Biodiversity and Reproductive Status of Beach-Cast Seaweeds from Espírito Santo, Southeastern Brazil: Sustainable Use and Conservation
Abstract
1. Introduction
2. Materials and Methods
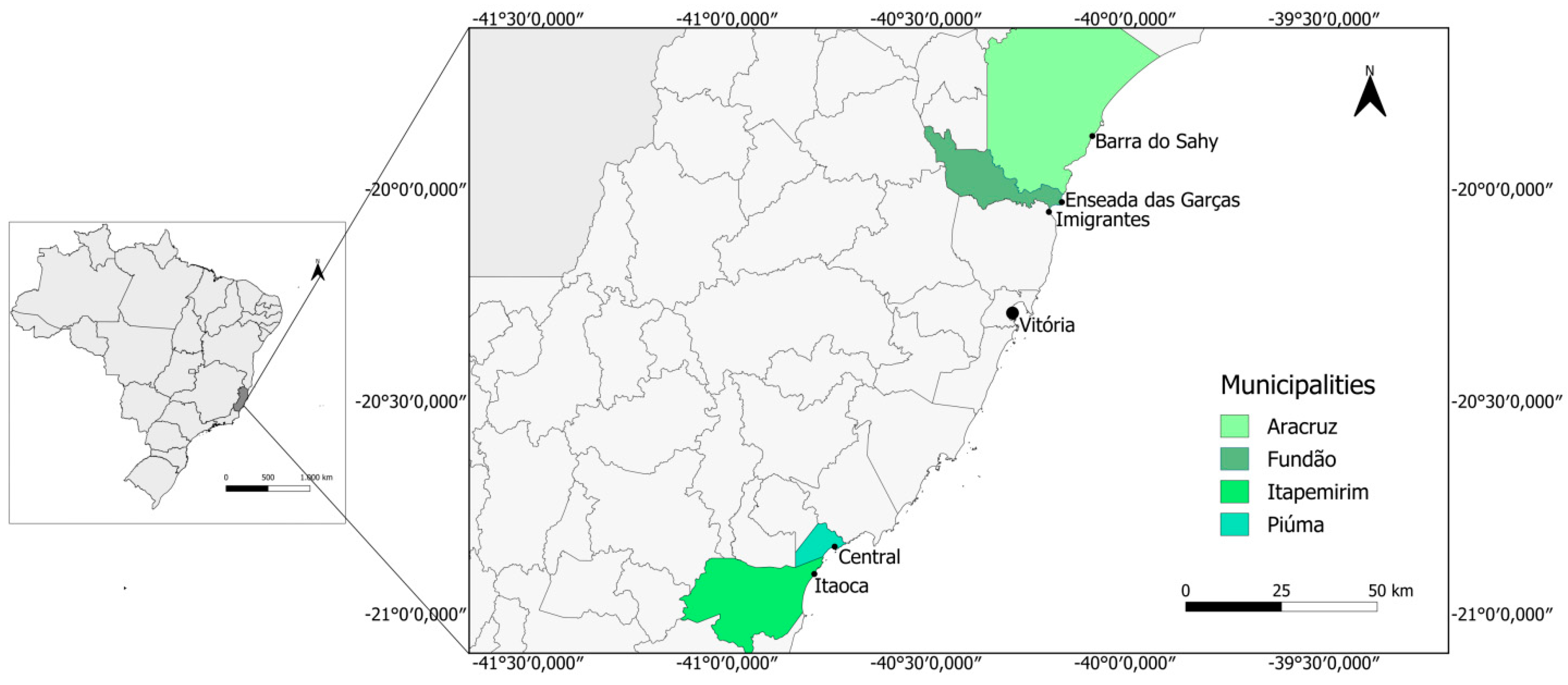
2.1. Study Area and Sampling
2.2. Taxonomic Analysis and Assessment of Reproductive Status
2.3. Statistical Analyses
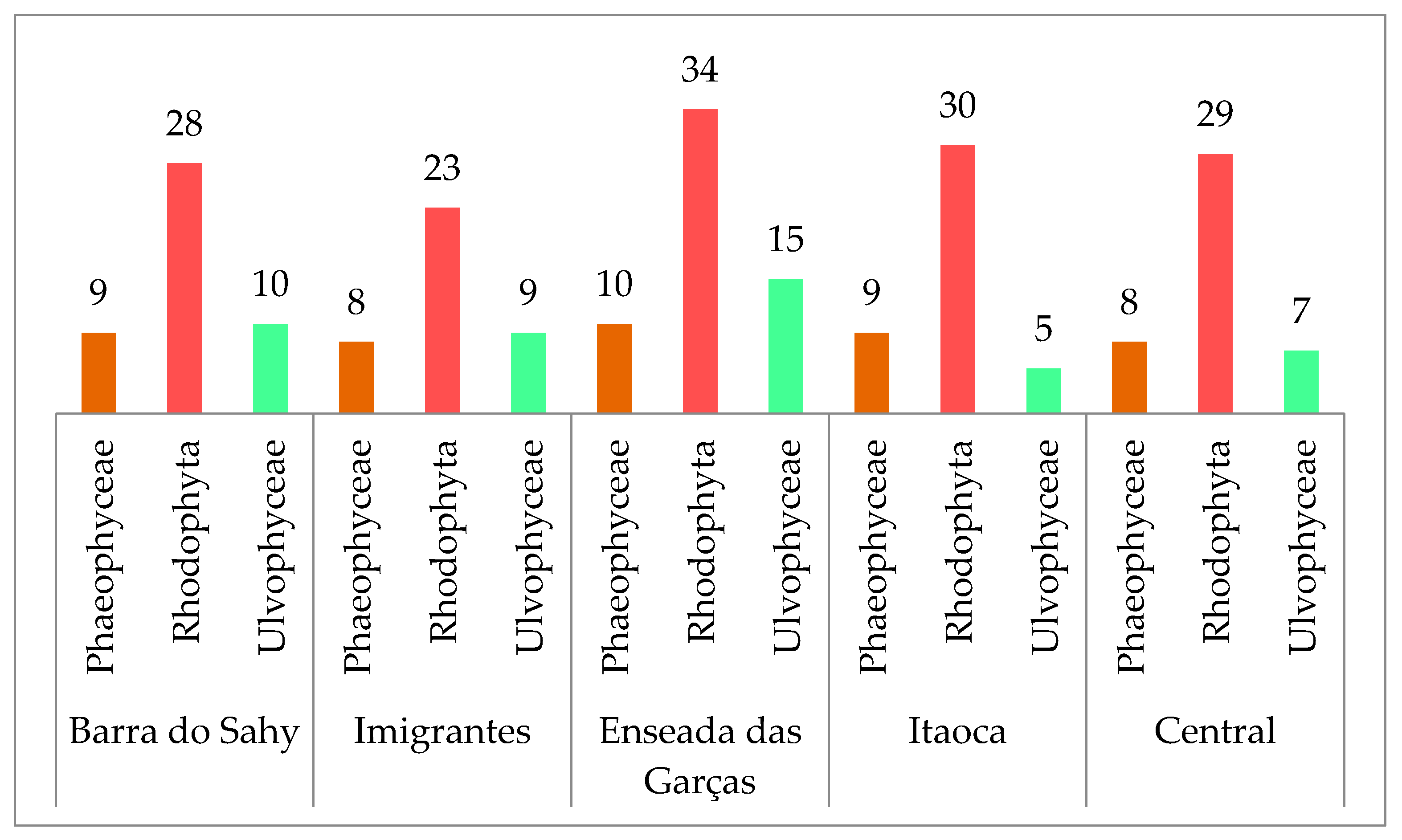
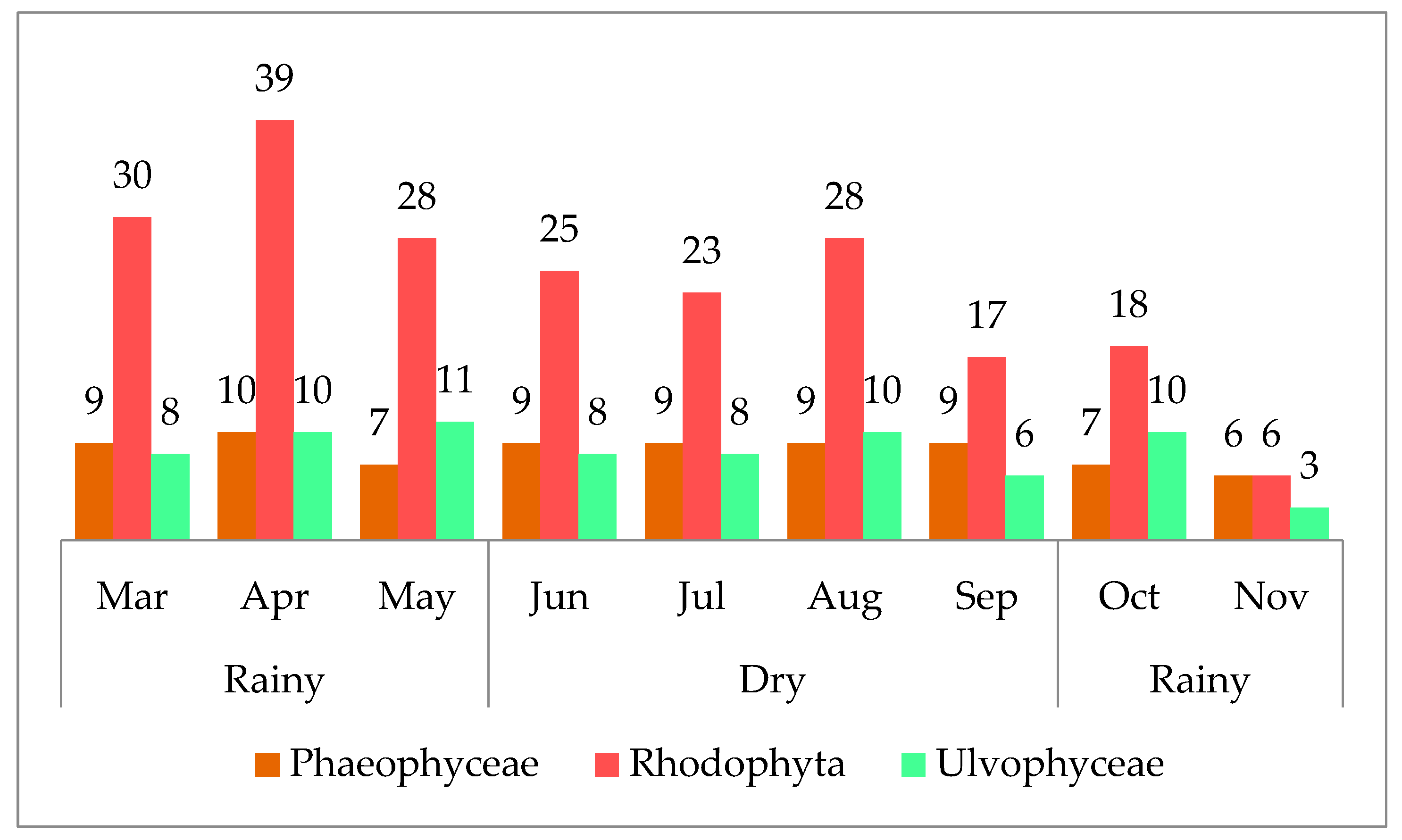
3. Results
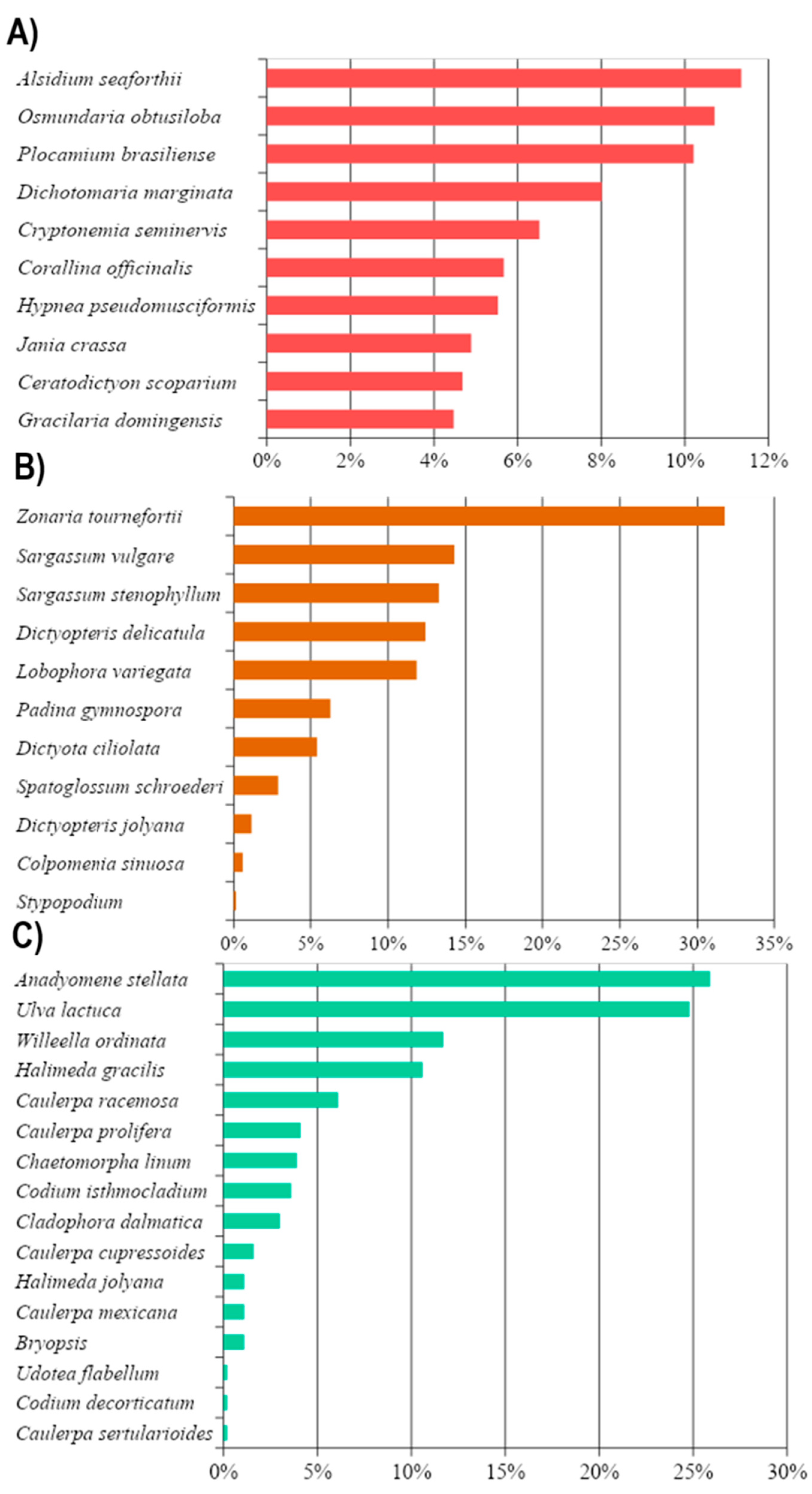
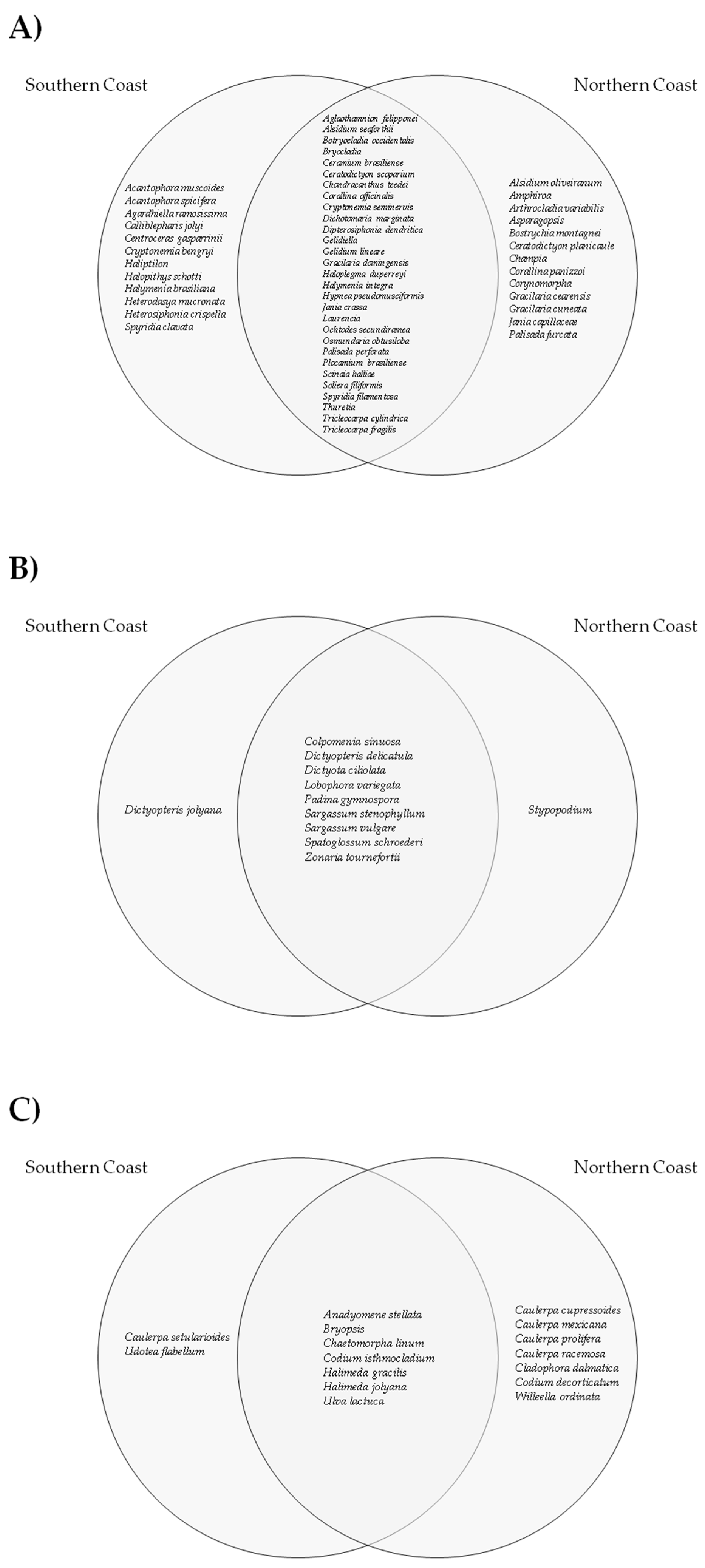
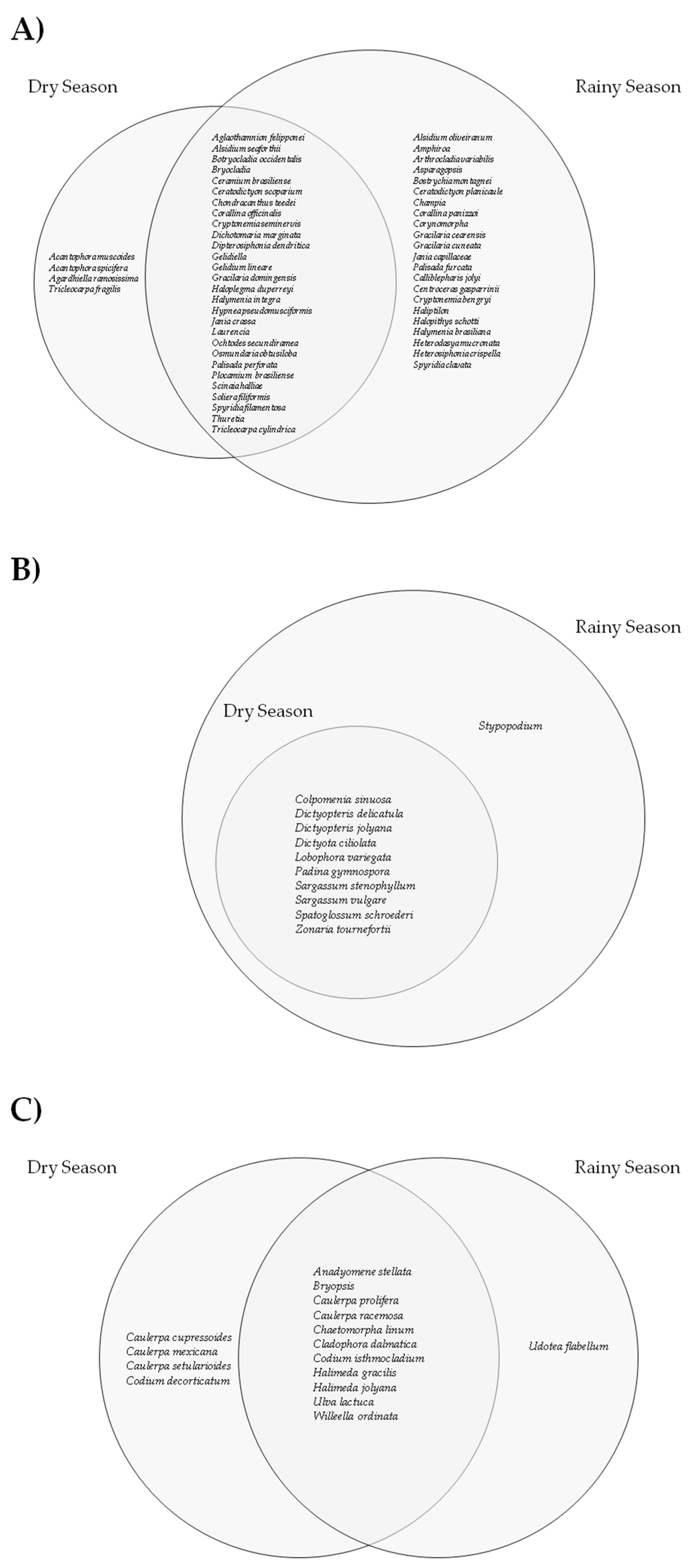
3.1. Taxonomic Data
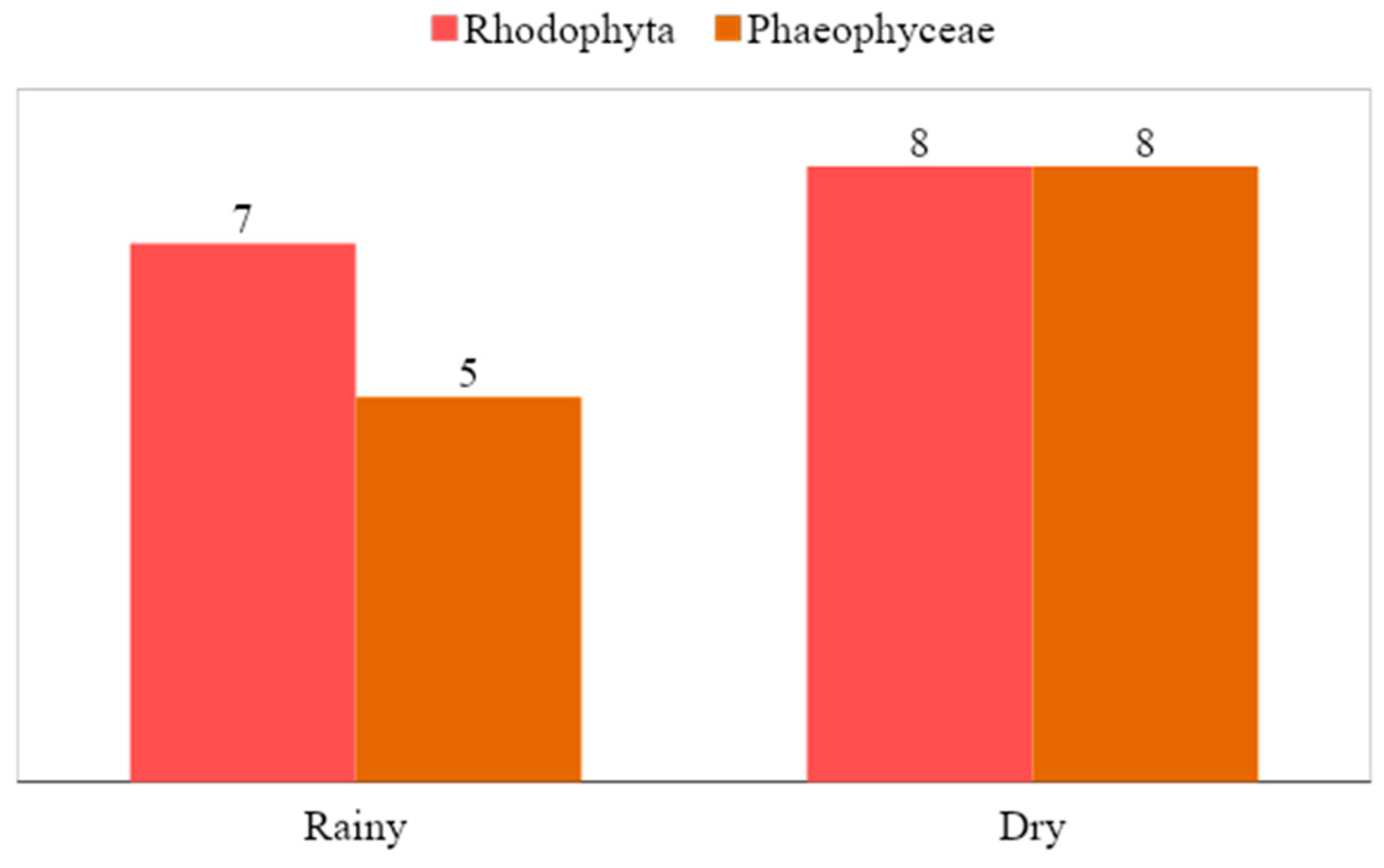
3.2. Reproductive Structures
4. Discussion
5. Conclusions
- Beach-cast macroalgae occur throughout the year, with higher richness during the rainy season;
- Temporal factors influence the occurrence and composition of stranded macroalgae, with the rainy season exhibiting greater richness;
- The taxonomic composition of the stranded macroalgae did not differ significantly between the northern and southern coasts of Espírito Santo;
- Stranded macroalgae along the coast of Espírito Santo do not appear to play a substantial role in the production and supply of propagules for their respective populations;
- Most species with reproductive structures did not show significant spatial or temporal patterns.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menezes, M.; Bicudo, C.E.M.; Moura, C.W.N.; Alves, A.M.; Santos, A.A.; Pedrini, A.G.; Araújo, A.; Tucci, A.; Fajar, A.; Malone, C.; et al. Update of the Brazilian floristic list of Algae and Cyanobacteria. Rodriguésia 2015, 66, 1047–1062. [Google Scholar] [CrossRef]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. 2022. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 20 January 2024).
- Bicudo, C.E.M.; Menezes, M. Introdução: As algas do Brasil. In Catálogo de Plantas e Fungos do Brasil; Forzza, R.C., Ed.; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Andrea Jakobsson Estúdio: Rio de Janeiro, Brazil, 2010; Volume 1, pp. 49–60. [Google Scholar]
- Cavalcanti, M.I.L.G.; Fujii, M.T. Macroalgas Arribadas da Costa Brasileira: Biodiversidade e Potencial de Aproveitamento, 1st ed.; Editora CRV: Curitiba, Brazil, 2021. [Google Scholar]
