An Easy and Non-Hazardous Extraction Method for Phycobiliproteins and Pigments from Anabaena cylindrica
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Cyanobacteria
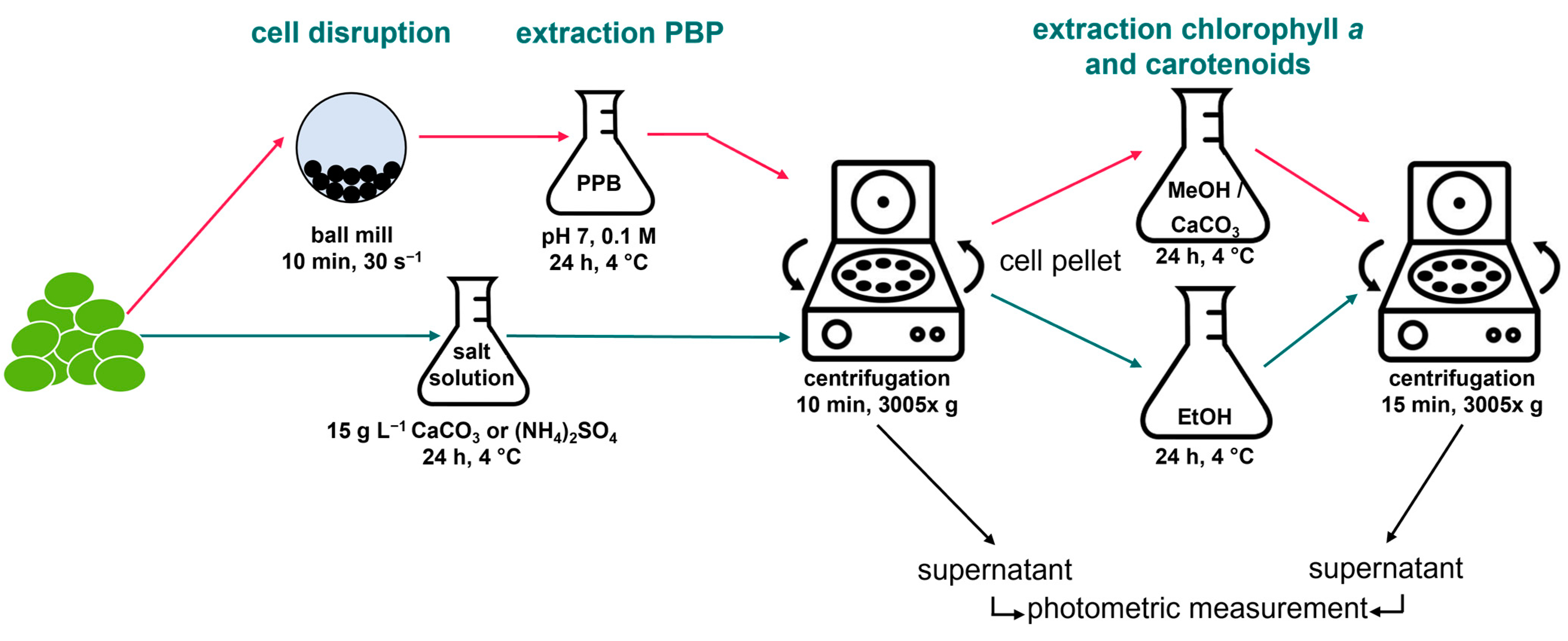
2.2. Extraction of Phycobiliproteins and Pigments
2.3. Statistical Analysis
3. Results and Discussion
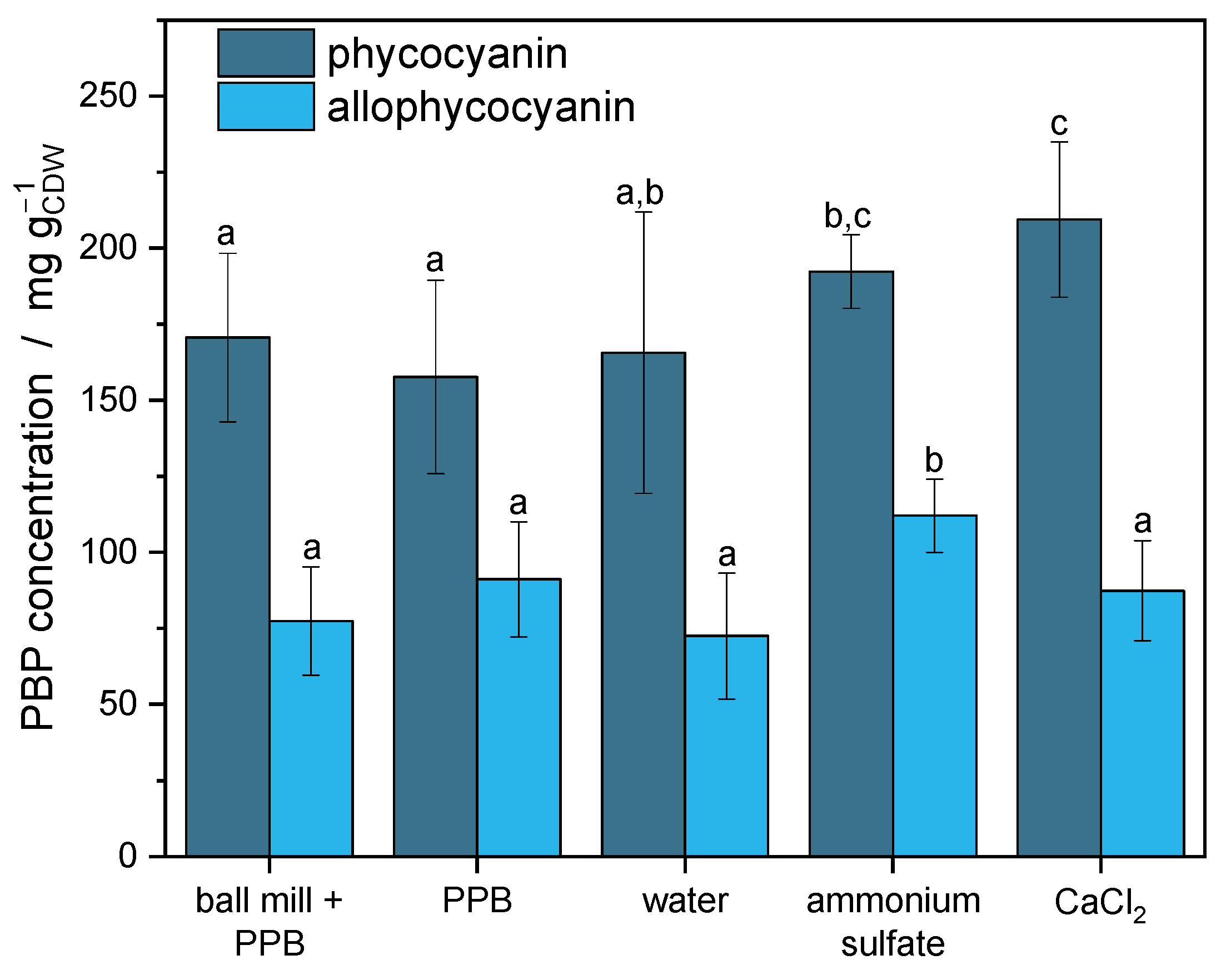
3.1. Extraction of Phycobiliproteins
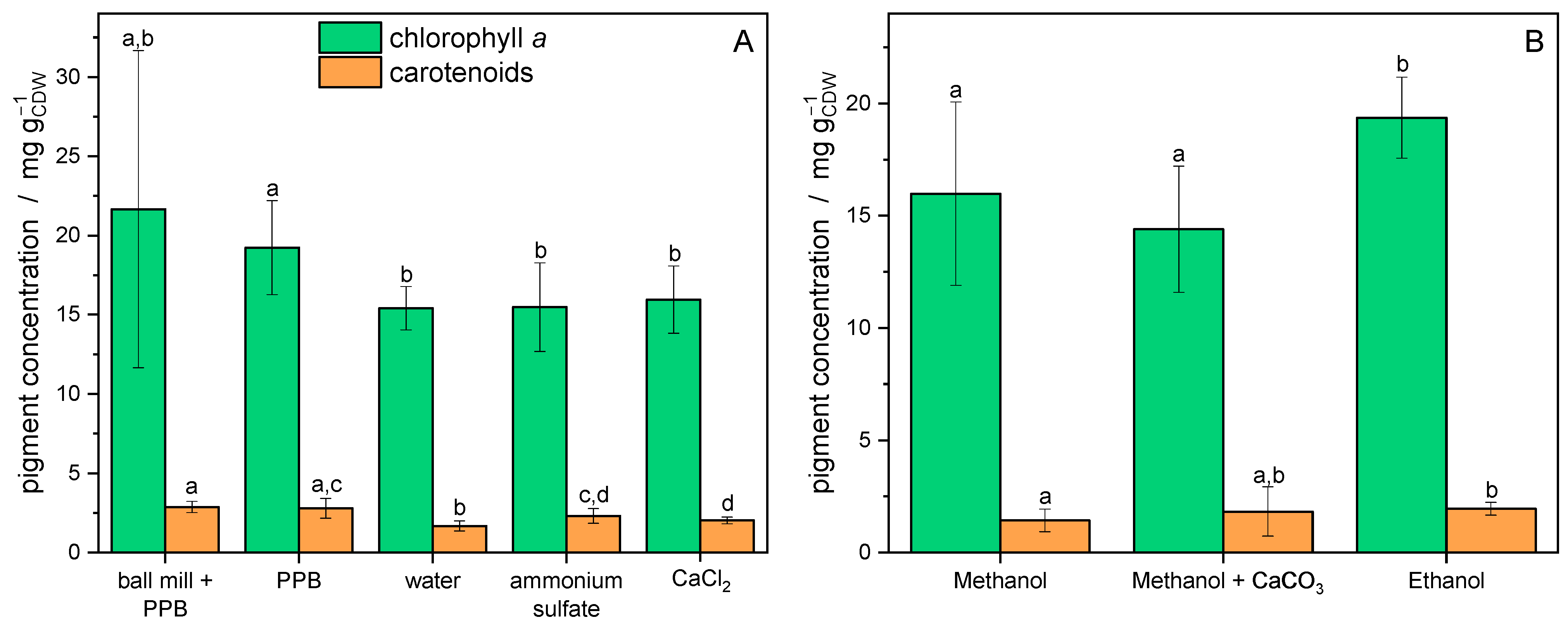
3.2. Extraction of Chlorophyll a and Carotenoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Allophycocyanin |
| EPS | Extracellular polymeric substance |
| PBP | Phycobiliprotein |
| PC | Phycocyanin |
| PE | Phycoerythrin |
| PPB | Potassium phosphate buffer |
References
- Athiyappan, K.D.; Routray, W.; Paramasivan, B. Phycocyanin from Spirulina: A comprehensive review on cultivation, extraction, purification, and its application in food and allied industries. Food Humanit. 2024, 2, 100235. [Google Scholar] [CrossRef]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Saer, R.G.; Blankenship, R.E. Light harvesting in phototrophic bacteria: Structure and function. Biochem. J. 2017, 474, 2107–2131. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal Uses of Chlorophyll: A Critical Overview. In Chlorophyll: Structure, Production and Medicinal Uses, Online-ausg; Le, H., Salcedo, E., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; ISBN 978-1-62100-015-0. [Google Scholar]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. Potential applications of blue green algae. J. Sci. Ind. Res. India 2012, 71, 13–20. [Google Scholar]
