Sexual Propagation in the Green Seaweed Codium tomentosum—An Emerging Species for Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Gametogenesis
2.2. Optimisation of Gamete Release
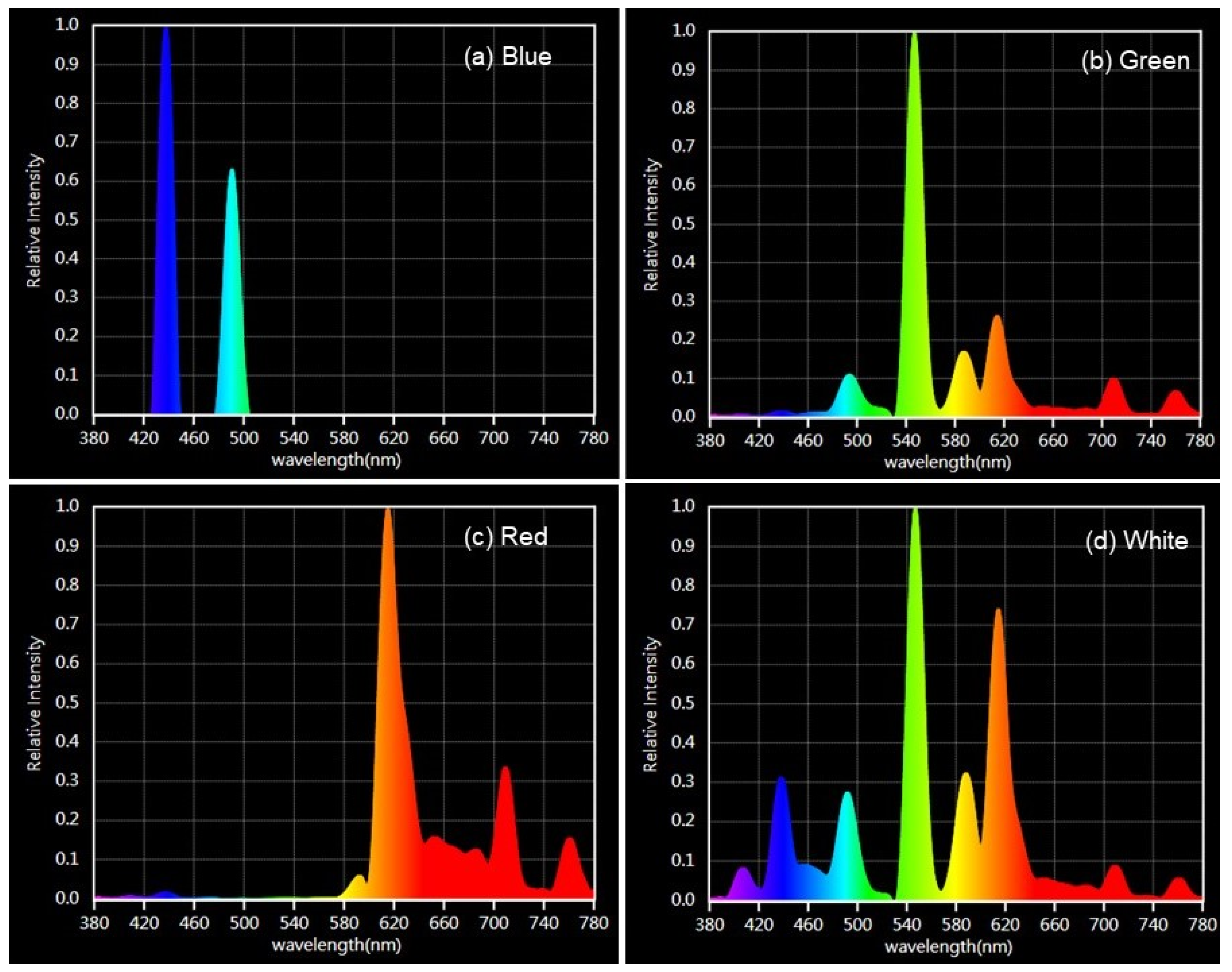
2.3. Light Intensity and Spectrum
2.4. Statistical Analysis
3. Results
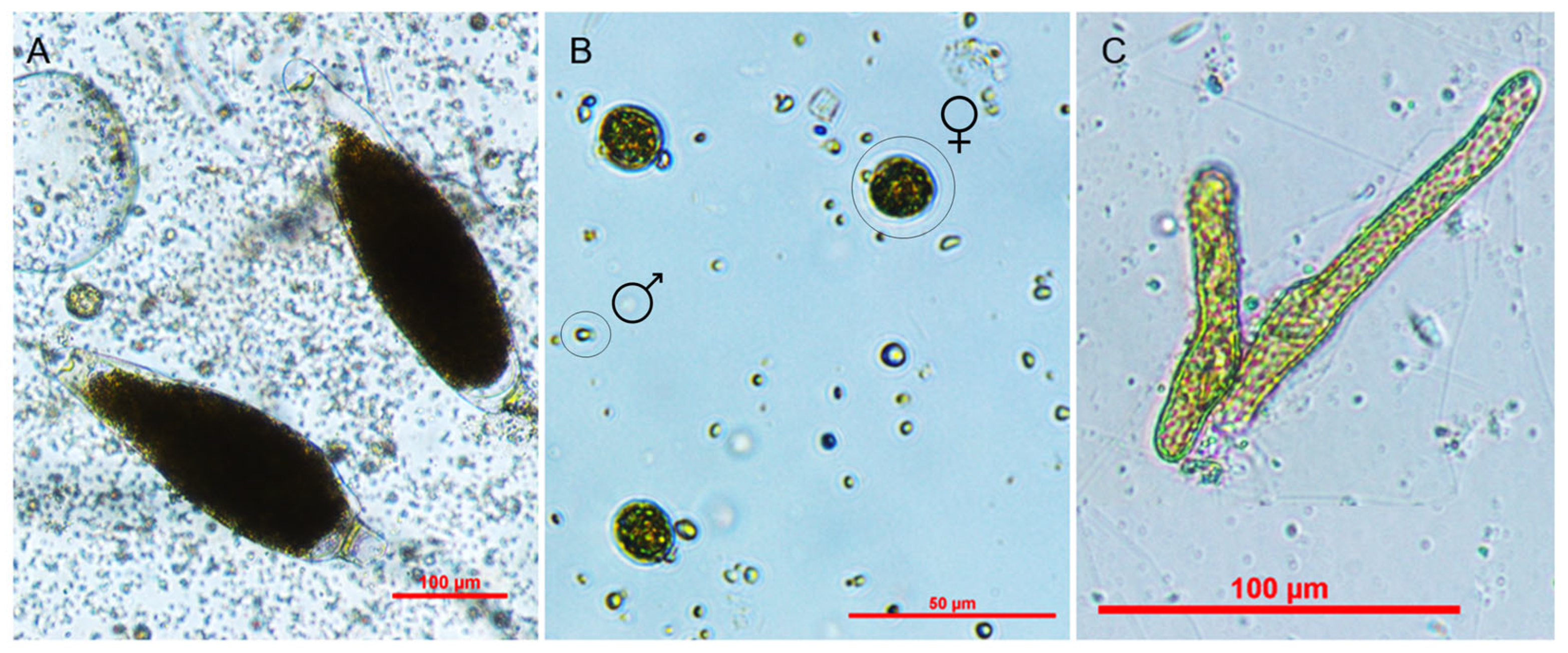
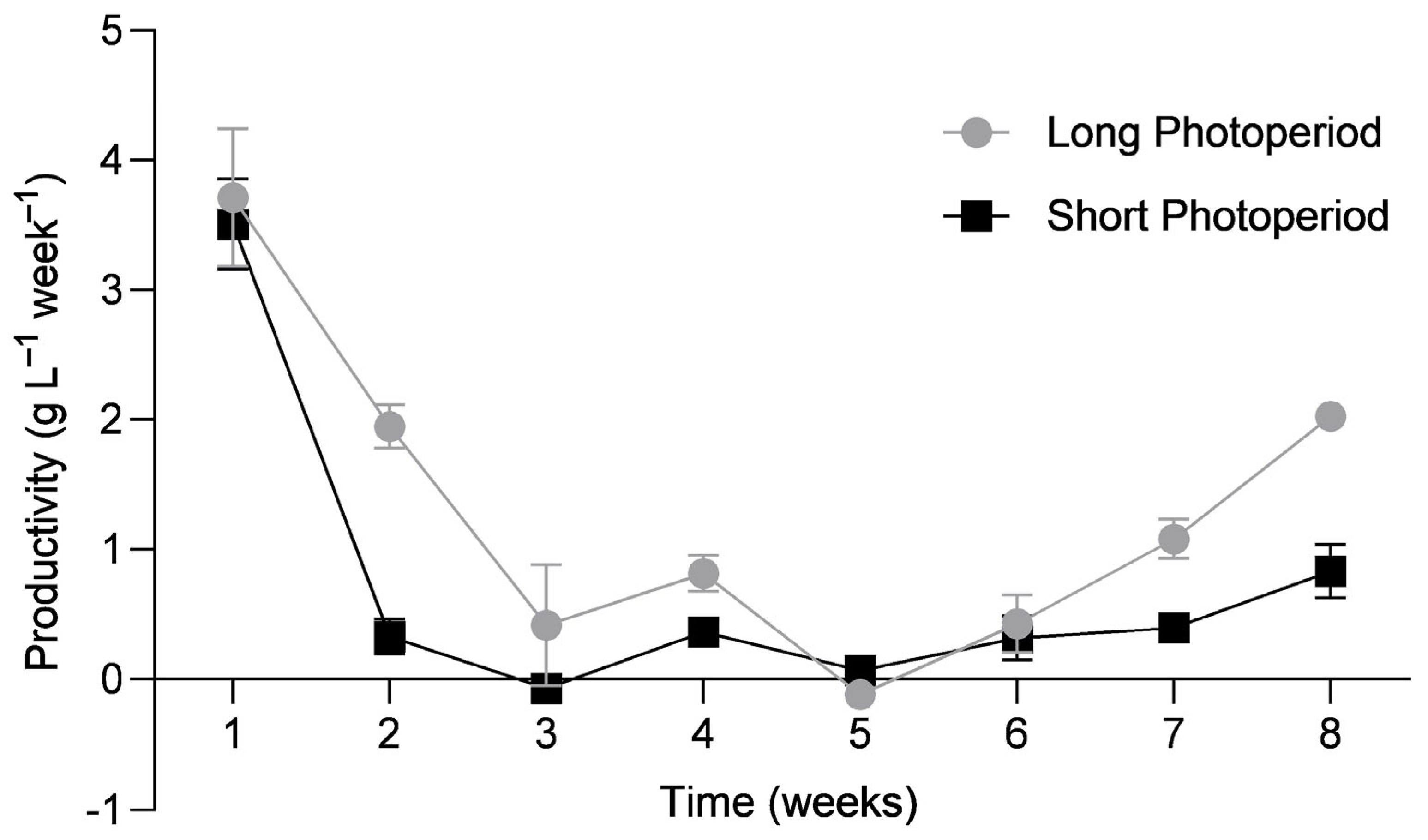
3.1. Induction of Gametogenesis
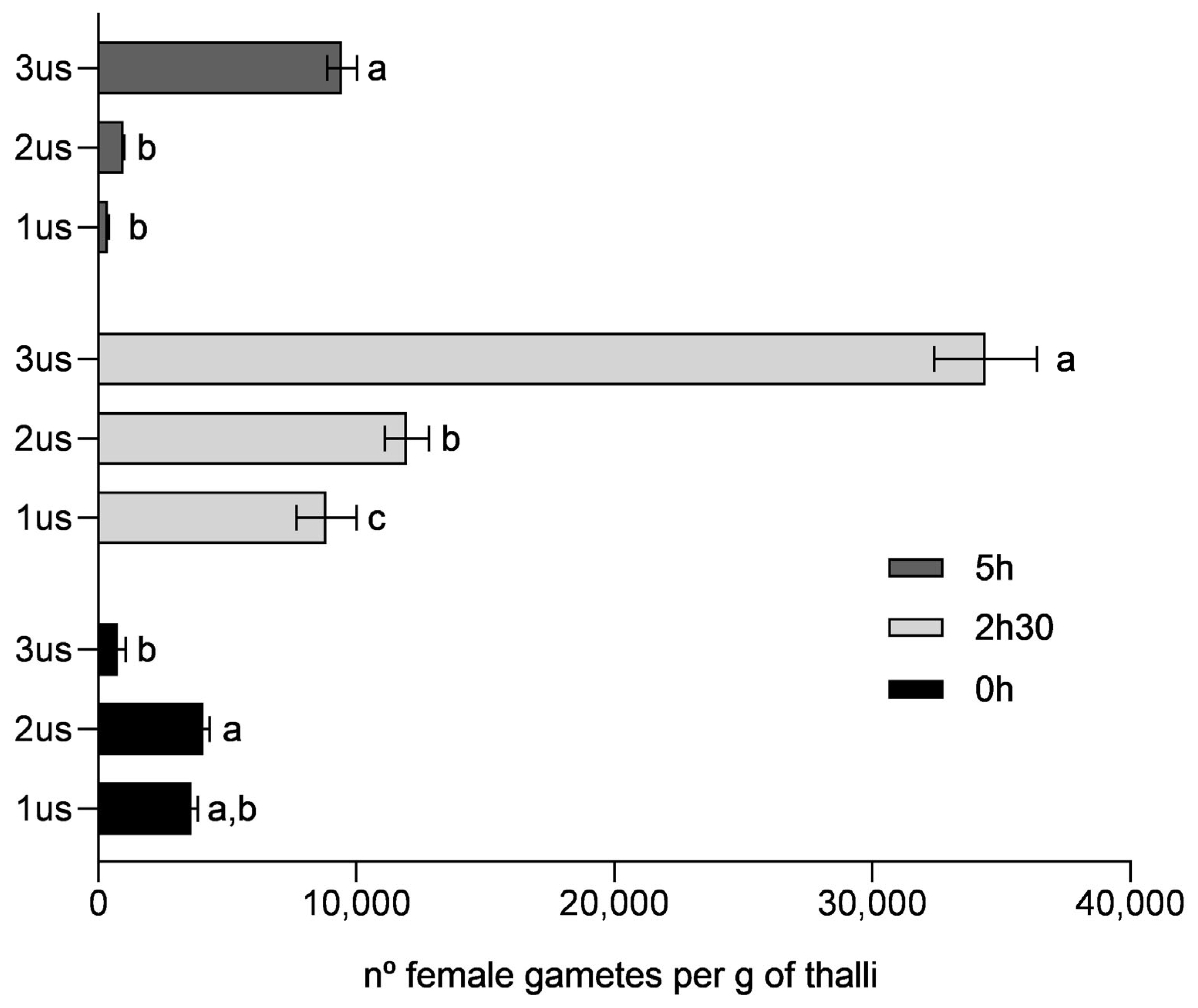
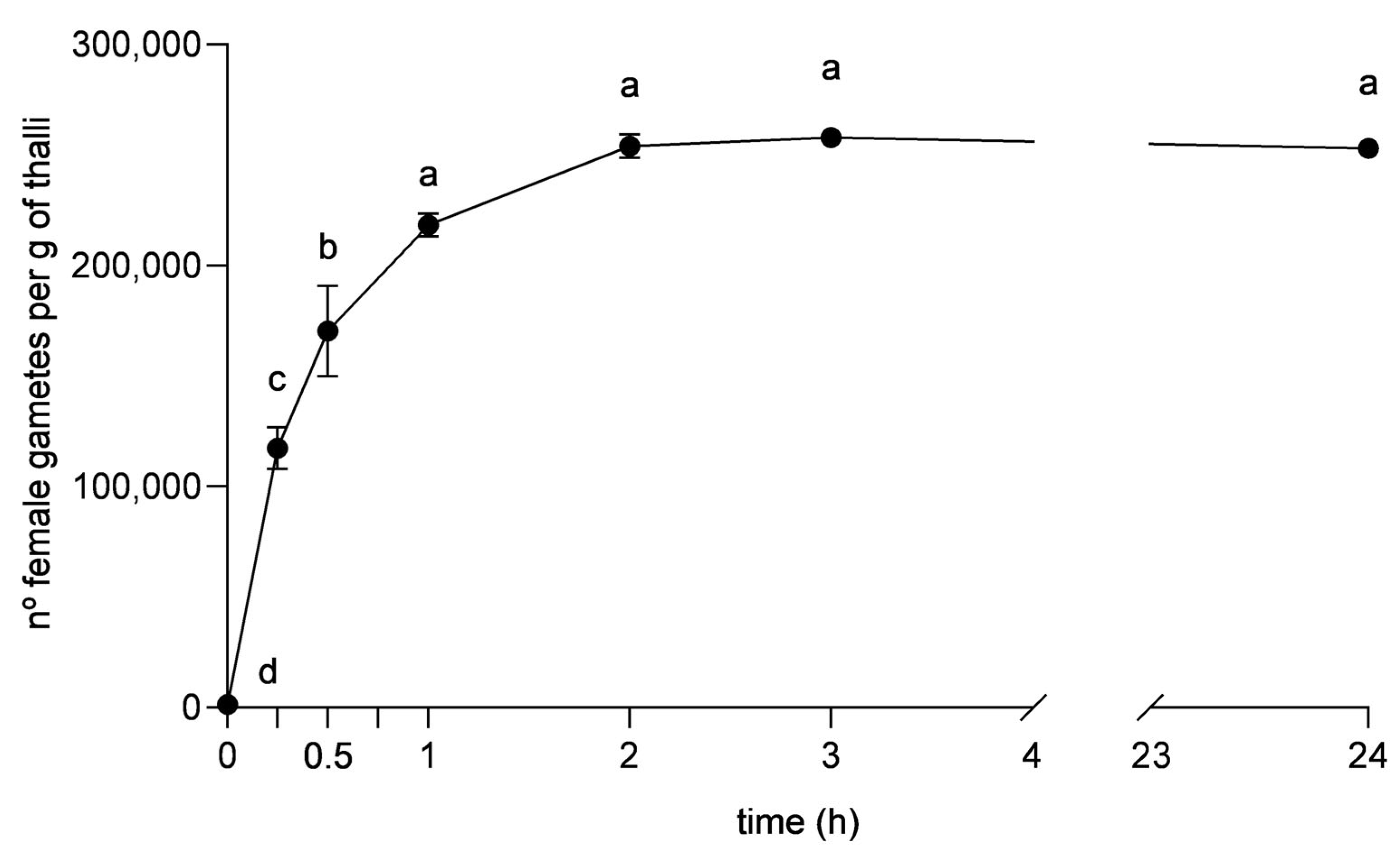
3.2. Optimisation of Gamete Release
3.3. Light Intensity and Spectrum
4. Discussion
4.1. Induction of Gametogenesis
4.2. Optimisation of Gamete Release
4.3. Light Intensity and Spectrum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.L.; Jacquemin, B.; Rebours, C. Pegasus—Phycommorph European Guidelines for Sustainable Aquaculture of Seaweeds. In Proceedings of the COST Action FA1406; Barbier, M., Charrier, B., Eds.; Roscoff, France, 2019. Available online: https://www.phycomorph.org/pegasus-phycomorph-european-guidelines-for-a-sustainable-aquaculture-of-seaweeds (accessed on 6 August 2024).