- Horta, P.A.; Amâncio, E.; Coimbra, C.S.; Oliveira, E.C. Considerações sobre a distribuição e origem da flora de macroalgas marinhas brasileiras. Hoehnea 2001, 28, 243–265. [Google Scholar]
- Abreu, S.F. Feições morfológicas e demográficas do litoral do Espírito Santo. Rev. Bras. De Geogr. 1943, 5, 215–234. [Google Scholar]
- Mabesoone, J.M.; Coutinho, P.N. Littoral and shallow marine geology of northern and northeastern Brazil. Trab. Ocean 1970, 12, 214. [Google Scholar] [CrossRef]
- Petri, S.; Fúlfaro, V.J. Geologia Do Brasil; Queiroz, T.A., Ed.; University of São Paulo: São Paulo, Brazil, 1983; 631p. [Google Scholar]
- Suguio, K.; Tessler, M.G. Planícies de cordões litorâneos quaternários do Brasil: Origem e nomenclatura. In Restingas, Origem, Estrutura, Processos; UFF: Niteroi, Brazil, 1984; 477p. [Google Scholar]
- Amado-Filho, G.M.; Pereira-Filho, G.H. Rhodolith beds in Brazil: A new potential habitat for marine bioprospection. Rev. Bras. De Farmacogn. 2012, 22, 782–788. [Google Scholar] [CrossRef]
- Guimarães, S.M.P.B. Uma análise da diversidade da flora marinha bentônica do estado do Espirito Santo, Brasil. Hoehnea 2003, 30, 11–19. [Google Scholar]
- Guimarães, S.M.P.B. A revised checklist of benthic marine Rhodophyta from the State of Espírito Santo, Brazil. Bol. Inst. Botânica 2006, 17, 143–194. [Google Scholar]
- Harb, T.B.; Chow, F. An overview of stranded seaweeds: Potential and opportunities for the valorization of underused waste biomass. Algal Res. 2022, 62, 102643. [Google Scholar] [CrossRef]
- Basilio, T.H.; Chow, F.; Harb, T.B.; Soares, L.P.; Araújo, P.G.; Machado, L.P.; Hohn, G.R.; Dos Santos, I.L.F.; Mattos, H.L.; Fujii, M.T. Algas Marinhas IN: Biodiversidade e Conservação Das Ilhas Costeiras do Litoral Sul Capixaba; Lura Editorial: São Paulo, Brazil, 2020; 252p. [Google Scholar]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef]
- Széchy, M.T.; Guedes, P.M.; Baeta-Neves, M.H.; Oliveira, E.N. Verification of Sargassum natans (Linnaeus) Gaillon (Heterokontophyta: Phaeophyceae) from the Sargasso Sea off the coast of Brazil, western Atlantic Ocean. Check List 2012, 8, 638–641. [Google Scholar] [CrossRef]
