From Inundations to Golden Opportunity: Turning Holopelagic Sargassum spp. into a Valuable Feed Ingredient through Arsenic Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation
2.2. Arsenic Removal Treatments
2.3. Arsenic Determination
2.4. Statistical Analysis
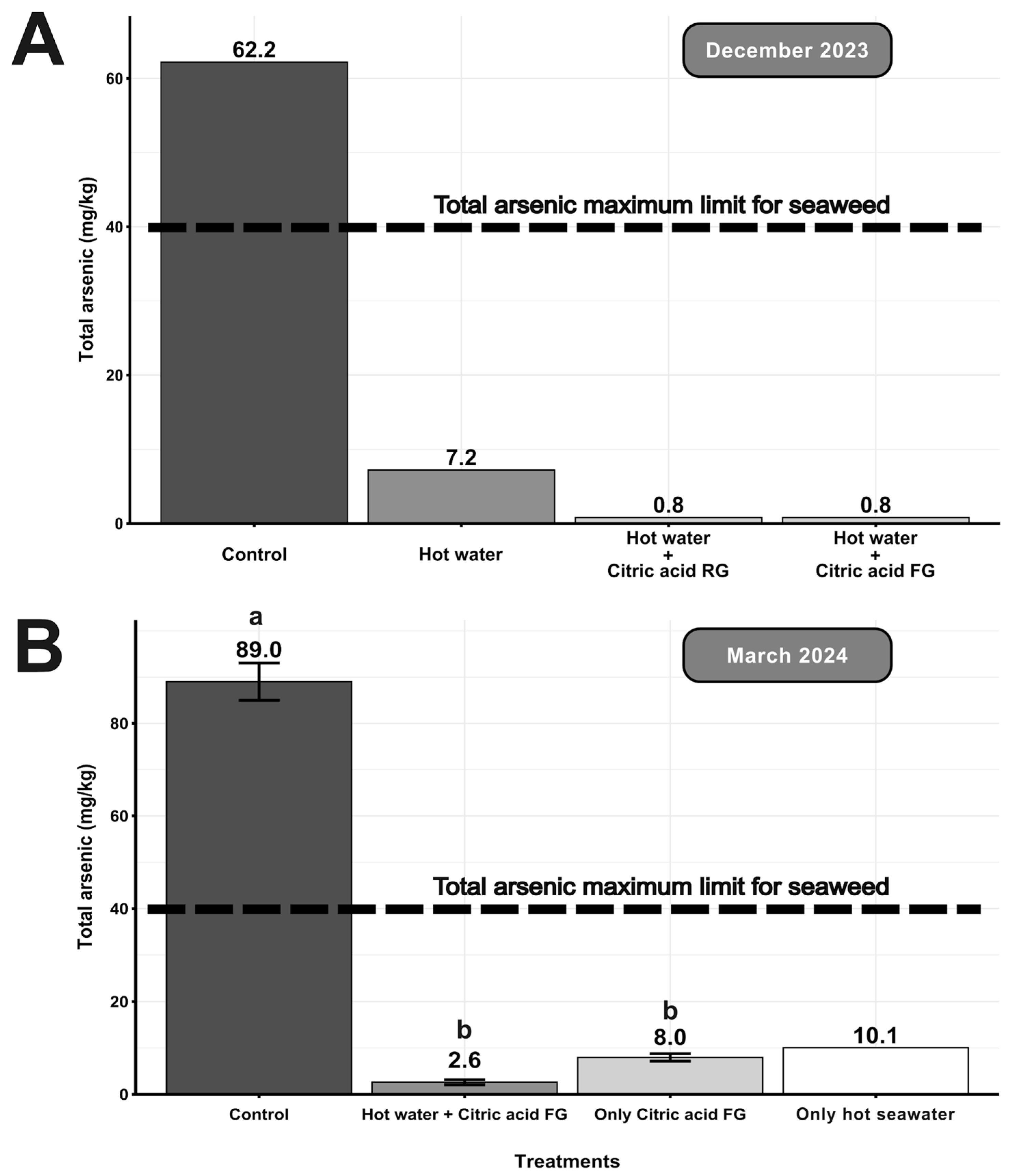
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations FishstatJ. FishStatJ-Software for Fishery and Aquaculture Statistical Time Series; Food and Agriculture Organization of the United Nations FishstatJ: Rome, Italy, 2020. [Google Scholar]
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Jordán-Dahlgren, E. Pelagic Sargassum cleanup cost in Mexico. Ocean. Coast. Manag. 2023, 237, 106542. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.A.; Oxenford, H.A.; van Tussenbroek, B.I. Pelagic Sargassum—A Guide to Current and Potential Uses in the Caribbean; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/en/c/cc3147en (accessed on 20 June 2024).