- Sugumaran, R.; Padam, B.S.; Yong, W.T.L.; Saallah, S.; Ahmed, K.; Yusof, N.A. A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects. Int. J. Environ. Res. Public Health 2022, 19, 7087. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The Underexplored Potential of Green Macroalgae in Aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Trowbridge, C.D.; Farnham, W.F.; White, L.F. Thriving Populations of the Native Macroalga Codium tomentosum on Guernsey Rocky Shores. J. Mar. Biol. Assoc. U. K. 2004, 84, 873–877. [Google Scholar] [CrossRef]
- Fernández, P.V.; Arata, P.X.; Ciancia, M. Polysaccharides from Codium Species: Chemical Structure and Biological Activity. Their Role as Components of the Cell Wall; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, ISBN 9780124080621. [Google Scholar]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta Macroalgae: A Source of Health Promoting Phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Moreira, A.S.P.; Alves, E.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Decoding Bioactive Polar Lipid Profile of the Macroalgae Codium tomentosum from a Sustainable IMTA System Using a Lipidomic Approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Hwang, E.K.; Park, C.S. Seaweed Cultivation and Utilization of Korea. Algae 2020, 35, 107–121. [Google Scholar] [CrossRef]
- Hwang, E.K.; Baek, J.M.; Park, C.S. Artificial Seed Production Using the Reproduction Methods in Codium fragile (Chlorophyta). Korean J. Fish. Aquat. Sci. 2005, 38, 164–171. [Google Scholar]
- Hwang, E.K.; Baek, J.M.; Park, C.S. Cultivation of the Green Alga, Codium fragile (Suringar) Hariot, by Artificial Seed Production in Korea. J. Appl. Phycol. 2008, 20, 469–475. [Google Scholar] [CrossRef]
- Nanba, N.; Kado, R.; Ogawa, H.; Nakagawa, T.; Sugiura, Y. Effects of Irradiance and Water Flow on Formation and Growth of Spongy and Filamentous Thalli of Codium fragile. Aquat. Bot. 2005, 81, 315–325. [Google Scholar] [CrossRef]
