
- Phalacroma rapa Stein (Figure 9, images 126–129)
- Dimensions: L 80–98 µm, W 65–80 µm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (locality o, r, s, aa, ad, ah, al, bi, bo, bq, bs).
- Phalacroma capitulatum (Balech) Hernández-Becerril (Figure 9, images 135–137)
- Dimensions: L 40–50 µm, W 38–40 µm.
- Local distribution: Gulf of California, at 30 °C and 35 salinity, and central Mexican Pacific (localities i, k, m, n, o, p, and r).
- Phalacroma mitra Schütt (Figure 9, images 130–132)
- Dimensions: L 40–72 µm, W 38–67 µm.
- Local distribution: Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities i, k, m, n, o, p, r, t, aa, ae, af, al, bc, bd, bk, and bn).
- Phalacroma favus Kofoid et Michener (Figure 9, images 133 and 134)
- Dimensions: LT 90–97.5 µm, W 62.5–77.5 µm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities n, p, and az).

- Phalacroma expulsum Kofoid et Michener (Figure 10, images 138–141)
- Dimensions: LT 50 µm, W 45 µm.
- Local distribution: Gulf of California, at 28–29 °C and 34.9–35 salinity (localities b and d).
- Section Doryphorum Kofoid et Skogsberg
- Phalacroma doryphorum Stein (Figure 8, images 110–112)
- Dimensions: LT 65–95 µm, W 55–88 µm.
- Local distribution: Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities i, k, m, n, q, t, and ah).
- Phalacroma porodictyum Stein (Figure 8, images 113 and 114)
- Dimensions: LT 68–90 µm, W 62.5–77.5 µm.
- Local distribution: Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities d, e, f, h, i, l, m, n, o, q, r, s, ah, ak, am, and at).
- Genus: Oxyphysis Kofoid
- Oxyphysis oxytoxoides Kofoid (Figure 10, images 151–154)
- Synonym: Phalacroma oxytoxoides (Kofoid) F. Gómez, P. Lopez-Garcia et D. Moreira

- Dimensions: L 65 µm, W 15 µm.
- Local distribution: western coast of Baja California, central Mexican Pacific, and Gulf of Mexico (localities o, u, bn, and bo).
- Genus: Dinofurcula Kofoid et Skogsberg
- Dinofurcula pseudoultima Hernández-Becerril (Figrue 10, image 150)