- Kollmen, J.; Strieth, D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- Shahid, A.; Khan, A.Z.; Jabeen, F.; Liu, C.-G.; Asif, M.; Mehmood, M.A. Cyanobacteria-Based Biorefineries for a Sustainable Future of Bioindustry. In A Sustainable Green Future; Oncel, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 525–539. ISBN 978-3-031-24941-9. [Google Scholar]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Rippka, R.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Kollmen, J.; Rech, M.; Lorig, F.; Di Nonno, S.; Stiefelmaier, J.; Strieth, D. New easy lab methods for the extraction of phycobiliproteins and pigments from cyanobacteria. J. Appl. Phycol. 2025. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar] [CrossRef]
- Reed, R.H.; Warr, S.R.; Kerby, N.W.; Stewart, W.D. Osmotic shock-induced release of low molecular weight metabolites from free-living and immobilized cyanobacteria. Enzym. Microb. Technol. 1986, 8, 101–104. [Google Scholar] [CrossRef]
- Casteel, S.N.; Chien, S.H.; Gearhart, M.M. Field Evaluation of Ammonium Sulfate versus Two Fertilizer Products Containing Ammonium Sulfate and Elemental Sulfur on Soybeans. Commun. Soil Sci. Plant Anal. 2019, 50, 2941–2947. [Google Scholar] [CrossRef]
- Bakeer, S.M. Effect of ammonium nitrate fertilizer and calcium chloride foliar spray on fruit cracking and sunburn of Manfalouty pomegranate trees. Sci. Hortic. 2016, 209, 300–308. [Google Scholar] [CrossRef]
- Kim, I.S.; Nguyen, G.-H.; Kim, S.-Y.; Lee, J.-W.; Yu, H.-W. Evaluation of Methods for Cyanobacterial Cell Lysis and Toxin (Microcystin-LR) Extraction Using Chromatographic and Mass Spectrometric Analyses. Environ. Eng. Res. 2009, 14, 250–254. [Google Scholar] [CrossRef]