- Miravalles, A.B.; Leonardi, P.I.; Cáceres, E.J. Female Gametogenesis and Female Gamete Germination in the Anisogamous Green Alga Codium fragile subsp. novae-zelandiae (Bryopsidophyceae, Chlorophyta). Phycol. Res. 2012, 60, 77–85. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014; Volume 53, ISBN 9788578110796. [Google Scholar]
- Silva, P.C. The Dichotomous Species of Codium in Britain. J. Mar. Biol. Assoc. U. K. 1955, 34, 565–577. [Google Scholar] [CrossRef]
- Marques, R.; Cruz, S.; Calado, R.; Lillebø, A.; Abreu, H.; Pereira, R.; Pitarma, B.; da Silva, J.M.; Cartaxana, P. Effects of Photoperiod and Light Spectra on Growth and Pigment Composition of the Green Macroalga Codium tomentosum. J. Appl. Phycol. 2020, 33, 471–480. [Google Scholar] [CrossRef]
- Borden, C.A.; Stein, J.R. Reproduction and Early Development in Codium fragile (Suringar) Hariot: Chlorophyceae. Phycologia 1969, 8, 91–99. [Google Scholar] [CrossRef]
- Ramus, J. Differentiation of the Green Alga Codium fragile. Am. J. Bot. 1972, 59, 478. [Google Scholar] [CrossRef]
- Hanisak, M.D. Growth Patterns of Codium fragile ssp. tomentosoides in Response to Temperature, Irradiance, Salinity, and Nitrogen Source. Mar. Biol. 1979, 50, 319–332. [Google Scholar] [CrossRef]
- Churchill, A.C.; Moeller, H.W. Seasonal Patterns of Reproduction in New York Populations of Codium fragile (Sur.) Hariot subsp. tomentosoides (Van Goor) Silva. J. Phycol. 1972, 8, 147–152. [Google Scholar] [CrossRef]
- Bast, F. An Illustrated Review on Cultivation and Life History of Agronomically Important Seaplants. In Seaweed: Mineral Composition, Nutritional and Antioxidant Benefits and Agricultural Uses; Nova Publishers: New York, NY, USA, 2014; pp. 39–70. ISBN 9781631175756. [Google Scholar]
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The Cultivation of European Kelp for Bioenergy: Site and Species Selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper 441; FAO: Rome, Italy, 2003; p. 105. [Google Scholar]
- Santelices, B. Patterns of Reproduction; Dispersal and Recruitment in Seaweeds. Eanography Mar. Biol. 1990, 28, 177–276. [Google Scholar]
- Dring, M.J. Photoperiodism and Phycology. Prog. Phycol. Res. 1984, 3, 159–192. [Google Scholar]
- Dring, M.J. Photocontrol of Development in Algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 174. [Google Scholar] [CrossRef]
- Brawley, S.H.; Johnson, L.E. Garnet Ogenesis, Gametes and Zygotes: An Ecological Perspective on Sexual Reproduction in the Algae. Br. Phycol. J. 1992, 27, 233–252. [Google Scholar] [CrossRef]
- Sá, M.F. Reproductive Status and Early Stages of Development in the Green Macroalgae Codium tomentosum. Master’s Thesis, University of Porto, Porto, Portugal, 2021. Available online: https://hdl.handle.net/10216/138709 (accessed on 1 June 2024).
- Pacheco, T. Propagation of Macroalga Codium tomentosum for Aquaculture Production. Master’s Thesis, University of Porto, Porto, Portugal, 2022. Available online: https://hdl.handle.net/10216/146493 (accessed on 1 June 2024).
