1. Introduction
Using biofuels made from biomass can partly replace fossil fuels and contribute towards reducing greenhouse gas emissions [
1,
2,
3,
4,
5]. Extensive research has delved into utilizing biomass such as wood, land-based, agricultural, and algae sources for bioenergy and biofuel applications. Early identified biomass, or first-generation biomass, originated from commodity sources such as sugarcane, potatoes, soybeans, and palm oil [
6,
7,
8]. The initial iteration of biomass faced sustainability challenges primarily due to its competition with food and feed crops, potentially causing an escalation in food prices. Non-edible biomass, like lignocellulosic feedstocks derived from agricultural and forest residue, emerged as a more promising alternative. Notably, lignocellulosic feedstocks boast higher yields compared to first-generation biomass. Nevertheless, its use for biofuel production presents significant drawbacks, including ecological concerns and the substantial land requirements to meet global biofuel demands [
9,
10].
Microalgae have garnered significant interest compared to other biomass sources since they do not compete directly with food or feed production. Compared to biomass derived from land, algae have higher photosynthetic efficiency, leading to faster growth rates and effective CO
2 utilization. Cultivating algae extensively is challenging due to the high production costs. This is one of the main challenges of utilizing algae to produce an alternative energy source [
11,
12,
13]. Moreover, ash content generally arises from the precipitation of salts, such as calcium, magnesium, iron, and phosphorus, in the cultivation medium due to high pH conditions. It can also be influenced by external ash deposition. In the case of diatoms, a notable factor is the significant silicate content [
14,
15]. Algae accumulate these elements throughout their growth, but the primary source of ash in microalgae is often not direct nutrient uptake [
16]. Numerous studies have reported that elevated ash levels negatively impact machinery and equipment during the conversion of algae into biofuels, chemicals, and other bioproducts [
17,
18,
19]. The ash can deactivate the catalysts that cover the active sites or chemically interact with the catalyst material. The ash deposits can also insulate heat transfer surfaces, leading to uneven heating and inefficient thermal conversion of biomass. In addition, at high temperatures, the inorganic components in the ash can melt and form a sticky, molten slag. This slag can adhere to the walls and internal surfaces of the reactor, obstructing the flow of feedstock and gases. In addition, ash can contain corrosive elements like alkali metals (i.e., sodium, potassium, etc.) and chlorine, which can chemically react and corrode metal surfaces and other reactor materials. Thus, high temperatures during thermochemical reactions such as pyrolysis or gasification result in ash volatilization and deposition, which lowers the efficiency of bioproducts that can be recovered from algal biomass conversion processes [
20,
21].
Numerous treatments have been explored to eliminate physiological ash (containing essential minerals for growth) and non-physiological ash (containing non-essential minerals or contaminants) from biomass. Jenkins et al. [
22] investigated the removal of inorganic components from the rice straw and wheat straw using water. Washing biomass with deionized water has proven effective for reducing ash, particularly non-physiological ash (soil). The non-physiological ash is easier to remove compared to the physiological ash because they are directly attached to the structure from the algae biomass [
23]. Turn et al. [
24] explored using distilled water to remove approximately 40% ash from switchgrass. Key factors considered for effective ash removal included the biomass-to-water ratio, the temperature of the water, and the contact time of the biomass with the water [
25]. Conversely, other research groups have examined the integration of weak and strong acids, and the results effectively removed the ash content from the biomass. The following authors, Stylianos et al. [
26], Yoo et al. [
27], and Liu and Bi [
28], revealed that applying nitric acid can significantly reduce the ash percentage in biomass. Despite reports that acid is effective in removing inorganic materials, its application has adverse effects on biomass, including the breakdown of specific organic components of the biomass and the generation of a waste stream that may require neutralization and safe handling of effluents [
29].
An alternative approach involves the application of chelating agents, which have recently garnered significant attention due to their active interaction with metal ions, altering their solubility within algae. Chelating agents typically extract inorganic metals from biomass because they coordinate with two or more electron-donating atoms, allowing them to form complex bonds with a single metal atom. The most frequently utilized chelating agents include ethylenediaminetetraacetic acid (EDTA), citric acid, and nitrilotriacetic acid (NTA). Edmunds et al. [
30] examined the application of EDTA for producing a low-ash bioenergy feedstock. A significant concern highlighted was the environmental impact of repeated EDTA usage since it is a non-biodegradable chelating agent. Wang et al. [
31] found that NTA acts as a chelating agent to bind and remove metal ions from algal biomass, contributing to ash content. According to Biller and Ross [
32], NTA treatment can improve biofuel yield from algal biomass by reducing ash content through hydrothermal liquefaction.
This study focuses on developing an ash removal process using NTA from three distinct algae species containing low, medium, and very high ash contents. Two benthic algal polyculture species that grow in planktonic form (Solid-State Algal Turf Scrubber and Green Algal Turf Scrubber) and the Scenedesmus sample were chosen for different treatment conditions. The objectives of this study were to evaluate the effectiveness of NTA and deionized water treatments and to understand the role of temperature in deashing algae biomass. One of the objectives was to learn about the impact of these treatments on the algal biomass composition, which is vital for converting it to biofuels. Several analytical techniques, including Fourier Transform Infrared Spectroscopy, high-pressure liquid chromatography, Total Carbon (TC) and Nitrogen (TN), ultimate analysis, and Flame Atomic Absorption Spectroscopy (AAS), were used in this study to understand the % ash removal, metals extraction, and changes in algae biomass composition before and after NTA treatment. To date, no data has been published on the effectiveness of NTA for ash removal across three different types of algae representing a wide range of ash contents, nor on its potential for regeneration within a closed-loop, environmentally benign system. These findings advance understanding of using biodegradable chelating agents such as NTA for sustainable ash removal and demonstrate their applicability in developing efficient, eco-friendly algal biomass processing methods.