- Reference: [36] (p. 5, figs 1–8).
- Local distribution: central Mexican Pacific (locality v). N = 1.
- Family: Amphisoleniaceae Lindemann
- Genus: Amphisolenia Stein
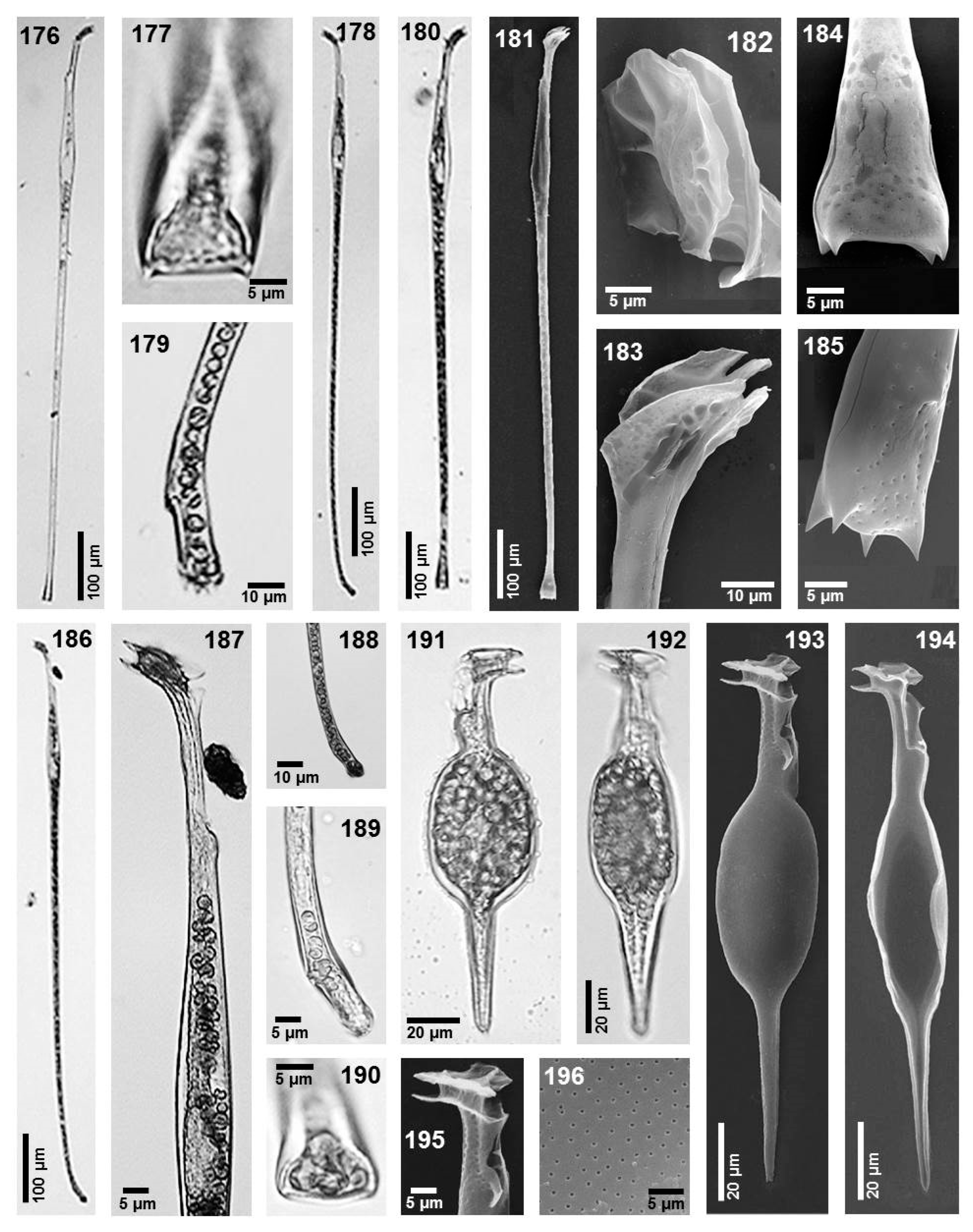
- Amphisolenia bidentata Schröder (Figure 11, images 162–165)
- Dimensions: L 625–800 µm, W 20–35 µm.
- Local distribution: Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities m, t, aa, ab, ad, af, am, ap, ar, au, ay, az, bc, bh, bi, bl, bm, bo, and br).
- Amphisolenia brevicauda Kofoid (Figure 11, images 166–171)
- Dimensions: L 475 µm, W 18 µm.
- Local distribution: central Mexican Pacific and Gulf of Mexico (localities t, af, ah, an, ax, az, bc, bl, bn, br).
- Remarks: This species is a new record for the Mexican Pacific.
- Amphisolenia globifera Stein (Figure 11, images 155–161)
- Dimensions: L 102 μm, W 12 μm.
- Local distribution: Gulf of California, at 28 °C and 35 salinity, and Gulf of Mexico (localities a and ao).
- Amphisolenia inflata Murray et Whitting (Figure 12, images 191–196)
- Dimensions: L 110 µm, W 30 µm.
- Local distribution: central Mexican Pacific, 29 °C and 32 salinity (locality t).
- Amphisolenia laticincta Kofoid (Figure 11, images 172 and 173)
- Dimensions: L 103–110 µm, W 10 µm.
- Local distribution: Gulf of California, at 28 °C and 35 salinity (localities a and h).
- Amphisolenia lemmermannii Kofoid (Figure 12, images 176 and 177)
- Dimensions: L 780–790 µm, W 30 µm.
- Local distribution: Gulf of California, central Mexican Pacific, and Gulf of Mexico (localities a, t, and ao).
- Amphisolenia palmata Stein (Figure 12, images 178 and 179)
- Dimensions: L 780 µm, W 25 µm.
- Local distribution: Gulf of California (locality a).
- Amphisolenia rectangulata Kofoid (Figure 12, images 180–185)
- Dimensions: LT 695 µm, W 15 µm.
- Local distribution: Gulf of California, 28 °C and 35 salinity (locality a).
- Amphisolenia schroederi Kofoid (Figure 11, image 174 and 175)
- Dimensions: L 380 µm, W 18 µm.