- Arita, J.T.; Iporac, L.A.; Bally, N.K.; Fujii, M.T.; Collado-Vides, L. Integrative literature analysis of Holopelagic Sargassum (sargasso) in the Western Atlantic (2011–2022): Status, trends, and gaps. Phycology 2023, 3, 447–458. [Google Scholar] [CrossRef]
- Sissini, M.N.; de Barros Barreto, M.B.; Széchy, M.T.; de Lucena, M.B.; Oliveira, M.C.; Gower, J.; Liu, G.; de Oliveira Bastos, E.; Milstein, D.; Gusmão, F.; et al. The floating Sargassum (Phaeophyceae) of the South Atlantic Ocean—Likely scenarios. Phycologia 2017, 56, 321–328. [Google Scholar] [CrossRef]
- Cavalcanti, M.I.L.G.; Fujii, M.T. Stranded seaweeds from Itaqui Beach, coast of the State of Piauí, Northeast of Brazil. Pesqui. Botânica 2021, 75, 381–414. [Google Scholar]
- Harb, T.B.; Pereira, M.S.; Cavalcanti, M.I.L.G.; Fujii, M.T.; Chow, F. Antioxidant activity and related chemical composition of extracts from Brazilian stranded marine algae: Opportunities of turning a waste into a resource. J. Appl. Phycol. Dordr. 2021, 33, 1–13. [Google Scholar]
- Câmara-Neto, C. Contribuição ao conhecimento qualitativo e quantitativo das “arribadas” da Redinha. Bol. Inst. Biol. Mar. 1971, 5, 3–30. [Google Scholar]
- Praciano, P.R.S. Composição e Estimativa das Algas Depositadas em Praias do ESTADO do CEARÁ, no Período de Julho de 1976 a Junho de 1977; Monografia de Bacharelado em Engenharia de Pesca; Universidade Federal do Ceará: Fortaleza, Brazil, 1977; 14p. [Google Scholar]
- Câmara-Neto, C.; Araújo, R.A.; Melo Filho, N.R.; Soares, M.L.; Costa, P.N. Composição e estimativa da biomassa das algas arribadas, em praias do Rio Grande do Norte. SUDENE Estud. Pesca 1981, 9, 85–95. [Google Scholar]
- Guedes, E.A.C.; Moura, A.N. Estudos da biomassa e composição mineral de algas arribadas em praias do litoral norte de Alagoas. Bol. Estud. Ciências Mar. 1996, 9, 19–30. [Google Scholar]
- Ferreira, G.S.; Brito, P.O.B.; Aderaldo, F.I.C.; Carneiro, P.B.M.; Rocha, A.M.; Gondim, F.A. Algas arribadas da Praia do Pacheco, Ceará, Brasil. Rev. Verde Agroecol. E Desenvolv. Sustentável 2020, 15, 208–214. [Google Scholar] [CrossRef]