- Corbett, L.L.; Parker, D.L. Viability of lyophilized cyanobacteria (blue-green algae). Appl. Environ. Microbiol. 1976, 32, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Dincoglu, B.; Tensi, G.; Demirel, Z.; Imamoglu, E. Optimization of phycobiliprotein extraction from triple algal co-culture. Syst. Microbiol. Biomanufacturing 2025, 5, 326–334. [Google Scholar] [CrossRef]
- Julianti, E.; Susanti, S.; Singgih, M.; Mulyani, L.N. Optimization of Extraction Method and Characterization of Phycocyanin Pigment from Spirulina platensis. J. Math. Fund. Sci. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Pott, R.W.M. The release of the blue biological pigment C-phycocyanin through calcium-aided cytolysis of live Spirulina sp. Color. Technol. 2019, 135, 17–21. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and purification of phycobiliproteins from algae and their applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef]
- Doke, J.M. An Improved and Efficient Method for the Extraction of Phycocyanin from Spirulina sp. Int. J. Food Eng. 2005, 1. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of phycocyanin-A natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Kuhnholz, J.; Glockow, T.; Siebecke, V.; Le, A.T.; Tran, L.-D.; Noke, A. Comparison of different methods for extraction of phycocyanin from the cyanobacterium Arthrospira maxima (Spirulina). J. Appl. Phycol. 2024, 36, 1725–1735. [Google Scholar] [CrossRef]
- Lauceri, R.; Bresciani, M.; Lami, A.; Morabito, G. Chlorophyll a interference in phycocyanin and allophycocyanin spectrophotometric quantification. J. Limnol. 2015, 77, 169–177. [Google Scholar] [CrossRef]
