Phylogenetic Proximity vs. Environmental Adaptation: Exploring Photosynthetic Performances in Mediterranean and Andean Isolated Microalgae Under Different Light Intensities
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strains and Growth Conditions
2.2. Different Light Intensity Treatments
2.3. Extraction and Determination of Photosynthetic Pigments
2.4. Determination of Mycosporine-Like Amino Acids
2.5. Extraction and Determination of Total Antioxidant Compounds
2.6. Chlorophyll Fluorescence Analysis
2.7. Statistical Analysis
3. Results
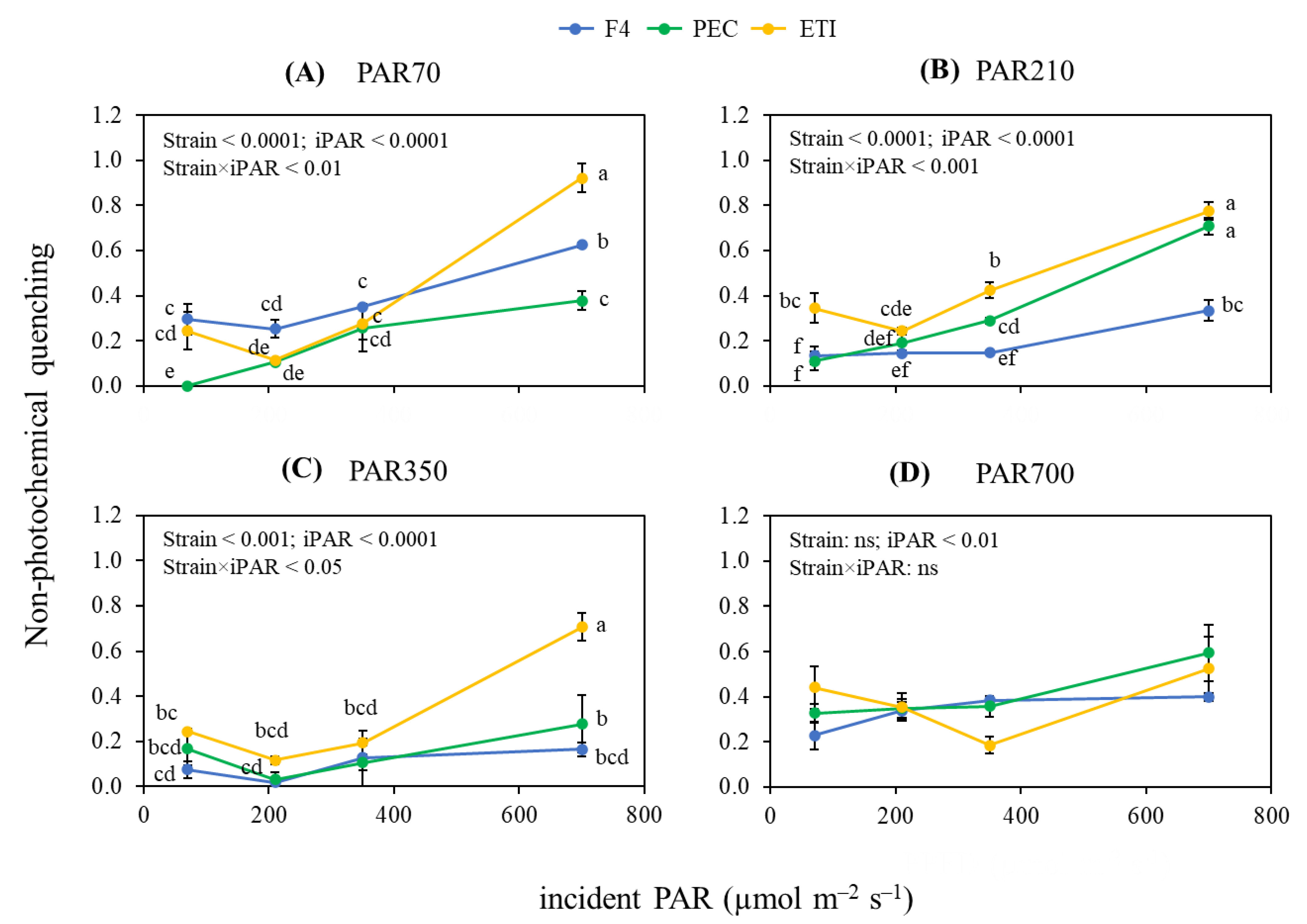
3.1. Chlorophyll Fluorescence
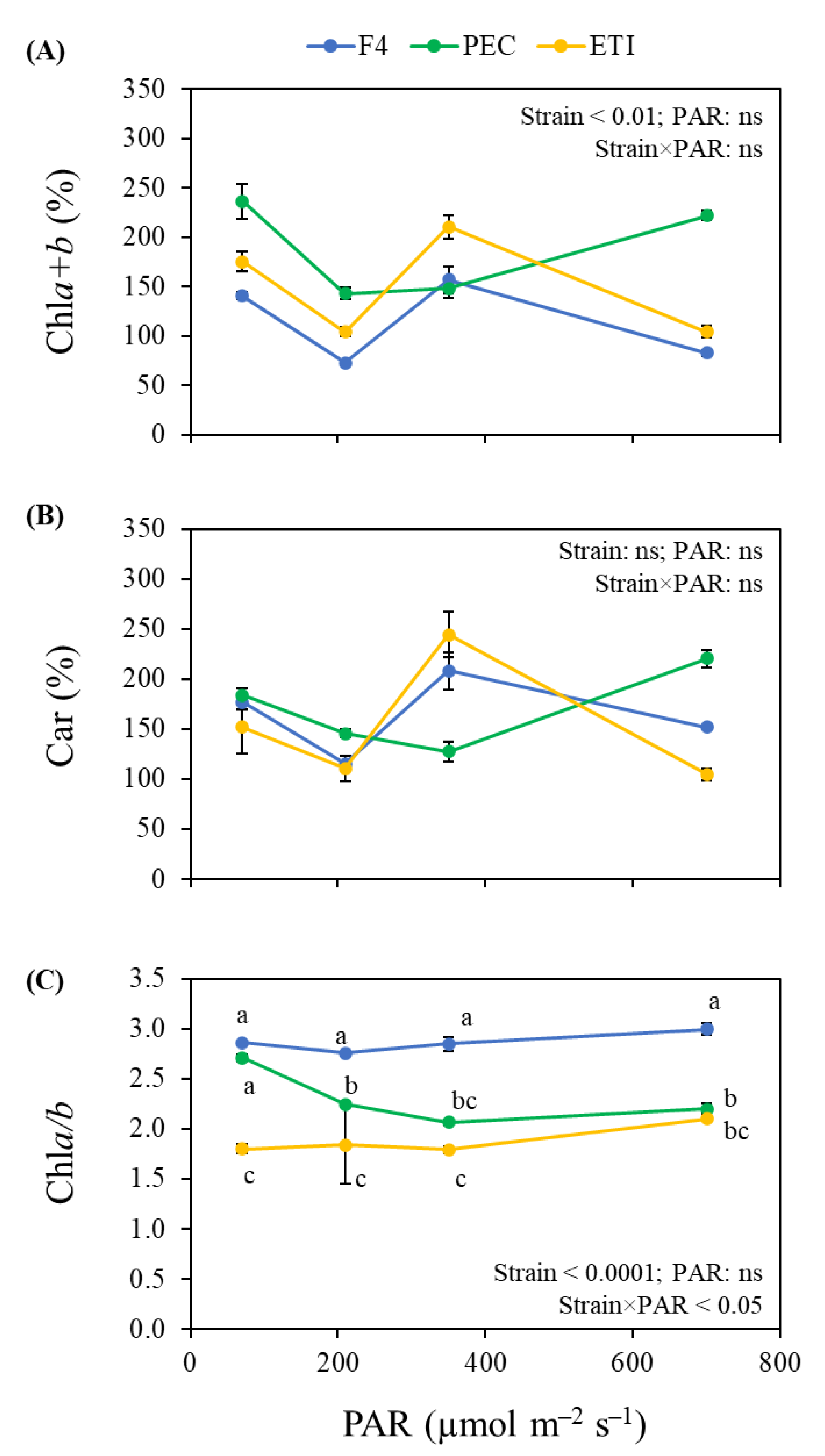
3.2. Photosynthetic Pigments
3.3. Antioxidant Systems
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 21–36. [Google Scholar]
- Levin, G.; Kulikovsky, S.; Liveanu, V.; Eichenbaum, B.; Meir, A.; Isaacson, T.; Tadmor, Y.; Adir, N.; Schuster, G. The desert green algae Chlorella ohadii thrives at excessively high light intensities by exceptionally enhancing the mechanisms that protect photosynthesis from photoinhibition. Plant J. 2021, 106, 1260–1277. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.; Wakao, S.; Niyogi, K.K. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015, 82, 449–465. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Minagawa, J. High light acclimation in green microalgae. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 445–469. ISBN 978-94-017-9031-4. [Google Scholar]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Solovchenko, A.; Neverov, K. Carotenogenic response in photosynthetic organisms: A colorful story. Photosynth. Res. 2017, 133, 31–47. [Google Scholar] [CrossRef]
- Nascimento, L.B.d.S.; Tattini, M. Beyond photoprotection: The multifarious roles of flavonoids in plant terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (Maas): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Virtanen, O.; Khorobrykh, S.; Tyystjärvi, E. Acclimation of Chlamydomonas reinhardtii to extremely strong light. Photosynth. Res. 2021, 147, 91–106. [Google Scholar] [CrossRef]
- Smith, B.M.; Morrissey, P.J.; Guenther, J.E.; Nemson, J.A.; Harrison, M.A.; Allen, J.F.; Melis, A. Response of the photosynthetic apparatus in Dunaliella salina (green algae) to irradiance stress. Plant Physiol. 1990, 93, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Yasmin, M.; Liveanu, V.; Burstein, C.; Hanna, R.; Kleifeld, O.; Simanowitz, M.C.; Meir, A.; Tadmor, Y.; Hirschberg, J.; et al. A desert Chlorella sp. that thrives at extreme high-light intensities using a unique photoinhibition protection mechanism. Plant J. 2023, 115, 510–528. [Google Scholar] [CrossRef]