- Cavalcanti, M.I.L.G.; Sánchez, P.M.G.; Fujii, M.T. Comparison of the diversity and biomass of beach-cast seaweeds from NE and Se Brazil. Eur. J. Phycol. 2022, 57, 367–376. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Santos, J.P.; Torres, P.; Amorim, A.M.; da Silva BN, T.; dos Santos DY, A.C.; Chow, F. Biostimulant potential of Brazilian macroalgae: Seasonal variations and effects on early growth and germination of lettuce. Braz. J. Bot. 2023, 46, 767–774. [Google Scholar] [CrossRef]
- Araújo, N.H. Avaliação de Produção de Compostos a Partir de Algas Marinhas Arribadas Como Alternativa Para a Adubação de Hortaliças. Dissertação de Mestrado, UFPB, Programa de Desenvolvimento em Meio Ambiente–PRODEMA, Paraíba, Brasil, 2016. [Google Scholar]
- Mandalka, A.; Cavalcanti, M.I.; Harb, T.B.; Fujii, M.T.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional composition of stranded marine algae from the Brazilian coast: Added value for algal biomass considered as waste. Foods 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.; Kendrick, G.A. Ecological significance and commercial harvesting of drifting and stranded macroalgae and seagrasses in Australia: A review. J. Appl. Phycol. 1997, 9, 311–326. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Sanchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Mancini Filho, J.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef]
- McKenzie, P.F.; Bellgrove, A. Dispersal of Hormosira banksii (Phaeophyceae) via detached fragments: Reproductive viability and longevity. J. Phycol. 2008, 44, 1108–1115. [Google Scholar] [CrossRef]
- Ulaski, B.P.; Otis, E.O.; Konar, B. How landscape variables influence the relative abundance, composition, and reproductive viability of macroalgal wrack in a high latitude glacial estuary. Estuar. Coast. Shelf Sci. 2023, 280, 108169. [Google Scholar] [CrossRef]
- Feldmann, J. Recherches sur la végétation marine de la Méditerranée. La Côte des Albères. Rev. Algol. 1937, 10, 1–339. [Google Scholar]
- Cheney, D.P. R and C/P-new and improved ratio for comparing seaweed floras. J. Phycol. 1977, 13, 12. [Google Scholar]