- López-Contreras, A.M.; Van Der Geest, M.; Deetman, B.; Van Den Burg, S.; Brust, H.; de Vrije, T. Opportunities for Valorisation of Pelagic Sargassum in the Dutch Caribbean; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2021; Volume 2137, Available online: https://library.wur.nl/WebQuery/wurpubs/582759 (accessed on 20 June 2024).
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of pelagic Sargassum biomass into sustainable applications: Current trends and challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.; Morton, P.L.; Brewton, R.A.; Hu, C.; Kelly, T.B.; Solow, A.R.; Lapointe, B.E. Nutrient and arsenic biogeochemistry of Sargassum in the western Atlantic. Nat. Commun. 2023, 14, 6205. [Google Scholar] [CrossRef] [PubMed]
- Alleyne, K.S.T.; Neat, F.; Oxenford, H.A. An analysis of arsenic concentrations associated with sargassum influx events in Barbados. Mar. Pollut. Bull. 2023, 192, 115064. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Flores, P.A.; Gobert, T.; Méndez-Rodríguez, L.C.; Serviere-Zaragoza, E.; Connan, S.; Robledo, D.; Freile-Pelegrín, Y.; de Anda Montañez, J.A.; Waeles, M. Inorganic arsenic in holopelagic Sargassum spp. stranded in the Mexican Caribbean: Seasonal variations and comparison with international regulations and guidelines. Aquat. Bot. 2023, 188, 103674. [Google Scholar] [CrossRef]
- Ortega-Flores, P.A.; Serviere-Zaragoza, E.; De Anda-Montañez, J.A.; Freile-Pelegrín, Y.; Robledo, D.; Méndez-Rodríguez, L.C. Trace elements in pelagic Sargassum species in the Mexican Caribbean: Identification of key variables affecting arsenic accumulation in S. fluitans. Sci. Total Environ. 2022, 806, 150657. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [PubMed]
- Petursdottir, A.H.; Sloth, J.J.; Feldmann, J. Introduction of regulations for arsenic in feed and food with emphasis on inorganic arsenic, and implications for analytical chemistry. Anal. Bioanal. Chem. 2015, 407, 8385–8396. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Lim, R.P. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012, 116, 118–135. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.R.; Oh, S.; Paik, M.J.; Je, J.G.; Heo, J.H.; Lee, T.K.; Fu, X.; Xu, J.; Gao, X.; et al. Arsenic removal from the popular edible seaweed Sargassum fusiforme by sequential processing involving hot water, citric acid, and fermentation. Chemosphere 2022, 292, 133409. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Domínguez, S.; Rodríguez-Martínez, R.E.; Díaz-Martínez, M.; Magaña-Gallegos, E.; Cuchillo-Hilario, M. Potential application of pelagic Sargassum spp. in animal feeding. J. Appl. Phycol. 2023, 35, 433–444. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or Golden Opportunity? The Valorisation of Sargassum from Beach Inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- López Miranda, J.L.; Celis, L.B.; Estévez, M.; Chávez, V.; van Tussenbroek, B.I.; Uribe-Martínez, A.; Cuevas, E.; Pantoja, I.R.; Masia, L.; Cauich-Kantun, C.; et al. Commercial Potential of Pelagic Sargassum spp. in Mexico. Front. Mar. Sci. 2021, 8, 768470. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal changes in the composition and biomass of beached pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2020, 167, 103275. [Google Scholar] [CrossRef]
- Parr, A. Quantitative observations on the pelagic Sargassum vegetation of the western North Atlantic. With preliminary discussion of morphology and relationships. Bull. Bingham. Oceanogr. Collect. 1939, 6, 1–94. [Google Scholar]
- Siuda, A.N.S.; Blanfuné, A.; Dibner, S.; Verlaque, M.; Boudouresque, C.; Connan, S.; Goodwin, D.S.; Stiger-Pouvreau, V.; Viard, F.; Rousseau, F.; et al. Morphological and Molecular Characters Differentiate Common Morphotypes of Atlantic Holopelagic Sargassum. Phycology 2024, 4, 256–275. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2019/1869. Amending and correcting Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for certain undesirable substances in animal feed. Off. J. Eur. Union 2019, L 289, 32–36. [Google Scholar]
- NORMA Oficial Mexicana NOM-127-SSA1-2021; Agua Para uso y Consumo Humano. Límites Permisibles de la Calidad del Agua. DOF: Ciudad de México, Mexico, 2022.