2. Materials and Methods
2.1. Materials
Nitrilotriacetic acid (C6H9NO6, 99%), sodium sulfide (Na2S, 98%), calcium hydroxide (Ca(OH)2, 95%), and hydrosulfuric acid (H2SO4, 99%) reagents were purchased from Sigma-Aldrich Solutions (Burlington, MA, USA), American Elements (Bluffdale, UT, USA), ThermoFisher Scientific (Waltham, MA, USA), and Fisher Scientific (Pittsburgh, PA, USA), respectively. Sandia National Laboratories provided three algal samples: two benthic algae polyculture species that grow in planktonic form (Solid-State and Green Algal Turf Scrubber (ATS)) and a Scenedesmus sample. Six algae samples were collected, consisting of three duplicate pairs. All samples were collected and stored at room temperature overnight before further processing.
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
The infrared spectra (400–4000 cm−1) were utilized to examine the functional groups of algae samples. Before analysis, the samples were finely ground and then dried at 60 °C for 24 h. For each sample, approximately 0.05–0.07 g of the dried material was placed on the diamond surface of the instrument (Agilent Technologies Cary 630 FTIR—Santa Clara, CA, USA) and pressed against the zinc crystal using a rod. Spectral data were collected using the 300 background scans coupled with the attenuated total reflection (ATR) method to obtain rapid molecular information while preserving the integrity of the samples, which remained intact after analysis.
2.3. Ash Treatment
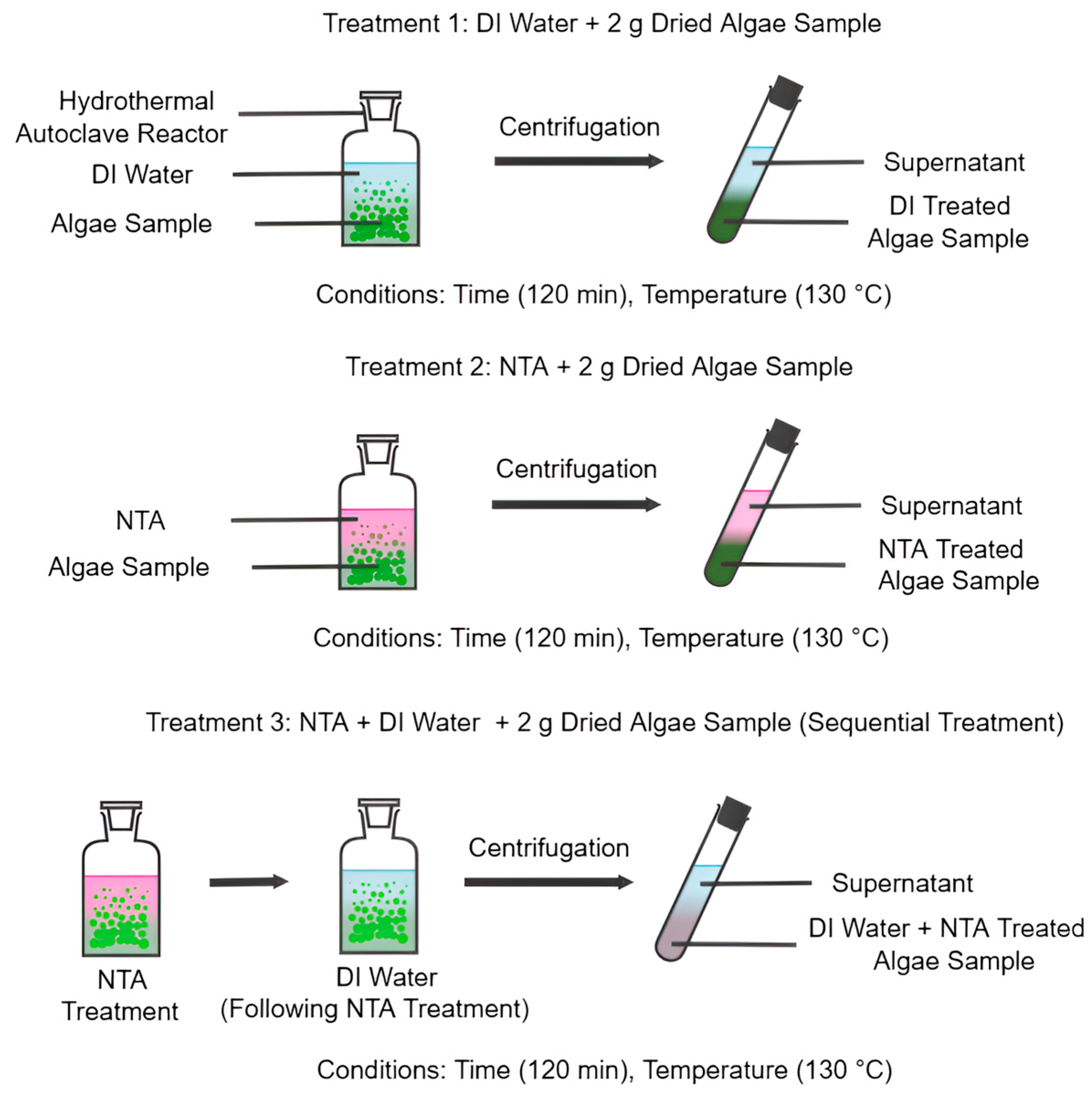
Initially, 2 g of each algae sample was weighed and oven-dried at 60 °C to remove residual moisture. Once cooled, the sample was combined with 100 mL of deionized (DI) water and transferred into a hydrothermal autoclave reactor. The sealed reactor was heated at 130 °C for 120 min to extract loosely bound, non-structural ash and surface-associated inorganics. This initial treatment using DI water alone served as Treatment 1, establishing a baseline for comparison as illustrated in
Figure 1.
For Treatment 2, the dried algal biomass was added to 100 mL of 0.05 M nitrilotriacetic acid (NTA) solution and placed in the hydrothermal reactor. The system was maintained at controlled temperatures (90, 100, 110, 120, and 130 °C) for 120 min to facilitate the chelation and extraction of metal ions and other ash-forming compounds. This treatment evaluated the efficacy of NTA under hydrothermal conditions without additional rinsing.