- Local distribution: Gulf of California, at 30 °C and 35 salinity (locality i, ag, ai, au, and bc).
4. Discussion
4.1. Taxonomy of Some Dinophysoid Species
4.2. Diversity and New Records
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez, F. Diversity and classification of dinoflagellates. In Dinoflagellates: Classification, Evolution, Physiology and Ecological Significance; Subba Rao, V.D., Ed.; Nova: New York, NY, USA, 2020; pp. 1–38. [Google Scholar]
- Guiry, D.R. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- Esqueda-Lara, K.; Hernández-Becerril, D.U. Two new species of the dinoflagellate genus Phalacroma Stein (Dinophyceae) from the tropical Mexican Pacific. Nova Hedwig. 2017, 105, 301–312. [Google Scholar] [CrossRef]
- Gómez, F. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Oceánides 2012, 27, 65–140. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Pospelova, V. A determination key for modern dinoflagellate cysts. Palynology 2015, 39, 387–409. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J.; Hallegraeff, G.M. Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO-IOC: Paris, France, 2003; pp. 283–318. [Google Scholar]
- Fensome, R.A.; Taylor, F.J.R.; Norris, G.; Sarjeant, W.A.S.; Wharton, D.I.; Williams, G.L. A Classification of Living and Fossil Dinoflagellates. Micropaleontol, Spec Publ No. 7; Sheridan Press: Hanover, PA, USA, 1993; p. 147. [Google Scholar]
- Sournia, A. Red tide and toxic marine phytoplankton of the world ocean: An inquiry into diversity. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erand, E., Gentienand, E., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 103–112. [Google Scholar]
- Hallegraeff, G.M.; Eriksen, R.S.; Davies, C.H.; Uribe-Palomino, J. Marine planktonic dinophysoid dinoflagellates (order Dinophysales): 60 years of species-level distributions in Australian waters. Austral. Syst. Bot. 2022, 35, 469–500. [Google Scholar] [CrossRef]
- Sournia, A. Atlas du Phytoplancton Marin. Volume I. Introduction, Cyanophycées, Dictyochophycées, Dinophycées, Raphidophycées; Éditions du Centre National de la Recherche Scientifique: Paris, France, 1986; p. 219. [Google Scholar]
- Zinssmeister, C.; Wilke, T.; Hoppenrath, M. Species diversity of dinophysoid dinoflagellates in the Clarion-Clipperton fracture zone, eastern Pacific. Mar. Biodiv. 2017, 47, 271–287. [Google Scholar] [CrossRef]
- Tarangkoon, W.; Hansen, G.; Hansen, P.J. Spatial distribution of symbiont-bearing dinoflagellates in the Indian Ocean in relation to oceanographic regimes. Aquat. Microb. Ecol. 2010, 58, 197–213. [Google Scholar] [CrossRef]
- Reguera, B.; García-Portela, M.; Velasco-Senovilla, E.; Rial, P.; Escalera, L.; Díaz, P.A.; Rodríguez, F. Dinophysis, a highly specialized mixoplanktonic protist. Front. Protistol. 2024, 1, 1328026. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Gaines, G.; Elbrächter, M. Heterotrophic nutrition. In The Biology of Dinoflagellates; Taylor, F.J.R., Ed.; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 224–268. [Google Scholar]
- Schnepf, E.; Elbrächter, M. Dinophyte chloroplasts and phylogeny—A review. Grana 1999, 38, 81–97. [Google Scholar] [CrossRef]
- Reguera, B.; González-Gil, S. Small cell and intermediate cell formation in species of Dinophysis (Dinophyceae, Dinophysiales). J. Phycol. 2001, 37, 318–333. [Google Scholar] [CrossRef]
- Koike, K.; Nishiyama, A.; Sayito, K.; Imai, K.; Koike, K.; Kobiyama, A.; Ogata, T. Mechanisms of gamete fusion in Dinophysis fortii (Dinophyceae, Dinophyta): Light microscopic and ultrastructural observation. J. Phycol. 2006, 42, 1247–1256. [Google Scholar] [CrossRef]