- World Health Organization. Preventing disease through healthy environments. In Exposure to Arsenic: A Major Public Health Concern; Department of Public Health, Environmental and Social Determinants of Health: Geneva, Switzerland, 2019. [Google Scholar]
- Fernández, F.; Boluda, C.J.; Olivera, J.; Guillermo, L.A.; Gómez, B.; Echavarría, E.; Aris, M.G. Análisis Elemental Prospectivo de la Biomasa algal acumulada en las costas de la República Dominicana durante 2015. Cent. Azúcar. 2017, 44, 11–22. [Google Scholar]
- Thompson, T.; Young, B.; Baroutian, S. Efficiency of hydrothermal pretreatment on the anaerobic digestion of pelagic Sargassum for biogas and fertiliser recovery. Fuel 2020, 279, 18527. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef] [PubMed]
- Tonon, T.; Machado, C.B.; Webber, M.; Webber, D.; Smith, J.; Pilsbury, A.; Cicéron, F.; Herrera-Rodriguez, L.; Jimenez, E.M.; Suarez, J.V.; et al. Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean. Phycology 2022, 2, 204–215. [Google Scholar] [CrossRef]
- Cipolloni, O.A.; Couture, P.; Cordonnier, S.; Pascal, P.Y. Temporal fluctuation of metallic trace elements concentrations in three morphotypes of floating holopelagic Sargassum from the Caribbean coast (Guadeloupe, French West Indies). Mar. Pollut. Bull. 2024, 201, 116229. [Google Scholar] [CrossRef]
- Liranzo-Gómez, R.E.; Gómez, A.M.; Gómez, B.; González-Hernández, Y.; Jauregui-Haza, U.J. Characterization of sargassum accumulated on Dominican beaches in 2021: Analysis of heavy, alkaline and alkaline-earth metals, proteins and fats. Mar. Pollut. Bull. 2023, 193, 115120. [Google Scholar] [CrossRef] [PubMed]
- Addico, G.; deGraft-Johnson, K.A.A. Preliminary investigation into the chemical composition of the invasive brown seaweed Sargassum along the West Coast of Ghana. Afr. J. Biotechnol. 2016, 28, 2184–2191. [Google Scholar] [CrossRef]
- Sahu, A.; Rane, N.V.; Lodaya, B.G.; Pandit, A.B. Green synthesis and kinetic study of eco-friendly chelating agent by hydrothermal process for remediation of heavy metals. Indian Chem. Eng. 2021, 64, 227–242. [Google Scholar] [CrossRef]
- Książek, E. Citric Acid: Properties, Microbial Production, and Applications in Industries. Molecules 2023, 29, 22. [Google Scholar] [CrossRef]
- Shinta, Y.C.; Zaman, B.; Sumiyati, S. Citric Acid and EDTA as chelating agents in phytoremediation of heavy metal in polluted soil: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012023. [Google Scholar] [CrossRef]
- Farjami, T.; Sharma, A.; Hagen, L.; Jensen, I.; Falch, E. Comparative study on composition and functional properties brewer´s spent grain proteins precipitated by citric acid and hydrochloric acid. Food Chem. 2024, 446, 13886341. [Google Scholar] [CrossRef] [PubMed]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.R.; Hernández-Carteño, J.; Estévez, M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum spp. J. Mar. Sci. Eng. 2020, 8, 899. [Google Scholar] [CrossRef]

| Country/Territory | Month and Year | Locality | As Content (mg/kg) | Reference |
|---|---|---|---|---|
| DR | October 2015 | Ojeda Beach, Boca Chica Beach, Guayacanes | 13.6–42.3 | [27] |