Treatment 3 was designed to assess the synergistic deashing effect of sequential application of NTA followed by DI water (NTA+DI). The algae were first treated with 0.05 M NTA under the same conditions as Treatment 2 (
Figure 1) in this method. After centrifugation and removal of the NTA solution, the solid biomass was resuspended in 100 mL of fresh DI water and subjected to a second hydrothermal reaction at the same temperature and duration (130 °C, 120 min). This second step aimed to remove residual solubilized metals and inorganics. This dual-stage treatment leveraged the benefits of both DI and NTA protocols to enhance overall ash and metal removal.
After completion of each treatment, the suspension was centrifuged (4000×
g, 10 min), the supernatant was collected for analysis, and the remaining solid was dried at 60 °C for 24 h. All treatments were carried out independently using a hydrothermal autoclave reactor to ensure uniform thermal control and to prevent the evaporation of water or exceeding the thermal degradation limit (approximately 170 °C) of the chelating agent. Each sample was analyzed for ash content using the method developed by the National Renewable Energy Laboratory (NREL) [
33]. This allowed for comparative evaluation of the deashing efficiency and compositional integrity across all tested conditions.
2.4. Stages for Nitrilotriacetic Acid Recycling
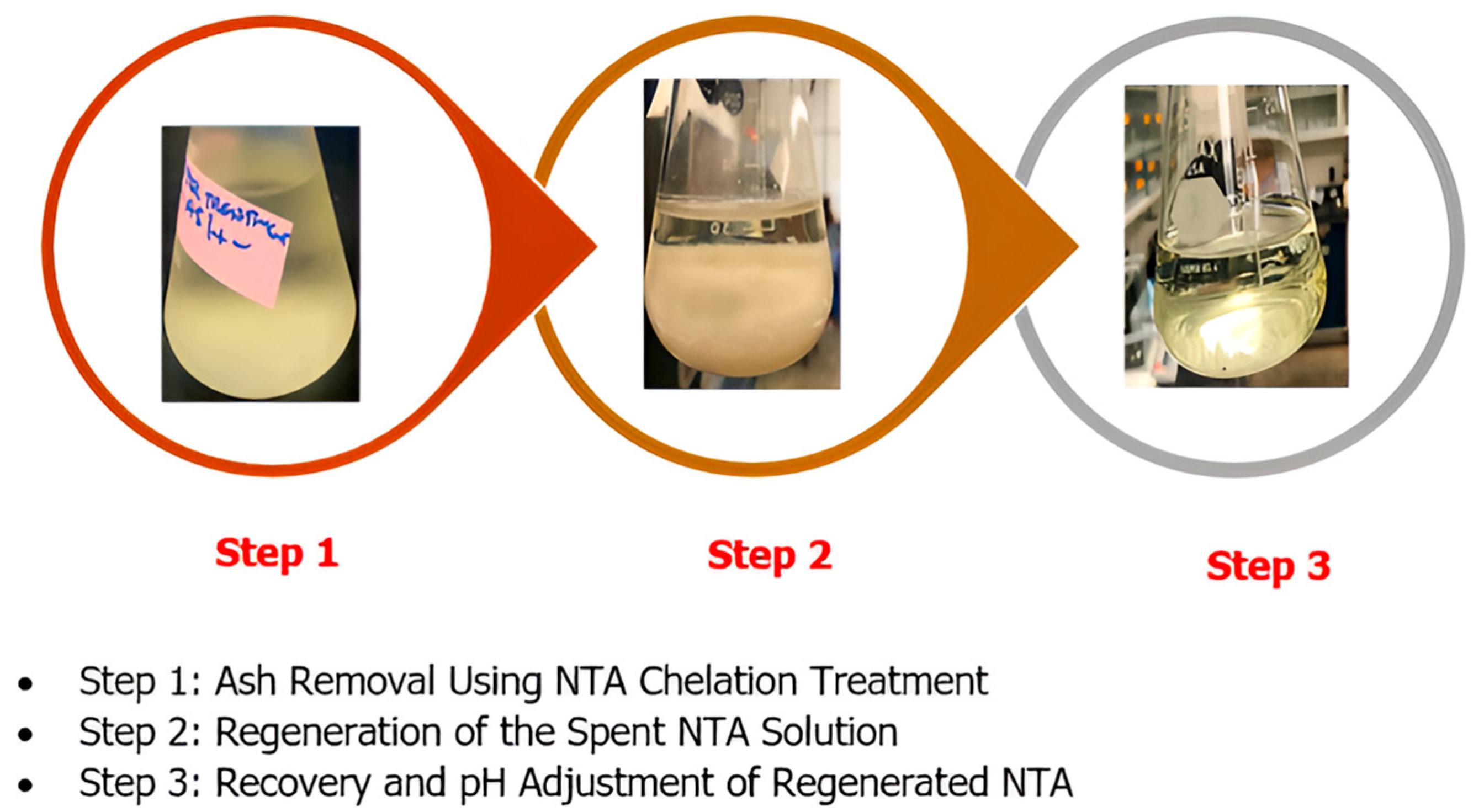
The process for step 1 (
Figure 2) began by mixing 2 g of pretreated
Scenedesmus biomass with 100 mL of 0.05 M NTA in a 250 mL round-bottom flask, which was then sealed with a reflux condenser and heated to 130 °C in an oil bath while stirring at 200 rpm for 2 h. This high-temperature treatment enhanced mineral solubilization and metal chelation by NTA. After cooling, the mixture was centrifuged (4000×
g, 10 min) to retrieve the biomass, which was dried (60 °C, 24 h) for analysis, while the spent NTA supernatant was collected for regeneration.
In step 2, the spent NTA solution, containing dissolved metals, was regenerated via metal sulfide precipitation. The supernatant was stirred while 0.05 M Na2S was added dropwise until visible precipitates formed, displacing metals and freeing NTA. To improve sedimentation, 5 mL of 0.1 M Ca(OH)2 was added as a coagulant, enhancing particle aggregation. This mixture was left overnight in a fume hood to allow complete settling of metal sulfides, ensuring a clear, regenerated NTA solution for reuse.