- Boltovskoy, D.; Valentin, J.L. Overview of the history of biological oceanography in the southwestern Atlantic, with emphasis on plankton. In Plankton Ecology of the Southwestern Atlantic: From the Subtropical to the Subantarctic Realm; Springer: Cham, Switzerland, 2018; pp. 3–34. [Google Scholar] [CrossRef]
- Basilio, T.H. Unidades Ambientais e a Pesca Artesanal em Piúma, Espí-Rito Santo, Brasil, 1st ed.; Laura Editorial: São Paulo, Brazil, 2016. [Google Scholar]
- Bernardi, J. Variação da Atividade Antioxidante em Macroalgas Recifais de Pernambuco. Dissertação de Mestrado, Universidade Federal de Pernambuco Centro de Tecnologia e Geociências, Recife, Brazil, 2018; 140p. [Google Scholar]
- Pereira, L.M. Biodiversidade de Algas Marinhas Arribadas na Praia de Candeias-PE e Avaliação do Potencial Como Substrato Para Cultivo de Hortaliças Microverdes. Dissertação de Mestrado, Instituto de Pesquisas Ambientais, da Secretaria de Meio Ambiente, Infraestrutura e Logística, São Paulo, Brazil, 2023; 59p. [Google Scholar]
- Braga, M.R.A.; Fujii, M.T.; Yokoya, N.S.; Eston, V.R.; Plastino, E.M.; Cordeiro-Marino, M. Macroalgal Reproductive Patterns in Mangroves of Ilha do Cardoso, SP, Brazil. In Anais do 2th Simpósio de Ecossistemas da Costa Sul e Sudeste Brasileira–Estrutura, Função e Manejo; ACIESP n. 71-1; ACIESP–Academia de Ciências do Estado de São Paulo: São Paulo, Brazil, 1990; pp. 314–326. [Google Scholar]
- de Paula, É.J. O Gênero Sargassum, C. Ag. (Phaeophyta-Fucales) no Litoral do Estado de São Paulo, Brasil. Bolm. Botânica 1988, 10, 65–118. [Google Scholar] [CrossRef][Green Version]
- Nunes, J.M.C.; de Paula, É.J. Composição e distribuição das Phaeophyta nos recifes da região metropolitana de Salvador, Bahia, Brasil. Iheringia. Ser. Bot. 2002, 57, 113–130. [Google Scholar]

| Taxa | North Coast | South Coast | Reproductive Status | Season | ||||
|---|---|---|---|---|---|---|---|---|
| BS | EG | IM | IT | PC | Dry | Rainy | ||
| Rhodophyta | ||||||||
| Acantophora muscoides (Linnaeus) Bory | x | x | ||||||
| Acantophora spicifera (M.Vahl) Børgesen | x | x | ||||||
| Agardhiella ramosissima (Harvey) Kylin | x | x | x | |||||
| Aglaothamnion felipponei (Howe) Aponte, Ballantine and J.N.Norris | x | x | x | x | x | |||
| Alsidium oliveiranum S.M.Guimarães and M.T.Fujii | x | x | ||||||