| Barbados | June 2018 | Consett Bay | 35.2 | [28] |
| Mexico | June 2018–May 2019 | Puerto Morelos | 48.2–175.0 | [10] |
| Mexico | August 2018–June 2019 | Contoy Island, Puerto Morelos, Cozumel, Mahahual, Chinchorro, Xahuayxol, Xcalak | 24.0–172.0 | [11] |
| Mexico | September 2018 | Tulum, Akumal, Playa del Carmen, Puerto Morelos, Cancún | 29.0–65.7 | [29] |
| Turks & Caicos | June 2019 | Shark Bay | 123.9 | [30] |
| Guadeloupe | May 2020–September 2021 | Petit Cul-du-Sac Marin | 53.5–145.2 | [31] |
| Mexico | July 2020 | Cancun, Puerto Morelos | 55.9 | [30] |
| Jamaica | August 2020 | Hellshire bay | 86.6 | [30] |
| Mexico | January 2021 | Cancun, Puerto Morelos | 53.9 | [30] |
| DR | February 2021 | Punta Cana | 21.4 | [30] |
| Barbados | February–August 2021 | Consett Bay | 18.3–64.5 | [8] |
| DR | March–August 2021 | Bávaro, Punta Cana, Juan Dolio, Guayacanes, San Andrés, Nigua, Enriquillo, Juancho | 35.0–101.0 | [32] |
| Mexico | December 2023, March 2024 | Puerto Morelos | 62.2–89.0 | This study |
| Ghana | NA | Western coastline | 13.0–53.5 | [33] |
| December 2023 | March 2024 | |||||
|---|---|---|---|---|---|---|
| 14% | 62% | 82% | 14% | 62% | 82% | |
| Control | 8.7 | 38.6 | 50.8 | 12.5 | 55.2 | 72.7 |
| Hot (sea) water | 1.0 | 4.5 | 5.9 | 1.4 | 6.8 | 8.2 |
| Only acid citric food-grade | ND | ND | ND | 1.1 | 4.9 | 6.5 |
| Hot water + citric acid reagent-grade | 0.1 | 0.5 | 0.7 | ND | ND | ND |
| Hot water + citric acid food-grade | 0.1 | 0.5 | 0.7 | 1.1 | 1.6 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisneros-Ramos, K.I.; Gutiérrez-Castañeda, M.; Magaña-Gallegos, E.; Villegas-Pañeda, A.G.; Monroy-Velázquez, L.V.; Barba-Santos, M.G.; Gaxiola-Cortés, M.G.; van Tussenbroek, B.I. From Inundations to Golden Opportunity: Turning Holopelagic Sargassum spp. into a Valuable Feed Ingredient through Arsenic Removal. Phycology 2024, 4, 384-393. https://doi.org/10.3390/phycology4030021
Cisneros-Ramos KI, Gutiérrez-Castañeda M, Magaña-Gallegos E, Villegas-Pañeda AG, Monroy-Velázquez LV, Barba-Santos MG, Gaxiola-Cortés MG, van Tussenbroek BI. From Inundations to Golden Opportunity: Turning Holopelagic Sargassum spp. into a Valuable Feed Ingredient through Arsenic Removal. Phycology. 2024; 4(3):384-393. https://doi.org/10.3390/phycology4030021
Chicago/Turabian StyleCisneros-Ramos, Karla Itzel, Montserrat Gutiérrez-Castañeda, Edén Magaña-Gallegos, Alejandra G. Villegas-Pañeda, Luz Verónica Monroy-Velázquez, María Guadalupe Barba-Santos, Martha Gabriela Gaxiola-Cortés, and Brigitta I. van Tussenbroek. 2024. "From Inundations to Golden Opportunity: Turning Holopelagic Sargassum spp. into a Valuable Feed Ingredient through Arsenic Removal" Phycology 4, no. 3: 384-393. https://doi.org/10.3390/phycology4030021
APA StyleCisneros-Ramos, K. I., Gutiérrez-Castañeda, M., Magaña-Gallegos, E., Villegas-Pañeda, A. G., Monroy-Velázquez, L. V., Barba-Santos, M. G., Gaxiola-Cortés, M. G., & van Tussenbroek, B. I. (2024). From Inundations to Golden Opportunity: Turning Holopelagic Sargassum spp. into a Valuable Feed Ingredient through Arsenic Removal. Phycology, 4(3), 384-393. https://doi.org/10.3390/phycology4030021