After overnight settling, the clear regenerated NTA solution was filtered (0.45 µm PTFE) to remove residual particles. Initially alkaline (~pH 10.5) from Na2S and Ca(OH)2, the solution was adjusted to pH 4.5 using 10% HNO3 to optimize the metal chelating efficiency of NTA. The pH-adjusted solution was stored for reuse in subsequent biomass treatments, maintaining consistent performance across cycles, as shown in step 3.
The effectiveness of the process was evaluated through total ash analysis, which was conducted following the laboratory analytical procedure developed by NREL [
33]. The summary of the NTA recycling process is presented in
Figure 2, while the ash content in the algae for each treatment was computed using Equation (1).
where
Wi is the initial total dry biomass (g),
Wf is the final total dry biomass (g),
Ci is the initial ash content (%), and
Cf is the final ash content (%).
2.5. Ultimate Analysis
The chemical composition of the algae samples was analyzed using standardized methods. These analyses included the quantification of total organic carbon, total nitrogen, moisture content, oven dry weight (ODW), and protein content. Thus, the algae samples were treated with DI water, followed by NTA and DI water at 130 °C for 2 h. Ash analyses were conducted on both the raw and treated algae samples. The NTA solution saturated with ash was regenerated using Na2S with Ca(OH)2. The regenerated solution was recycled for subsequent ash removal cycles.
The elemental composition of the algae samples was determined at various stages of treatment, including the untreated state, using a Flash 2000 Organic Elemental Analyzer (Sunnyvale, CA, USA) with the CHNS module. This analysis directly quantified the carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) content. In contrast, the oxygen content was computed by subtracting the sum of these elements from the total sample mass.
To ensure reliability and statistical significance, all samples were thoroughly dried by placing them in an oven at 60 °C for 24 h before analysis, and each analysis was performed in triplicate. This drying step was crucial for obtaining measurements on a dry basis, eliminating any potential interference from moisture content. This analytical technique provided a comprehensive profile of the elemental changes occurring throughout the treatment process, offering valuable insight into the effectiveness of each stage and the overall transformation of the algae biomass.
2.6. Flame Atomic Absorption Spectroscopy (AAS)
The inorganic elemental composition of the algae samples at various treatment stages was analyzed using AA-7000 Shimadzu Flame AAS (Laval, QC, Canada). The procedure involved adding 250 µL of 72% (w/w) H2SO4 to 0.025 g of dried algae in a glass tube. The mixture was then incubated in a 30 °C water bath for an hour and vortexed every 10 min to ensure proper mixing. Subsequently, 7 mL of ultrapure water (18.2 MΩ) was introduced to dilute the sulfuric acid concentration to 4% (w/w). The prepared samples were placed in an autoclave-compatible rack and subjected to a 121 °C sterilization cycle. After an hour of cooling, the samples were filtered through a 0.22 µm filter with a disposable syringe. The filtered samples were then stored in a refrigerator at 4 °C for more than 24 h before undergoing inorganic elemental analysis.
2.7. Total Carbon (TC) and Total Nitrogen (TN) Determination
The determination of Total Carbon (TC) and Total Nitrogen (TN) in the supernatant was conducted using a Shimadzu Total Organic Carbon AnalyzerTOC-VCSN (Laval, QC, Canada) coupled with a Shimadzu Total Nitrogen Measuring Unit TNM-1 (Laval, QC, Canada. Before the analysis, the TC analyzer and TN measuring unit were calibrated using standard solutions prepared according to the manufacturer’s specifications. The liquid sample was filtered through a 0.45 μm membrane to remove particulates and placed into autosampler vials for automated injection. The Shimadzu TOC-VCSN analyzer uses a high-temperature combustion catalytic oxidation method to break down organic compounds in the sample into carbon dioxide (CO2). The generated CO2 was detected using a non-dispersive infrared (NDIR) detector, providing the TC in the sample.
For TN analysis, the TNM-1 module facilitated the measurement of nitrogen content by utilizing catalytic oxidation followed by chemiluminescence detection. During this process, nitrogen-containing compounds in the sample were oxidized to nitrogen monoxide (NO) at high temperatures in the presence of a catalyst. The generated NO was detected using chemiluminescence, and the TN concentration was quantified. Quality control measures, such as periodic analysis of blank samples and certified materials, were employed to validate the accuracy and precision of the results.
2.8. Data and Statistical Analysis
All experiments were performed in triplicate, and the data were reported as mean ± standard deviation. Ash removal efficiency, elemental composition, and biochemical changes were analyzed using analysis of variance (ANOVA) to determine significant differences between treatments, with a confidence level of 95% (p < 0.05). Post hoc Tukey tests were applied to identify specific differences among treatment groups. Statistical software such as OriginPro 2024b was used for data processing, visualization, and interpretation.
3. Results
This study conducted a comprehensive analysis to evaluate the effectiveness of various deashing treatments: deionized (DI) water, nitrilotriacetic acid (NTA), and their combination on algal biomass. Experimental assessments included ash content quantification, metal ion removal efficiency, and changes in biochemical composition such as protein content. Elemental analysis provided insights into removing major ash-forming elements (Ca, K, and Mg) and heavy metals (Cu, Zn, and Pb). Additionally, temperature-dependent deashing efficiency was investigated, and statistical analysis using mean ± standard deviation helped determine treatment consistency and reproducibility. The Scenedesmus sample underwent detailed characterization due to its excellent response to treatment.