- Reguera, B.; González-Gil, S.; Delgado, M. Dinophysis diegensis is a life history stage of Dinophysis caudata (Dinophyceae, Dinophysiales). J. Phycol. 2007, 43, 1083–1093. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, S.; Kim, H.S.; Yi, G.M.; Kang, G.; Yih, W. First establishment of the marine dinoflagellate Dinophysis acuminata in culture. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Sakiyama, S.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis caudata (Dinophyceae). Plankton Benthos Res. 2008, 3, 78–85. [Google Scholar] [CrossRef]
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villareal, M. Bloom dynamics of Dinophysis acuta in an upwelling system: In situ growth versus transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Okolodkov, Y. Dinophysiales (Dinophyceae) of the National Park Sistema Arrecifal Veracruzano, Gulf of Mexico, with a key for identification. Act. Bot. Mex. 2014, 106, 9–71. [Google Scholar] [CrossRef]
- Steidinger, K.A.; Tangen, K. Dinoflagellates. In Identifying Marine phytoplankton; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 387–584. [Google Scholar]
- Parra-Toriz, D.; Esqueda-Lara, K.; Hernández-Becerril, D.U. Morphological observations of the marine planktonic dinoflagellate Phalacroma turbineum Kofoid et Michener (Dinophyta), with discussion on its taxonomy and distribution. Eur. J. Protistol. 2012, 48, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Esqueda-Lara, K.; Parra-Toriz, D.; Hernández-Becerril, D.U. Morphology and taxonomy of Dinophysis species of the section Hastata (Dinoflagellata), including the description of Dinophysis conjucta sp. nov., from the Mexican marine waters. J. Mar. Biol. Assoc. UK 2013, 93, 1187–1202. [Google Scholar] [CrossRef]
- Wilke, T.; Zinssmeister, C.; Hoppenrath, M. Morphological variability within the marine dinoflagellate Ornithocercus quadratus (Dinophysales, Dinophyceae)—Evidence for three separate morphospecies. Phycology 2018, 57, 555–571. [Google Scholar] [CrossRef]
- Handy, S.M.; Bachvaroff, T.R.; Timme, R.E.; Wayne Coasts, W.D.; Kim, S.; Delwiche, C.F. Phylogeny of our dinophysiacean genera (Dinophyceae, Dinophysiales) based on rDNA sequences from single cells and environmental samples. J. Phycol. 2009, 45, 1163–1174. [Google Scholar] [CrossRef]
- Jensen, M.H.; Daugbjerg, N. Molecular phylogeny of selected species of the order Dinophysiales (Dinophyceae): Testing the hypothesis of a dinophysiod radiation. J. Phycol. 2009, 45, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; López-García, P.; Moreira, D. Molecular phylogeny of dinophysoid Dinoflagellates the systematic position on Oxyphysis oxytoxoides and the Dinophysis Hastata group (Dinophysales, Dinophyceae). J. Phycol. 2011, 47, 303–406. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Meave del Castillo, M.E.; Flores-Granados, C. Dinoflagelados del Orden Dinophysiales en las costas mexicanas. In Planctología Mexicana; Barreiro Güemes, M.T., Meave del Castillo, M.E., Signoret Poillon, M., Figueroa-Torres, M.G., Eds.; Sociedad Mexicana de Planctología; Universidad Autónoma Metropolitana: Mexico City, Mexico, 2003; pp. 19–42. [Google Scholar]
- Okolodkov, Y.; Gárate-Lizárraga, I. An annotated checklist of dinoflagellates (Dinophyceae) from the Mexican Pacific. Acta Bot. Mex. 2006, 74, 1–154. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Ceballos-Corona, J.G.A.; Esqueda-Lara, K.; Tovar-Salazar, M.A.; León-Álvarez, D. Marine planktonic dinoflagellates of the order Dinophysiales (Dinophyta) from coasts of the tropical Mexican Pacific, including two new species of the genus. Amphisolenia. J. Mar. Biol. Assoc. UK 2008, 88, 1–15. [Google Scholar] [CrossRef]