- Belgio, E.; Trsková, E.; Kotabová, E.; Ewe, D.; Prášil, O.; Kaňa, R. High light acclimation of Chromera velia points to photoprotective NPQ. Photosynth. Res. 2018, 135, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Bonente, G.; Pippa, S.; Castellano, S.; Bassi, R.; Ballottari, M. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J. Biol. Chem. 2012, 287, 5833–5847. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Sabia, A.; Giovanardi, M.; Ferroni, L.; Maglie, M.; Pancaldi, S. Chlorophyta microalgae as dietary protein supplement: A comparative analysis of productivity related to photosynthesis. J. Appl. Phycol. 2022, 34, 1323–1340. [Google Scholar] [CrossRef]
- Cecchin, M.; Simicevic, J.; Chaput, L.; Hernandez Gil, M.; Girolomoni, L.; Cazzaniga, S.; Remacle, C.; Hoeng, J.; Ivanov, N.V.; Titz, B.; et al. Acclimation strategies of the green alga Chlorella vulgaris to different light regimes revealed by physiological and comparative proteomic analyses. J. Exp. Bot. 2023, 74, 4540–4558. [Google Scholar] [CrossRef] [PubMed]
- Suwannachuen, N.; Leetanasaksakul, K.; Roytrakul, S.; Phaonakrop, N.; Thaisakun, S.; Roongsattham, P.; Jantasuriyarat, C.; Sanevas, N.; Sirikhachornkit, A. Palmelloid formation and cell aggregation are essential mechanisms for high light tolerance in a natural strain of Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2023, 24, 8374. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Chiellini, C.; Barozzi, E.; Sandoval, C.; Echeverría, C.; Guglielminetti, L. Exploring the physiological multiplicity of native microalgae from the Ecuadorian highland, Italian lowland and indoor locations in response to UV-B. Int. J. Mol. Sci. 2023, 24, 1346. [Google Scholar] [CrossRef]
- Chiellini, C.; Mariotti, L.; Huarancca Reyes, T.; de Arruda, E.J.; Fonseca, G.G.; Guglielminetti, L. Remediation capacity of different microalgae in effluents derived from the cigarette butt cleaning process. Plants 2022, 11, 1770. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic performance of five cool-season turfgrasses under UV-B exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Huang, J.J.; Bunjamin, G.; Teo, E.S.; Ng, D.B.; Lee, Y.K. An enclosed rotating floating photobioreactor (RFP) powered by flowing water for mass cultivation of photosynthetic microalgae. Biotechnol. Biofuels 2016, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Mariotti, L.; Chiellini, C.; Guglielminetti, L.; Fonseca, G.G. UV-B irradiation effect on microalgae performance in the remediation of effluent derived from the cigarette butt cleaning process. Plants 2022, 11, 2356. [Google Scholar] [CrossRef]