3.1. FTIR Analysis of Raw and Treated Algae
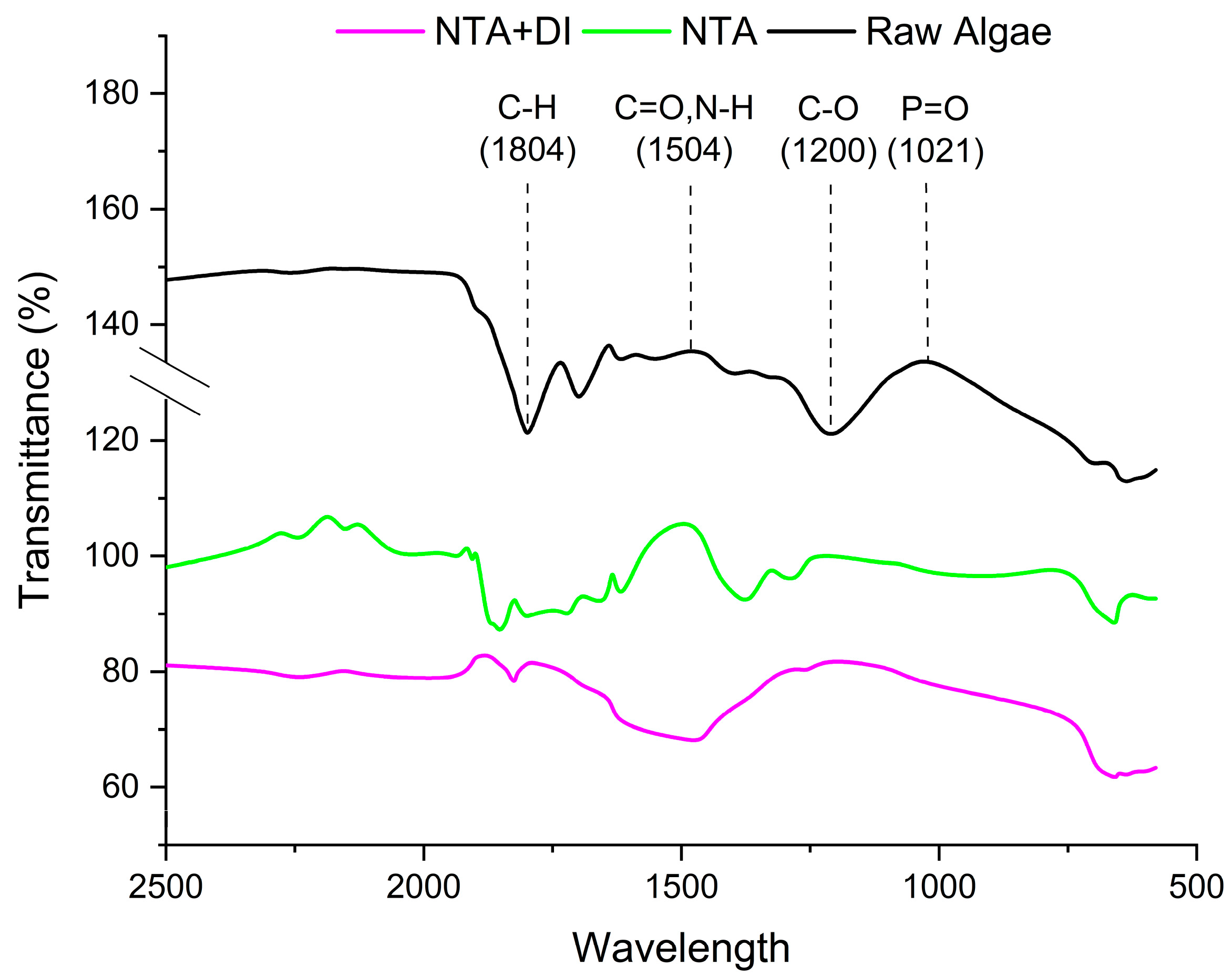
The FTIR spectra in
Figure 3 display the transmittance of raw and treated
Scenedesmus algae samples with NTA and NTA+DI. The results revealed that the C-H stretching (1804 cm
−1) peaks indicate the presence of aliphatic chains, likely from lipids and fatty acids in the algal biomass. However, a slight reduction in intensity could indicate degradation or modification of lipid structures during treatment. Further reduction suggests a continued breakdown or removal of lipid-like substances during the combined treatment [
34].
The strong peak in the raw sample at 1504 cm−1 is characteristic of carbonyl groups (C=O) in proteins (amide I band), lipids, and carbohydrates. Notwithstanding, this peak could be associated with the N-H bending vibrations of proteins (amide II band). The shift or reduction in peak intensity suggests chelation or interaction of NTA with carbonyl groups, possibly altering protein structure or binding to carboxylates. The NTA+DI treatment enhances this effect, as further removal of cell wall-bound minerals and organic components occurs, aligning with the significant reduction in ash content in the raw algae sample.
The peaks at 1200 cm
−1 (C-O stretching) and 1021 cm
−1 (P=O stretching) are associated with polysaccharides and phosphates, respectively, and exhibit a notable reduction in intensity after each treatment stage. These peaks are firm in raw algae due to structural polysaccharides and phosphate-containing compounds. Post-NTA treatment causes a decrease in intensity, signifying the chelation of metal–phosphate complexes and partial removal of polysaccharides [
35]. The NTA+DI treatment further reduces these peaks, highlighting the combined efficiency in breaking down the cell wall and removing phosphate minerals, key ash-forming components.
3.2. Ash Content and Removal Efficiency Analysis
Removing ash from algae is critical in enhancing their potential for bioenergy or biochemical production. In this study, NTA was used as a chelating agent to selectively remove ash from three (3) different algae samples: high ash (SS ATS), medium ash (Green ATS), and low ash (
Scenedesmus). The use of NTA for ash removal in the algae samples yielded varying degrees of success, as shown in
Table 1. The ash removal efficiencies were computed using a mass-corrected approach that accounts for the loss of total dry biomass during treatment. This corrected method ensures that reductions in ash content are not over- or underestimated due to changes in organic matter content. The efficiency was calculated using Equation (1), and the procedure was the same for all samples, adhering to the protocol outlined in
Figure 1.