- Park, C.S.; Soth, C.H. Effects of Light and Temperature on Morphogenesis of Codium fragile (Suringar) Hariot in Laboratory Culture. Korean J. Phycol. 1992, 7, 213–223. [Google Scholar]
- Cabral, J.P. Characterization and Multivariate Analysis of Patella intermedia, Patella ulyssiponensis and Patella vulgata from Povoa de Varzim (Northwest Portugal). Iberus 2003, 21, 1–17. [Google Scholar]
- Harrison, P.J.; Berges, J.A. Marine Culture Media. In Algal Culturing Techniques; Academic Press: Cambridge, MA, USA, 2005; pp. 21–33. [Google Scholar] [CrossRef]
- Marques, R.; Moreira, A.; Cruz, S.; Calado, R.; Cartaxana, P. Controlling Light to Optimize Growth and Added Value of the Green Macroalga Codium tomentosum. Front. Mar. Sci. 2022, 9, 906332. [Google Scholar] [CrossRef]
- Huo, Y.; Kim, J.K.; Yarish, C.; Augyte, S.; He, P. Responses of the Germination and Growth of Ulva prolifera Parthenogametes, the Causative Species of Green Tides, to Gradients of Temperature and Light. Aquat. Bot. 2021, 170, 103343. [Google Scholar] [CrossRef]
- Liu, X.; Bogaert, K.; Engelen, A.H.; Leliaert, F.; Roleda, M.Y.; De Clerck, O. Seaweed Reproductive Biology: Environmental and Genetic Controls. Bot. Mar. 2017, 60, 89–108. [Google Scholar] [CrossRef]
- Martins, N.; Tanttu, H.; Pearson, G.A.; Bartsch, I. Interactions of Daylength, Temperature and Nutrients Affect Thresholds for Life Stage Transitions in the Kelp Laminaria digitata (Phaeophyceae ). Bot. Mar. 2017, 60, 109–121. [Google Scholar] [CrossRef]
- Balar, N.; Jaiswar, S.; Mantri, V.A. Phycological Society Effects of Extrinsic Abiotic Factors on Induction of Gametogenesis and Efficacy of a Device for the Segregation of Non-Fused Gametes and Zygotes in the Green Alga Ulva lactuca. Appl. Phycol. 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Khoeyi, Z.A.; Seyfabadi, J.; Ramezanpour, Z. Effect of Light Intensity and Photoperiod on Biomass and Fatty Acid Composition of the Microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Streckaite, S.; Llansola-Portoles, M.J.; Pascal, A.A.; Ilioaia, C.; Gall, A.; Seki, S.; Fujii, R.; Robert, B. Pigment Structure in the Light-Harvesting Protein of the Siphonous Green Alga Codium fragile. Biochim. Biophys. Acta-Bioenerg. 2021, 1862, 148384. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, M.L.; Valenzuela, P.; Gaitán-Espitia, J.D.; Destombe, C. Evidence of Reproductive Cost in the Triphasic Life History of the Red Alga Gracilaria chilensis (Gracilariales, Rhodophyta). J. Appl. Phycol. 2014, 26, 569–575. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, Q.; Liu, S.; Zhang, S.; Tang, Y.; Lu, Z.; Yu, Y. Trade-off between Vegetative Regeneration and Sexual Reproduction of Sargassum thunbergii. Hydrobiologia 2011, 678, 127–135. [Google Scholar] [CrossRef]
- Lüning, K.; Dieck, I.T. Environmental Triggers in Algal Seasonality. Bot. Mar. 1989, 32, 389–398. [Google Scholar] [CrossRef]
- Tala, F.; Chow, F. Phenology and Photosynthetic Performance of Porphyra spp. (Bangiophyceae, Rhodophyta): Seasonal and Latitudinal Variation in Chile. Aquat. Bot. 2014, 113, 107–116. [Google Scholar] [CrossRef]
- Miravalles, A.B.; Leonardi, P.I.; Cáceres, E.J. Male Gametogenesis Ultrastructure of Codium fragile subsp. Novae-Zelandiae (Bryopsidophyceae, Chlorophyta). Phycologia 2011, 50, 370–378. [Google Scholar] [CrossRef]
- Prince, J.S.; Trowbridge, C.D. Reproduction in the Green Macroalga Codium (Chlorophyta): Characterization of Gametes. Bot. Mar. 2004, 47, 461–470. [Google Scholar] [CrossRef]
- McArthur, D.M.; Moss, B.L. Gametogenesis and Gamete Structure of Enteromorpha intestinalis (L.) Link. Br. Phycol. J. 1979, 14, 43–57. [Google Scholar] [CrossRef]