- Parra-Toriz, D.; Hernández-Becerril, D.U. Planktonic dinoflagellates (Dinophyceae) of the order Dinophysales from the southern Gulf of Mexico. Nova Hedwig. 2023, 116, 231–263. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Meza-García, P.; Villarreal-Martínez, A.M.; García-Anaya, T.M.; Vázquez-Montes, E.D.; Esqueda-Lara, K.; Arana-García, J. Observations on some planktonic dinophysoid dinoflagellates (Dinophysales, Dinophyta) from the Mexican Pacific, including the description of a new species, Dinofurcula pseudoultima sp. nov., and taxonomic and distribution notes about the genera Latifascia and Triposolenia. Protistology 2023, 17, 3–15. [Google Scholar] [CrossRef]
- Balech, E. Dinoflagelados. In Fitoplancton Marino; Balech, E., Ferrando, H.J., Eds.; Editorial Universitaria de Buenos Aires: Buenos Aires, Argentina, 1964. [Google Scholar]
- Balech, E. On thecal morphology of Dinoflagellates with special emphasis on cingular and sulcal plates. An. Centro Cienc. Mar. Limnol. UNAM 1980, 7, 57–68. [Google Scholar]
- Balech, E. Los Dinoflagelados del Atlántico Sudoccidental; Publicaciones Especiales, Instituto Español de Oceanografía: Madrid, Spain, 1988; p. 219. [Google Scholar]
- Yang, S.M.; Li, R.X. Atlas of Dinoflagellates in China’s Seas; Ocean Press: Beijing, China, 2014; 213p. [Google Scholar]
- Tai, L.-S.; Skogsberg, T. Studies on the Dinophysoidae, marine armored dinoflagellates, of Monterey Bay, california. Arch. Protistenk. 1934, 82, 380–482. [Google Scholar]
- Abé, T.H. The armored Dinoflagellata. II. Prorocentridae and Dinophysidae (B)—Dinophysis and its allied genera. Publ. Seto Mar. Biol. Lab. 1967, 15, 37–68. [Google Scholar] [CrossRef][Green Version]
- Kofoid, C.A.; Skogsberg, T. The Dinoflagellata: The Dinophysoidae. Mem. Mus. Comp. Zool. 1928, 21, 1–766. [Google Scholar][Green Version]
- Schiller, J. Dinoflagellatae (Peridineae). Teil I. Rabenhorst’s Kryptogamen-Flora; Akademie Verlag: Leipzig, Germany, 1933; p. 590. [Google Scholar][Green Version]
- Gul, D.; Saifullah, S.M. Taxonomic and ecological studies on three marine genera of Dinophysiales from Arabian Sea shelf of Pakistan. Pak. J. Bot. 2010, 42, 2647–2660. [Google Scholar][Green Version]
- Balech, E. Microplancton de la Campaña Productividad III. Rev. Mus. Arg. Cs. Nat. B Rivadavia Hidrobiol. 1971, 3, 1–202. [Google Scholar][Green Version]
- Jörgensen, E. Mediterranean Dinophysiaceae. In Report of the Danish Oceanographic Expedition II; Carlsbergfondet: Copenhagen, Denmark, 1923; Volume 2, pp. 1–48. [Google Scholar][Green Version]
- Wood, E.J.F. Dinoflagellates in the Australian region. Mar. Freshwat. Res. 1954, 5, 171–351. [Google Scholar] [CrossRef]
- Yang, S.M.; Li, R.X.; Dong, S.G. Dinoflagellates in the China’s Seas I (Prorocentrales, Dinophysiales); Ocean Press: Beijing, China, 2014; 156p. [Google Scholar]
- Taylor, F.J.R. Dinoflagellates from the International Indian Ocean Expedition: A Report on Material Collected by the R. V. “Anton Bruun” 1963–1964; Bibliotheca Botanica: Adendorf, Germany, 1976; 234p. [Google Scholar]
- Esqueda-Lara, K.; Hernández-Becerril, D.U. Dinoflagelados Microplanctónicos Marinos del Pacífico Central Mexicano (Isla Isabel, Nayarit y Costas de Jalisco y Colima); Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2010; p. 206. [Google Scholar]
- Matzenauer, L. Die Dinoflagellaten des Indischen Ozeans. Bot. Arch. 1933, 35, 437–510. [Google Scholar]