The NTA treatment achieved only a modest ash content reduction in SS ATS (≈2.8%), indicating a high presence of non-metallic or refractory ash components that NTA, which primarily targets metal ions, could not effectively address. Additionally, the presence of stable metal oxides, like aluminum or iron, further diminished ash removal efficiency under standard NTA conditions [
36]. The dense structure of SS ATS may have hindered the penetration of NTA into the biomass matrix, limiting its ability to access and chelate metals bound within the structure [
37].
The Green ATS showed a moderate reduction in ash content of about 8.7%. NTA was more effective at chelating ash-forming metals than SS ATS, likely due to a higher concentration of more easily removable metal oxides or ions, such as calcium and magnesium. Additionally, the structural properties of Green ATS may have enhanced its surface exposure to ash. However, the reaction time or NTA concentration may not have been optimal, resulting in only partial ash removal. Extending the treatment time or increasing the NTA concentration could improve efficiency [
38]. The organic compounds or other components in Green ATS could have interfered with the effectiveness of NTA, competing for the chelating sites and reducing its ability to target metal ions effectively.
Moreover,
Scenedesmus exhibited a dramatic reduction in ash content from 15.2% to 3.8%, indicating a removal efficiency of 75%. The effectiveness of NTA, in this case, could be attributed to the low initial ash content compared to the other two samples, which likely consisted of more readily chelatable metal ions (e.g., calcium and magnesium). NTA is highly effective in removing these ions, significantly reducing ash [
39]. Since
Scenedesmus has a relatively low initial ash content, most of the ash is likely in the form of metal salts or oxides that are more reactive with NTA, resulting in efficient chelation and removal [
40,
41]. In addition, the structure of
Scenedesmus may have allowed better penetration of NTA, increasing its effectiveness in targeting and removing ash-forming components.
Scenedesmus was selected for thorough evaluation in this study based on its significant ash reduction after NTA treatment. This is associated with relatively high ash removal efficiency. It is an ideal candidate for further analysis to optimize the ash removal process and understand the underlying mechanisms contributing to its effectiveness. In addition to ash reduction, the final dry mass of the algae was measured to quantify biomass recovery. Scenedesmus showed the highest relative loss (28.5%), reflecting a great degree of metal leaching compared to Green ATS (14%) and SS ATS (7%). These differences further support the selection of Scenedesmus as the focus of subsequent ash optimization trials.
3.2.1. Ash Removal Efficiency for Each Treatment Stage
Figure 4 presents the results as ash content percentages, and the corresponding ash removal efficiencies reveal the varying impacts of each treatment stage at 130 °C. The DI treatment effectively reduced ash content by approximately 24.2%. This initial reduction may result from the solubilization of easily removable inorganic compounds, particularly water-soluble salts (e.g., sodium, potassium) and loosely bound ash particles. However, the limited effectiveness suggests that a significant portion of the ash content may consist of metallic oxides or non-water-soluble components, which require more potent chelating agents for removal. This stage is preparatory, effectively removing surface-bound ash but not targeting deeply embedded or more chemically stable ash components.
The NTA treatment markedly increased ash removal, with an efficiency of 54%. As a chelating agent, NTA selectively binds to metal ions such as calcium, magnesium, and iron, forming soluble complexes that can be removed from the algae matrix. This significant decrease in ash content indicates that NTA effectively targets ash-forming metal ions that DI alone could not remove. The reduction also suggests that a substantial fraction of the algae ash is composed of metal oxides or carbonates, which are susceptible to chelation. This stage demonstrates the enhanced ash removal capability of NTA over DI, as it can access and bind metal ions within the cellular structure of the algae.
Following the NTA treatment with additional DI rinse resulted in a final ash content of 3.83%, corresponding to an overall ash removal efficiency of 56%. This additional DI rinse removes residual ash components and loosely bound NTA-metal complexes that may remain within the algae. The marginal increase in ash removal (2% over the NTA treatment phase) suggests that DI rinsing primarily aids in flushing out residual or weakly bound ash particles that the initial NTA chelation did not completely solubilize. This added step may not be justified practically, given the extra processing time, water use, and complexity it introduces.
This synergy approach maximizes ash removal, indicating that a post-chelation DI rinse can provide a finishing effect to remove residual ash effectively. This sequential approach can improve the quality of algae feedstocks for biofuel production by reducing inorganic impurities that can impact downstream process efficiency [
42,
43]. In addition, in real-world applications, a single-step NTA may be preferred for efficiency, unless higher purity is essential for downstream processing.
Moreover, it is essential to note that the ash removal efficiencies in
Table 1 and
Figure 4 differ due to variations in experimental design.
Table 1 shows the highest corrected efficiency of 75% for
Scenedesmus using a single optimized NTA treatment at 130 °C.
Figure 4 shows the results from individual treatments (DI and NTA) and a sequential treatment (NTA followed by DI), demonstrating a total removal of 56%. This lower efficiency stems from factors like partial re-adsorption and the sequential nature of the treatments, highlighting how treatment structure and application mode affect overall performance.
3.2.2. Impact of Temperature on Ash Removal Efficiency
Figure 5 illustrates the effectiveness of ash removal from Scenedesmus algae at various temperatures. The results show that higher temperatures improve ash removal efficiency. At 90 °C, the ash content is 13.6%, with a removal efficiency of 54%. Although moderate temperatures allow some ash removal, efficiency is low due to limited solubilization of inorganic compounds. Increasing the temperature to 100 °C enhances ash removal, decreasing ash content to 8.15% and achieving a 66.89% removal efficiency. This indicates that higher temperatures facilitate the breakdown of complex ash-forming compounds, allowing for more effective removal [
44].
At 110 °C, the ash content decreased slightly to 6.9%, with an ash removal efficiency of 68.18%. The modest increase in efficiency from 100 °C to 110 °C indicates that most easily removable ash compounds have already been eliminated, leaving behind more resistant components. Unlike 120 °C, the ash content reached 6.7%, achieving an ash removal efficiency of 71.56%. This temperature facilitates further ash removal, but the efficiency gains are moderate due to the persistence of resistant ash compounds. At 130 °C, the ash content dropped to 4.2%, with a high ash removal efficiency of 83.07%. This suggests that higher temperatures are crucial for removing the most resilient ash-forming compounds, likely due to the breakdown of refractory compounds that only dissolve at elevated temperatures [
45].