| Alsidium seaforthii (Turner) J.Agardh | x | x | x | x | x | T | x | x |
| Amphiroa sp. | x | x | x | |||||
| Arthrocladia variabilis (Harvey) Weber Bosse | x | x | x | |||||
| Asparagopsis taxiformis (Delile) Trevisan | x | x | ||||||
| Bostrychia montagnei Harvey | x | x | ||||||
| Botryocladia occidentalis (Børgesen) Kylin | x | x | x | x | x | |||
| Bryocladia sp. | x | x | x | x | ||||
| Calliblepharis jolyi E.C.Oliveira | x | x | T, C | x | ||||
| Centroceras gasparrinii (Meneghini) Kützing | x | x | ||||||
| Ceramium brasiliense A.B.Joly | x | x | x | x | x | |||
| Ceratodictyon planicaule M.J.Wynne | x | x | x | x | ||||
| Ceratodictyon scoparium (Montagne and Millardet) R.E.Norris | x | x | x | x | x | x | x | |
| Champia sp. | x | x | ||||||
| Chondracanthus teedei (Mertens ex Roth) Kützing | x | x | x | x | x | |||
| Corallina officinalis Linnaeus | x | x | x | x | x | x | ||
| Corallina panizzoi Schnetter and U.Richter | x | x | x | |||||
| Corynomorpha clavata (Harvey) J.Agardh | x | x | ||||||
| Cryptonemia bengryi W.R.Taylor | x | x | ||||||
| Cryptonemia seminervis (C.Agardh) J.Agardh | x | x | x | x | x | x | x | |
| Dichotomaria marginata (J.Ellis and Solander) Lamarck | x | x | x | x | x | x | x | |
| Dipterosiphonia dendritica (C.Agardh) F.Schmitz | x | x | x | x | ||||
| Geldium lineare Iha and Freshwater | x | x | x | x | x | x | ||
| Gelidiella sp. | x | x | x | x | ||||
| Gracilaria cearensis (A.B.Joly and Pinheiro) A.B.Joly and Pinheiro | x | x | ||||||
| Gracilaria cuneata J.E.Areschoug | x | C | x | |||||
| Gracilaria domingensis (Kützing) Sonder ex Dickie | x | x | x | x | x | C | x | x |
| Haliptilon sp. | x | x | ||||||
| Halopithys schotti (W.R.Taylor) L.E.Phillips and De Clerck | x | x | x | |||||
| Haloplegma duperreyi Montagne | x | x | x | x | x | |||
| Halymenia brasiliana S.M.P.B.Guimarães and M.T.Fujii | x | x | ||||||
| Halymenia integra M.Howe and W.R.Taylor | x | x | x | x | C | x | x | |
| Heterodasya mucronata (Harvey) M.J.Wynne | x | x | ||||||
| Heterosiphonia crispella (C.Agardh) M.J.Wynne | x | x | x | |||||
| Hypnea pseudomusciformis Nauer, Cassano and M.C.Oliveira | x | x | x | x | x | x | x | |
| Jania capillaceae Harvey | x | x | ||||||