- Balech, E. Dinoflagelados nuevos o interesantes del Golfo de México y Caribe. Rev. Mus. Arg. Cs. Nat. B Rivadavia Hidrobiol. 1967, 2, 77–126. [Google Scholar]
- Murray, G.; Whitting, F.G. New Peridiniaceae from the Atlantic. Trans. Linnean Soc. Lond. 2nd Ser. Bot. 1899, 5, 321–342. [Google Scholar] [CrossRef]
- Balech, E. Tintinnoinea y dinoflagellata del Pacífico según material de las expediciones Norpac y Downwind del Instituto Scripps de Oceanografía. Rev. Mus. Argent. Cienc. Nat. (Cienc. Zool.) 1962, 7, 1–253. [Google Scholar]
- Hernández-Becerril, D.U.; Barón-Campis, S.A.; Ceballos-Corona, J.G.A.; Alonso-Rodríguez, R.; Rincones-Reyes, K.M.; Becerra-Reynoso, R.T.; Arce-Rocha, G. Catálogo de Fitoplancton del Pacífico Central Mexicano. Cruceros “MareaR” (2009–2019) B/O “El Puma”; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2021; p. 254. [Google Scholar]
- Kofoid, C.A. On Oxyphysis oxytoxoides gen. nov. sp. nov. a dinophysoid dinoflagellate convergent toward the peridinioid type. Univ. Calif. Pub. Zool. 1926, 28, 203–216. [Google Scholar]
- Rampi, L.; Bernhard, M. Chiave per la Determinazione Delle Peridinee Pelagiche Mediterranee; Comitato Nazionale Energia Nucleare: Rome, Italy, 1980. [Google Scholar]
- Gul, D.; Saifullah, S.M. Genus Amphisolenia Stein from north-west Arabian sea shelf of Pakistan. Pak. J. Bot. 2007, 39, 561–576. [Google Scholar]
- Dodge, J.D. Taxonomy and systematics of dinoflagellates. In Out of the Past; Norton, T.A., Ed.; British Phycological Society: Belfast, UK, 2003; pp. 69–86. [Google Scholar]
- MacKenzie, L. Does Dinophysis (Dinophyceae) have a sexual life cycle? J. Phycol. 1992, 28, 399–406. [Google Scholar] [CrossRef]
- Gómez, F. A list of free-living dinoflagellate species in the world’s oceans. Acta Bot. Croat. 2005, 64, 129–212. [Google Scholar]
- Hernández-Becerril, D.U. Dinophysis taylorii, sp. nov., y otros Dinophysis de Baja California, México (Dinophyceae). Rev. Biol. Trop. 1992, 40, 101–109. [Google Scholar]
- Halim, Y. Dinoflagellates of the south-east Caribbean Sean (east-Venezuela). Int. Revue Ges. Hydrobiol Hydrogr. 1967, 52, 701–755. [Google Scholar] [CrossRef]
- Balech, E. Estructura de Amphisolenia bidentata Schröder (Dinoflagellata). Physis Sección A 1977, 37, 25–32. [Google Scholar]
| Species | No. Specimens | Study Areas | Localities |
|---|---|---|---|
| Citharistes regius | 2 | Gulf of California | g, i, n |
| Dinophysis acuminata | 18 | western coast of Baja California | u |
| central Mexican Pacific | bk | ||
| Dinophysis exigua | 3 | Gulf of California | n |
| central Mexican Pacific | t | ||
| Dinophysis fortii | 43 | western coast of Baja California | a |
| central Mexican Pacific | p, t, u | ||
| Gulf of Mexico | ab, ac, ae, ah, an, ao, as, bj, bk, bn | ||
| Dinophysis infundibulus | 2 | Gulf of Mexico | al |
| Dinophysis recurva | 2 | central Mexican Pacific | v |
| Dinophysis similis | 2 | Gulf of California | b |
| Dinophysis sourniae | 3 | central Mexican Pacific | e |
| Dinophysis balechii | 1 | Gulf of California in [27] | see [27] |
| central Mexican Pacific | see [27] | ||
| Dinophysis conjuncta | 3 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | see [27] | ||
| Dinophysis hastata | 17 | Gulf of California in [27] | see [27] |
| central Mexican Pacific | see [27] | ||
| Gulf of Mexico | an, ao, ap, as, ay, bb, bs | ||
| Dinophysis monacantha | 1 | Gulf of California in [27] | see [27] |
| Dinophysis nias | 4 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | see [27] | ||
| Dinophysis phalacromoides | 1 | Gulf of California in [27] | see [27] |