- Moles, T.M.; Guglielminetti, L.; Huarancca Reyes, T. Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Sci. Hortic. 2019, 257, 108730. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and growth responses of Arundo donax L. plantlets under different oxygen deficiency stresses and reoxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in excess: The molecular mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Popson, D.; D’Silva, S.; Wheeless, K.; Morgan-Kiss, R. Permanent stress adaptation and unexpected high light tolerance in the shade-adapted Chlamydomonas priscui. Plants 2024, 13, 2254. [Google Scholar] [CrossRef]
- Benito, X.; Luethje, M.; Schneider, T.; Fritz, S.C.; Baker, P.A.; Pedersen, E.J.; Gaüzère, P.; de Novaes Nascimento, M.; Bush, M.; Ruhi, A. Ecological resilience in tropical Andean lakes: A paleolimnological perspective. Limnol. Oceanogr. 2022, 67, S23–S37. [Google Scholar] [CrossRef]
- Quaas, T.; Berteotti, S.; Ballottari, M.; Flieger, K.; Bassi, R.; Wilhelm, C.; Goss, R. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic differences in the process of excess energy dissipation. J. Plant Physiol. 2015, 172, 92–103. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Cataldo, M.L.; Conversano, F.; Brunet, C. Growth and photophysiological responses of two picoplanktonic Minutocellus species, strains RCC967 and RCC703 (Bacillariophyceae). Eur. J. Phycol. 2012, 47, 408–420. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Orgnero, S.; Hüner, N.P.A.; Smith, D.R. The enigmatic loss of light-independent chlorophyll biosynthesis from an Antarctic green alga in a light-limited environment. New Phytol. 2019, 222, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Rommel, S.; Kalra, I.; D’Silva, S.; Hahn, M.M.; Popson, D.; Cvetkovska, M.; Morgan-Kiss, R.M. Cyclic electron flow (CEF) and ascorbate pathway activity provide constitutive photoprotection for the photopsychrophile, Chlamydomonas sp. UWO 241 (renamed Chlamydomonas priscuii). Photosynth. Res. 2022, 151, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Szyszka, B.; Ivanov, A.G.; Hüner, N.P.A. Psychrophily is associated with differential energy partitioning, photosystem stoichiometry and polypeptide phosphorylation in Chlamydomonas raudensis. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Jácome, G.; Valarezo, C.; Yoo, C. Assessment of water quality monitoring for the optimal sensor placement in lake Yahuarcocha using pattern recognition techniques and geographical information systems. Environ. Monit. Assess. 2018, 190, 259. [Google Scholar] [CrossRef]