| Jania crassa J.V.Lamouroux | x | x | x | x | x | x | x | |
| Laurencia dendroidea J.Agardh | x | x | x | x | x | x | ||
| Ochtodes secundiramea (Montagne) M.Howe | x | x | x | x | x | T | x | x |
| Osmundaria obtusiloba (C.Agardh) R.E.Norris | x | x | x | x | x | T | x | x |
| Palisada furcata (Cordeiro-Marino and M.T.Fujii) Cassano and M.T.Fujii | x | x | x | |||||
| Palisada perforata (Bory) K.W.Nam | x | x | x | x | x | x | ||
| Plocamium brasiliense (Greville) M.Howe and W.R.Taylor | x | x | x | x | x | T | x | x |
| Scinaia halliae (Setchell) Huisman | x | x | x | x | x | |||
| Soliera filiformis (Kützing) P.W.Gabrielson | x | x | x | x | x | |||
| Spyridia clavata Kützing | x | T | x | |||||
| Spyridia filamentosa (Wulfen) Harvey | x | x | x | x | x | x | ||
| Thuretia bornetii Vickers | x | x | x | x | x | |||
| Tricleocarpa cylindrica (J.Ellis and Solander) Huisman and Borowitzka | x | x | x | x | x | x | x | |
| Tricleocarpa fragilis Huisman and R.A.Townsend | x | x | x | x | x | x | x | |
| Ulvophyceae | ||||||||
| Anadyomene stellata (Wulfen) C.Agardh | x | x | x | x | x | x | x | |
| Bryopsis sp. | x | x | x | x | x | |||
| Caulerpa cupressoides (Vahl) C.Agardh | x | x | x | x | ||||
| Caulerpa mexicana Sonder ex Kützing | x | x | x | x | ||||
| Caulerpa prolifera (Forsskål) J.V.Lamouroux | x | x | x | x | ||||
| Caulerpa racemosa (Forsskål) J.Agardh | x | x | x | x | x | |||
| Caulerpa sertularioides (S.G.Gmelin) M.Howe | x | x | ||||||
| Chaetomorpha linum (O.F.Müller) Kützing | x | x | x | x | x | x | ||
| Cladophora dalmatica Kützing | x | x | x | x | x | |||
| Codium decorticatum (Woodward) M.Howe | x | x | x | |||||
| Codium isthmocladium Vickers | x | x | x | x | x | x | ||
| Halimeda gracilis J.Agardh | x | x | x | x | x | x | x | |
| Halimeda jolyana Ximenes, Bandeira-Pedrosa, Cassano, Oliveira-Carvalho, Verbruggen and S.M.B.Pereira | x | x | x | x | x | |||
| Udotea flabellum (J.Ellis and Solander) M.Howe | x | x | ||||||
| Ulva lactuca Linnaeus | x | x | x | x | x | x | x | |
| Willeella ordinata Børgesen | x | x | x | x | x | |||
| Phaeophyceae | ||||||||
| Colpomenia sinuosa (Mertens ex Roth) Derbès and Solier | x | x | x | x | x | |||