3.3. Successive Cycling
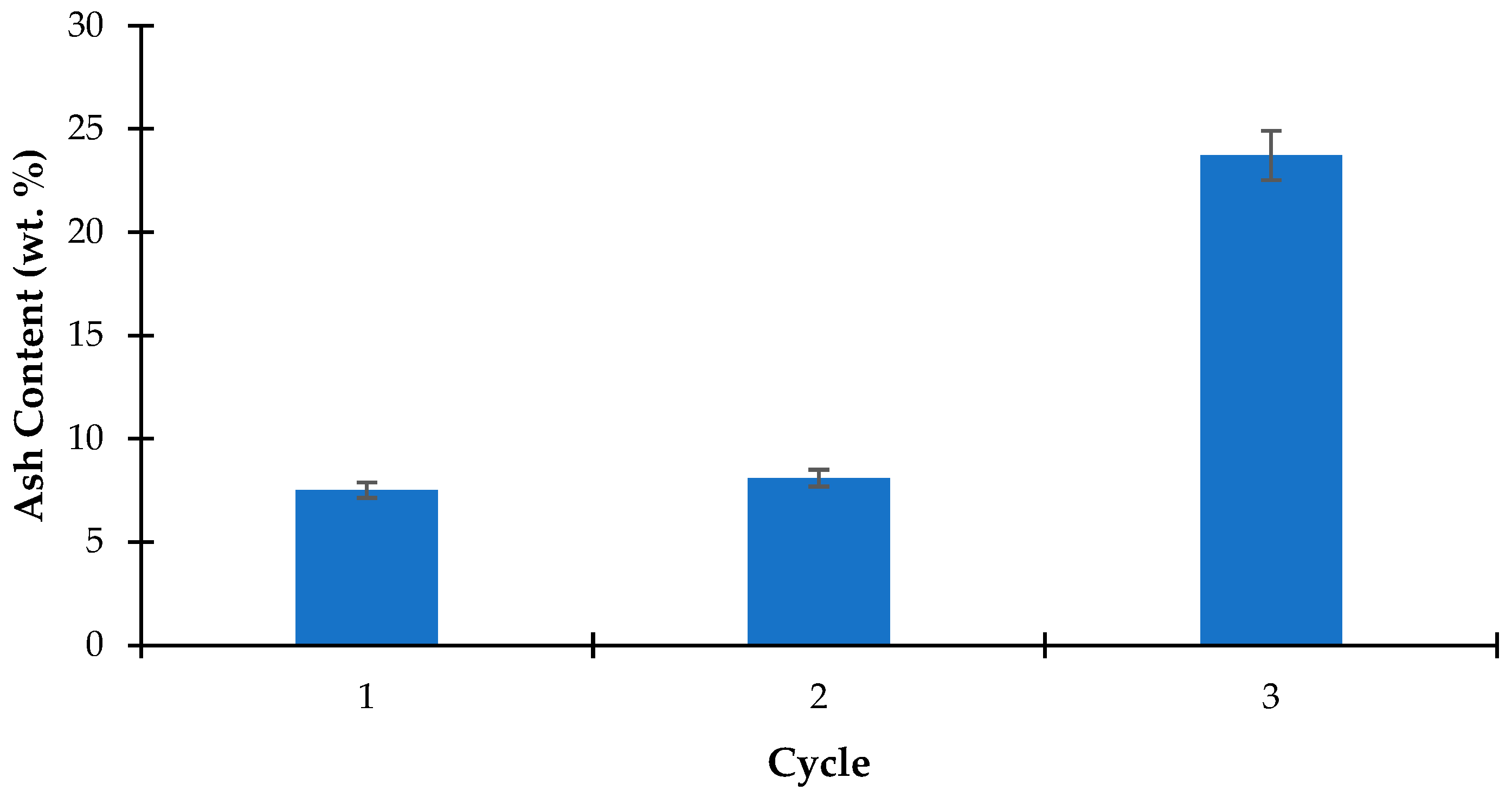
The ash content in the first recycling, as shown in
Figure 6, is 7.52%, indicating effective removal of inorganic components. This process establishes a baseline for ash removal efficiency. After the second recycling, the ash content increases slightly to 8.10%, suggesting a minor decline in removal efficiency, potentially due to partial saturation of the chelating agent or other compounds affecting ash extraction in the algae matrix [
46].
In the third recycling cycle, a significant increase in ash content is observed, reaching 23.72%. This substantial rise indicates a considerable decline in ash removal efficiency, likely due to the exhaustion or reduced efficacy of the recycling agent. However, this increase may be partially attributed to the cumulative loss of organic (ash-free) biomass during successive processing steps. As soluble organic matter is lost with each treatment, the biomass becomes enriched in inorganic components, resulting in a higher proportion of ash when expressed as a percentage of total mass. This normalization effect can artificially inflate the ash content, even if the absolute mass of ash remains constant. Over successive cycles, the chelating agent or other active compounds may lose their binding capacity, accumulating ash components rather than effective removal [
47].
3.4. Effects of DI and NTA Treatments on Algal Biomass Composition
3.4.1. CHN Elemental Analysis
The ultimate analysis of untreated and treated
Scendesmus biomass reveals compositional changes in ash, hydrogen (H), carbon (C), and nitrogen (N) following each treatment stage: raw algae, DI, NTA, and NTA+DI treatment (
Table 2). The ash content steadily decreases across the treatments, from 15.2% in raw algae to 3.8% after the final stage, confirming the cumulative ash-removal effectiveness. In contrast, carbon and hydrogen levels remain relatively stable throughout all treatments, indicating the preservation of the organic carbon framework of the biomass. However, nitrogen content notably decreases from 8.4% to 7.1% (approximately 15% reduction) across treatments. This loss may not solely reflect changes in elemental composition. Still, it could also result from biomass loss during washing, centrifugation, or solubilization of nitrogen-containing compounds (e.g., amino acids or small peptides). As ash is removed, the total dry biomass is reduced, which increases the proportional contribution of remaining components and may skew percentage-based elemental values.