| Dinophysis pusilla | 3 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | see [27] | ||
| Dinophysis schuettii | 13 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | ah, ak, ay, bc, bd | ||
| Dinophysis swezyae | 4 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | see [27] | ||
| Dinophysis uracantha | 3 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | af, ao, be, bf | ||
| Dinophysis uracantha var. mediterranea | 4 | Gulf of California in [27] | see [27] |
| central Mexican Pacific | see [27] | ||
| Dinophysis uracanthoides | 2 | Gulf of California in [27] | see [27] |
| Gulf of Mexico | see [27] | ||
| Dinophysis caudata | 70 | western coast of Baja California | u |
| Gulf of California | a, b, c, m | ||
| central Mexican Pacific | n, o, r, s, t | ||
| Gulf of Mexico | ab, ac, ad, af, ah, ai, aj, ak, al, am, ao, ap, aq, ar, as, at, au, aw, ax, ay, az, bb, bc, bd, bk, bn, bo, bq, br, bs | ||
| Dinophysis caudata var. diegensis | 2 | central Mexican Pacific | n |
| Dinophysis tripos | 40 | western coast of Baja California | a |
| Gulf of California | u | ||
| Metaphalacroma skogsbergi | 6 | Gulf of California | a, b, t |
| Pseudophalacroma nasutum | 1 | western coast of Baja California | u |
| Histioneis biremis | 1 | Gulf of California | a |
| Histioneis costata | 6 | Gulf of California | a |
| Gulf of Mexico | aa | ||
| Histioneis crateriforme | 1 | central Mexican Pacific | p |
| Histioneis depressa | 2 | central Mexican Pacific | v |
| Histioneis garrettii | 1 | central Mexican Pacific | r |
| Histioneis mitchellana | 1 | central Mexican Pacific | v |
| Ornithocercus assimilis | 8 | central Mexican Pacific | b, d, e, f, g, n |
| Gulf of Mexico | au | ||
| Ornithocercus cristatus | 1 | central Mexican Pacific | t |
| Ornithocercus francescae | 1 | central Mexican Pacific | t |
| Ornithocercus heteroporus | 2 | central Mexican Pacific | s |
| Ornithocercus magnificus | 14 | central Mexican Pacific | o, r, t |
| Gulf of Mexico | aa, ac, ad, ae, ao, au, bg | ||
| Ornithocercus orbiculatus | 5 | central Mexican Pacific | o, r |
| Gulf of Mexico | ac | ||
| Ornithocercus quadratus | 1 | Gulf of California | b |
| Ornithocercus schuetti | 1 | Gulf of California | e |
| Ornithocercus splendidus | 2 | Gulf of California | i, k |
| Gulf of Mexico | au | ||
| Ornithocercus steinii | 6 | Gulf of California | a, b, c, d, e, f, g, h, i, j, k, m |
| central Mexican Pacific | n, q, r, s, t | ||
| Gulf of Mexico | ao | ||
| Ornithocercus thumii | 4 | Gulf of California | d, f, g, h, j, k, l |
| central Mexican Pacific | q, r, s | ||
| Gulf of Mexico | ae | ||
| Phalacroma laeve | 5 | Gulf of California | m |
| central Mexican Pacific | t | ||
| Gulf of Mexico | am, bb, bo | ||
| Phalacroma operculoides | 9 | Gulf fo Mexico | ab, ah, aj, ak, as, bo |
| Phalacroma ornamentatum | 12 | central Mexican Pacific | a |
| Gulf of Mexico | aa, ah, ai, aj, aw, ax, ay, bb, bd, dj, bn | ||
| Phalacroma parvulum | 9 | central Mexican Pacific | n, o, r |
| Phalacroma rotundatum | 11 | Gulf of California | a, b, j |
| central Mexican Pacific | t | ||
| Gulf of Mexico | ac, af, ag, am, an, ao, bo | ||
| Phalacroma paulsenii | 5 | Gulf of California | a, c |
| Phalacroma rudgei | 1 | Gulf of California | k |
| Phalacroma whittingiae | 4 | Gulf of California | a, l, m |
| Gulf of Mexico | ab | ||
| Phalacroma apicatum | 1 | Gulf of California | d, e, m |
| central Mexican Pacific | o, r, s | ||
| Phalacroma argus | 7 | Gulf of California | d, e, m |
| central Mexican Pacific | o, r, s | ||