- Li, Y.; Gu, W.; Huang, A.; Xie, X.; Wu, S.; Wang, G. Transcriptome analysis reveals regulation of gene expression during photoacclimation to high irradiance levels in Dunaliella salina (Chlorophyceae). Phycol. Res. 2019, 67, 291–302. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 157. [Google Scholar] [CrossRef]
- Ballottari, M.; Dall’Osto, L.; Morosinotto, T.; Bassi, R. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J. Biol. Chem. 2007, 282, 8947–8958. [Google Scholar] [CrossRef]
- La Rocca, N.; Sciuto, K.; Meneghesso, A.; Moro, I.; Rascio, N.; Morosinotto, T. Photosynthesis in extreme environments: Responses to different light regimes in the Antarctic alga Koliella antarctica. Physiol. Plant. 2015, 153, 654–667. [Google Scholar] [CrossRef]
- Bartolini, A. La Riserva Naturale del Padule di Fucecchio. Dieci anni di Gestione (1996–2006); Centro di Ricerca, Documentazione e Promozione del Padule di Fucecchio: Larciano, Italy, 2007. [Google Scholar]


| Strain | Isolation Source | Geographic Location | Taxonomic Affiliation | Reference |
|---|---|---|---|---|
| F4 (MLIP M004) | Mediterranean Marshland (Italy) | 43°48′31″ N 10°48′18″ E | Chlorella sorokiniana | [21] |
| PEC | Andean Lake (Ecuador) | 00°22′10″ N 78°06′09″ W | Pectinodesmus pectinatus | [20] |
| ETI | Andean Lake (Ecuador) | 00°22′10″ N 78°06′09″ W | Ettlia pseudoalveolaris | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panicucci, G.; Chiellini, C.; Sbrana, C.; Echeverría, C.; Guglielminetti, L.; Huarancca Reyes, T. Phylogenetic Proximity vs. Environmental Adaptation: Exploring Photosynthetic Performances in Mediterranean and Andean Isolated Microalgae Under Different Light Intensities. Phycology 2025, 5, 24. https://doi.org/10.3390/phycology5020024
Panicucci G, Chiellini C, Sbrana C, Echeverría C, Guglielminetti L, Huarancca Reyes T. Phylogenetic Proximity vs. Environmental Adaptation: Exploring Photosynthetic Performances in Mediterranean and Andean Isolated Microalgae Under Different Light Intensities. Phycology. 2025; 5(2):24. https://doi.org/10.3390/phycology5020024
Chicago/Turabian StylePanicucci, Giulio, Carolina Chiellini, Cristiana Sbrana, Cristina Echeverría, Lorenzo Guglielminetti, and Thais Huarancca Reyes. 2025. "Phylogenetic Proximity vs. Environmental Adaptation: Exploring Photosynthetic Performances in Mediterranean and Andean Isolated Microalgae Under Different Light Intensities" Phycology 5, no. 2: 24. https://doi.org/10.3390/phycology5020024
APA StylePanicucci, G., Chiellini, C., Sbrana, C., Echeverría, C., Guglielminetti, L., & Huarancca Reyes, T. (2025). Phylogenetic Proximity vs. Environmental Adaptation: Exploring Photosynthetic Performances in Mediterranean and Andean Isolated Microalgae Under Different Light Intensities. Phycology, 5(2), 24. https://doi.org/10.3390/phycology5020024