The substantial reduction in ash content enhances the value of algal biomass for thermochemical conversion processes like hydrothermal liquefaction by minimizing catalytic interference and improving biocrude quality. While the reduced nitrogen content may limit protein-based applications (e.g., animal feed or biochemical recovery), it is advantageous for energy-focused valorization by lowering the formation of nitrogenous byproducts. Overall, the NTA-based ash removal method significantly improves the elemental purity of algal biomass while maintaining its core organic characteristics [
48].
This study evaluated the efficacy of NTA in deashing algal biomass while preserving its CHN composition post-treatment. However, long-term factors like biomass storage stability, microbial contamination, and downstream conversion efficiency are crucial for maintaining feedstock viability and may require further investigation. Smith et al. [
49] highlighted that moisture content significantly affects biomass stability and conversion performance, highlighting the importance of furthering this investigation.
3.4.2. Metal Ions Analysis
Table 3 presents the concentration of various metal ions (Ca, K, Cu, Zn, and Pb) in the
Scenedesmus algal biomass at the earlier stages. The raw algae contain various metal ions, with calcium (Ca) and potassium (K), the most abundant at 6720 ppm and 4430 ppm, respectively. Zinc (Zn) and lead (Pb) are also present, revealing potential contamination with heavy metals. After the DI treatment, Ca reduces marginally to 6520 ppm, indicating that DI water alone is ineffective at removing Ca. This is likely due to its stable association with the biomass. K decreases significantly to 2770 ppm, suggesting that K is more soluble and readily removed by DI water. In addition, Cu, Zn, and P decrease to 330, 3300, and 140 ppm, respectively, showing partial removal of these metals with DI water.
After NTA treatment, calcium (Ca) levels dropped below detectable limits, indicating effective chelation and removal from the biomass. Potassium (K) decreased to 2330 ppm; however, the reduction was less significant than the DI treatment. Copper (Cu), zinc (Zn), and lead (Pb) levels were also reduced, highlighting the superior ability of NTA to bind and remove these metals compared to DI water. The best results for metal ion removal were achieved with the combination of NTA and DI treatment, which brought both Ca and K to undetectable levels. Copper, zinc, and lead concentrations were further reduced to 200 ppm, 570 ppm, and 30 ppm, respectively. This significant reduction, especially the complete removal of Ca and K, enhances biomass purity, making it more suitable for thermochemical conversion processes. High alkali and heavy metal levels can lead to undesirable reactions during hydrothermal liquefaction or pyrolysis, resulting in char formation and lower quality of biocrude [
48]. Therefore, effectively removing these metals through NTA and DI treatment is expected to improve both the efficiency and quality of biocrude production. The results confirm that the NTA+DI treatment is a practical deashing approach, significantly reducing the inorganic contaminants and making the algal biomass suitable for applications requiring low ash and metal content in biocrude production.
3.4.3. Total Carbon and Nitrogen Content Analysis
Figure 7 illustrates the effects of different treatments on the elemental composition of biomass, focusing on TC and TN levels. After the DI treatment, the TC level averages 90 mg/L, with minimal impact on internal carbon content, while the TN remains high at 70 mg/L, indicating that nitrogenous compounds are largely retained. Following NTA treatment, TC increases to 120 mg/L due to the removal of inorganic ash, but TN significantly drops to 30 g/mL, likely because NTA chelates and removes nitrogen-rich impurities. Combined treatment results in TC decreasing to 70 mg/L and TN further declining to 20 mg/L, the lowest recorded. This sequential approach effectively reduces nitrogen content, enhancing biomass suitability for biocrude generation by minimizing nitrogen-related pollutants and improving the purity for thermochemical conversion [
49].
The C:N ratio (
Table 2) remained stable across treatments, indicating the organic framework in solid biomass was preserved. To analyze the organic leachate, total organic carbon (TOC) and total nitrogen (TN) were measured in the aqueous supernatant. The C:N ratios in the aqueous phase differed from the solid phase, with a lower C:N in the DI treatment, suggesting preferential solubilization of nitrogen-rich compounds. Conversely, the NTA-treated supernatant showed higher carbon content, indicating release of carbon-rich components from matrix disruption. The differences in C:N ratios reflect varied leaching behavior during treatments, not analytical inconsistencies.
4. Conclusions
This study assessed the effectiveness of deionized (DI) water, nitrilotriacetic acid (NTA), and their combined application (NTA+DI) for ash and heavy metal reduction in three algal biomasses: Solid-State Algal Turf Scrubber (ATS), Green ATS, and Scenedesmus. Among these, Scenedesmus demonstrated the highest deashing efficiency, with ash content reduced from 15.2% to 3.8% using the NTA+DI treatment. DI treatment primarily removed surface-bound impurities, while NTA effectively chelated ash-forming metals such as calcium, potassium, and magnesium. Increasing treatment temperatures from 90 °C to 130 °C enhanced ash removal, achieving up to 83.07% efficiency due to improved solubility and reaction kinetics. However, repeated NTA recycling showed declining efficiency beyond the second cycle, indicating the need for replenishment or regeneration.
Elemental analysis revealed minimal changes in CHN composition, confirming the preservation of the biomass organic structure. The substantial removal of ash, nitrogen, and heavy metals enhances the suitability of the treated biomass for thermochemical processes such as hydrothermal liquefaction, where low ash and nitrogen content improve biocrude yield and quality.
It is important to note that the NTA+DI approach proved most effective for moderately ash-containing algae like Scenedesmus. Its performance may be limited for biomass with significantly higher ash content or those cultivated in media heavily enriched with metals, where more aggressive or alternative treatments may be required. Nonetheless, NTA+DI offers a promising pathway to produce purified biomass suitable for biofuel applications for low to moderate-ash algae.