| Dictyopteris delicatula J.V.Lamouroux | x | x | x | x | x | x | x | |
| Dictyopteris jolyana E.C.Oliveira and R.P.Furtado | x | x | ND | x | x | |||
| Dictyota ciliolata Sonder ex Kützing | x | x | x | x | x | ND | x | x |
| Lobophora variegata (J.V.Lamouroux) Womersley ex E.C.Oliveira | x | x | x | x | x | ND | x | x |
| Padina gymnospora (Kützing) Sonder | x | x | x | x | x | ND | x | x |
| Sargassum stenophyllum C.Martius | x | x | x | x | x | ND | x | x |
| Sargassum vulgare C.Agardh | x | x | x | x | x | ND | x | x |
| Spatoglossum schroederi (C.Agardh) Kützing | x | x | x | x | x | ND | x | x |
| Stypopodium sp. | x | x | ||||||
| Zonaria tournefortii (J.V.Lamouroux) Montagne | x | x | x | x | x | ND | x | x |
| Locality/Municipality | Feldmann Index | Cheney Index |
|---|---|---|
| (R/P) | (R + U)/P | |
| Barra do Sahy/Aracruz | 3.1 | 4.2 |
| Enseada das Garças/Fundão | 3.5 | 5 |
| Imigrantes/Aracruz | 2.8 | 4 |
| Itaoca/Itapemirim | 3.3 | 3.8 |
| Central Beach/Piúma | 3.6 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, I.A.G.; Basílio, T.H.; dos Santos, I.L.F.; Fujii, M.T. Biodiversity and Reproductive Status of Beach-Cast Seaweeds from Espírito Santo, Southeastern Brazil: Sustainable Use and Conservation. Phycology 2024, 4, 427-442. https://doi.org/10.3390/phycology4030024
Martins IAG, Basílio TH, dos Santos ILF, Fujii MT. Biodiversity and Reproductive Status of Beach-Cast Seaweeds from Espírito Santo, Southeastern Brazil: Sustainable Use and Conservation. Phycology. 2024; 4(3):427-442. https://doi.org/10.3390/phycology4030024
Chicago/Turabian StyleMartins, Iago A. G., Thiago H. Basílio, Igor L. F. dos Santos, and Mutue T. Fujii. 2024. "Biodiversity and Reproductive Status of Beach-Cast Seaweeds from Espírito Santo, Southeastern Brazil: Sustainable Use and Conservation" Phycology 4, no. 3: 427-442. https://doi.org/10.3390/phycology4030024
APA StyleMartins, I. A. G., Basílio, T. H., dos Santos, I. L. F., & Fujii, M. T. (2024). Biodiversity and Reproductive Status of Beach-Cast Seaweeds from Espírito Santo, Southeastern Brazil: Sustainable Use and Conservation. Phycology, 4(3), 427-442. https://doi.org/10.3390/phycology4030024