- Tephly, T.R. The toxicity of methanol. Life Sci. 1991, 48, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Wessels, D.C.J.; Deutschewitz, K.; Dojani, S.; Reichenberger, H.; Büdel, B. Ecological characterization of soil-inhabiting and hypolithic soil crusts within the Knersvlakte, South Africa. Ecol. Process. 2013, 2, 231. [Google Scholar] [CrossRef]
- Pápista, É.; Ács, É.; Böddi, B. Chlorophyll-a determination with ethanol—A critical test. Hydrobiologia 2002, 485, 191–198. [Google Scholar] [CrossRef]
- Thao, N.T.; Binh, T.T.; Khanh Linh, P.T.; Son, V.H.; Minh Tu, N.T. Optimization of the extraction conditions for Chlorophyll a from fresh Spirulina. Food Sci. Appl. Biotechnol. 2022, 5, 99. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K. Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Exploration of marine genus Chroococcidiopsis sp.: A valuable source for antioxidant industry? J. Appl. Phycol. 2021, 33, 2169–2187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollmen, J.; Lorig, F.; Strieth, D. An Easy and Non-Hazardous Extraction Method for Phycobiliproteins and Pigments from Anabaena cylindrica. Phycology 2025, 5, 11. https://doi.org/10.3390/phycology5020011
Kollmen J, Lorig F, Strieth D. An Easy and Non-Hazardous Extraction Method for Phycobiliproteins and Pigments from Anabaena cylindrica. Phycology. 2025; 5(2):11. https://doi.org/10.3390/phycology5020011
Chicago/Turabian StyleKollmen, Jonas, Fabian Lorig, and Dorina Strieth. 2025. "An Easy and Non-Hazardous Extraction Method for Phycobiliproteins and Pigments from Anabaena cylindrica" Phycology 5, no. 2: 11. https://doi.org/10.3390/phycology5020011
APA StyleKollmen, J., Lorig, F., & Strieth, D. (2025). An Easy and Non-Hazardous Extraction Method for Phycobiliproteins and Pigments from Anabaena cylindrica. Phycology, 5(2), 11. https://doi.org/10.3390/phycology5020011