- Wheeler, A.E.; Page, J.Z. The Ultrastructure of Derbesia tenuissima (de Notaris) Crouan. I. Organization of the Gametophyte Protoplast, Gametangium, and Gametangial PORE1, 2, 3. J. Phycol. 1974, 10, 336–352. [Google Scholar] [CrossRef]
- Moeller, H. Ecology and Life History of Codium fragile subsp. tomentosoides; Rutgers: Newark, NJ, USA, 1969; p. 258. [Google Scholar]
- Hanisak, M.D. Nitrogen Limitation of Codium fragile ssp. tomentosoides as Determined by Tissue Analysis. Mar. Biol. 1979, 50, 333–337. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for Microalgal Cell Disruption and Product Extraction: A Review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of Osmotic Dehydration and Ultrasound Pre-Treatment on Cell Structure: Melon Dehydration. LWT 2008, 41, 604–610. [Google Scholar] [CrossRef]
- Oltmanns, F. Morphologie Und Biologie Der Algen: Chrysophyceae—Chlorophyceae, 2nd ed.; Gustav Fischer: Jena, Germany, 1922. [Google Scholar] [CrossRef][Green Version]
- Berthold, G. Zur Kenntnis Der Siphoneen Und Bangiaceen. Mitt. Zool. Sta. Neapel. 1881, 2, 72–82. [Google Scholar]
- Prince, J.S. Sexual Reproduction in Codium fragile subsp. tomentosoides (Chlorophyceae) from the Northeast Coast of North America. J. Phycol. 1988, 24, 112–114. [Google Scholar] [CrossRef]
- Leukart, P.; Lüning, K. Minimum Spectral Light Requirements and Maximum Light Levels for Long-Term Germling Growth of Several Red Algae from Different Water Depths and a Green Alga. Eur. J. Phycol. 1994, 29, 103–112. [Google Scholar] [CrossRef]
- Melo, R.; Sousa-pinto, I.; Antunes, S.C.; Costa, I. Temporal and Spatial Variation of Seaweed Biomass and Assemblages in Northwest Portugal. J. Sea Res. 2021, 174, 102079. [Google Scholar] [CrossRef]
- Seki, S.; Yamano, Y.; Oka, N.; Kamei, Y.; Fujii, R. Discovery of a Novel Siphonaxanthin Biosynthetic Precursor in Codium fragile That Accumulates Only by Exposure to Blue-Green Light. FEBS Lett. 2022, 596, 1544–1555. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Aguilera, J.; Niell, F.X. Red and Blue Light Regulation of Growth and Photosynthetic Metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur. J. Phycol. 1995, 30, 11–18. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue Light Reduces Photosynthetic Efficiency of Cyanobacteria through an Imbalance between Photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef]
- Ramus, J. Seaweed Anatomy and Photosynthetic Performance: The Ecological Significance of Light Guides, Heterogeneous Absorption Ans Multiple Scatter. J. Phycol. 1978, 14, 352–362. [Google Scholar] [CrossRef]
- Uragami, C.; Galzerano, D.; Gall, A.; Shigematsu, Y.; Meisterhans, M.; Oka, N.; Iha, M.; Fujii, R.; Robert, B.; Hashimoto, H. Light-Dependent Conformational Change of Neoxanthin in a Siphonous Green Alga, Codium intricatum, Revealed by Raman Spectroscopy. Photosynth. Res. 2014, 121, 69–77. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, M.F.; Pacheco, T.C.; Sousa-Pinto, I.; Marinho, G.S. Sexual Propagation in the Green Seaweed Codium tomentosum—An Emerging Species for Aquaculture. Phycology 2024, 4, 533-547. https://doi.org/10.3390/phycology4040029
Sá MF, Pacheco TC, Sousa-Pinto I, Marinho GS. Sexual Propagation in the Green Seaweed Codium tomentosum—An Emerging Species for Aquaculture. Phycology. 2024; 4(4):533-547. https://doi.org/10.3390/phycology4040029
Chicago/Turabian StyleSá, Maria Francisca, Teresa Cunha Pacheco, Isabel Sousa-Pinto, and Gonçalo Silva Marinho. 2024. "Sexual Propagation in the Green Seaweed Codium tomentosum—An Emerging Species for Aquaculture" Phycology 4, no. 4: 533-547. https://doi.org/10.3390/phycology4040029
APA StyleSá, M. F., Pacheco, T. C., Sousa-Pinto, I., & Marinho, G. S. (2024). Sexual Propagation in the Green Seaweed Codium tomentosum—An Emerging Species for Aquaculture. Phycology, 4(4), 533-547. https://doi.org/10.3390/phycology4040029