| Phalacroma ovum | 6 | Gulf of California | a, m |
| Gulf of Mexico | af | ||
| Phalacroma scrobiculatum | 41 | Gulf of California | a, b, j, k, l |
| central Mexican Pacific | aa, af, ah, ai, aj, al, ao, ap, aq, ar, as at, aw, ax, ay, az, bb, bf, bj, bk, bl, bn | ||
| Phalacroma cuneus | 13 | Gulf of California | k |
| central Mexican Pacific | m, n, q, r, s, t | ||
| Phalacroma rapa | 16 | central Mexican Pacific | o, r, s |
| Gulf of Mexico | aa, ad, ah, al, bj, bo, bq, bs | ||
| Phalacroma capitulatum | 3 | Gulf of California | i, k, m, n |
| central Mexican Pacific | o, p, r | ||
| Phalacroma mitra | 28 | Gulf of California | i, k, m |
| central Mexican Pacific | n, o, p, r, t | ||
| Gulf of Mexico | aa, ae, af, al, bc, bd, bk, bn | ||
| Phalacroma favus | 4 | central Mexican Pacific | n, p |
| Gulf of Mexico | az | ||
| Phalacroma expulsum | 10 | central Mexican Pacific | s, v |
| Gulf of Mexico | ac, af, ag, am, ah, az | ||
| Phalacroma stenopterygium | 1 | Gulf of California | b, d |
| Phalacroma doryphorum | 1 | Gulf of California | i, k, m |
| central Mexican Pacific | n, q, t | ||
| Gulf of Mexico | ah | ||
| Phalacroma porodictyum | 20 | Gulf of California | d, e, f, h, j, l, m |
| central Mexican Pacific | n, o, q, r, s | ||
| Gulf of Mexico | ah, ak, am, at | ||
| Oxyphysis oxytoxoides | 2 | western coast of Baja California | u |
| central Mexican Pacific | o | ||
| Gulf of Mexico | bn, bo | ||
| Dinofurcula pseudoultima | 1 | central Mexican Pacific | v |
| Amphisolenia bidentata | 25 | Gulf of California | m |
| central Mexican Pacific | t | ||
| Gulf of Mexico | aa, ab, ad, af, am, ap, ar, au, ay, az bc, bh, bi, bl, bm, bo, br | ||
| Amphisolenia brevicauda | 1 | Gulf of California | d |
| Amphisolenia deltiana | 10 | central Mexican Pacific | t |
| Gulf of Mexico | af, ah, an, ax, az, bc, bl, bn, br | ||
| Amphisolenia globifera | 2 | Gulf of California | a |
| Gulf of Mexico | ao | ||
| Amphisolenia inflata | 2 | central Mexican Pacific | t |
| Amphisolenia laticincta | 2 | Gulf of California | a, h |
| Amphisolenia lemmermanni | 3 | Gulf of California | a |
| central Mexican Pacific | t | ||
| Gulf of Mexico | ao | ||
| Amphisolenia palmata | 1 | Gulf of California | a |
| Amphisolenia rectangulata | 3 | Gulf of California | a |
| Amphisolenia schroederi | 1 | Gulf of California | j |
| Gulf of Mexico | ag, ai, au, bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esqueda-Lara, K.; Hernández-Becerril, D.U.; González-Gómez, J.P. Diversity and Morphology of Planktonic Species of the Order Dinophysales (Dinoflagellata) from the Tropical Mexican Pacific and the Gulf of Mexico. Phycology 2025, 5, 48. https://doi.org/10.3390/phycology5030048
Esqueda-Lara K, Hernández-Becerril DU, González-Gómez JP. Diversity and Morphology of Planktonic Species of the Order Dinophysales (Dinoflagellata) from the Tropical Mexican Pacific and the Gulf of Mexico. Phycology. 2025; 5(3):48. https://doi.org/10.3390/phycology5030048
Chicago/Turabian StyleEsqueda-Lara, Karina, David U. Hernández-Becerril, and Juan Pablo González-Gómez. 2025. "Diversity and Morphology of Planktonic Species of the Order Dinophysales (Dinoflagellata) from the Tropical Mexican Pacific and the Gulf of Mexico" Phycology 5, no. 3: 48. https://doi.org/10.3390/phycology5030048
APA StyleEsqueda-Lara, K., Hernández-Becerril, D. U., & González-Gómez, J. P. (2025). Diversity and Morphology of Planktonic Species of the Order Dinophysales (Dinoflagellata) from the Tropical Mexican Pacific and the Gulf of Mexico. Phycology, 5(3), 48. https://doi.org/10.3390/phycology5030048






