Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Sample Preparation
2.2. Extraction of Crude MAAs from Dulse
2.3. HPLC Analysis of MAAs
2.4. Protein, Phycoerythrin (PE), and Sugar Contents
2.5. Abiotic Data from Hakodate
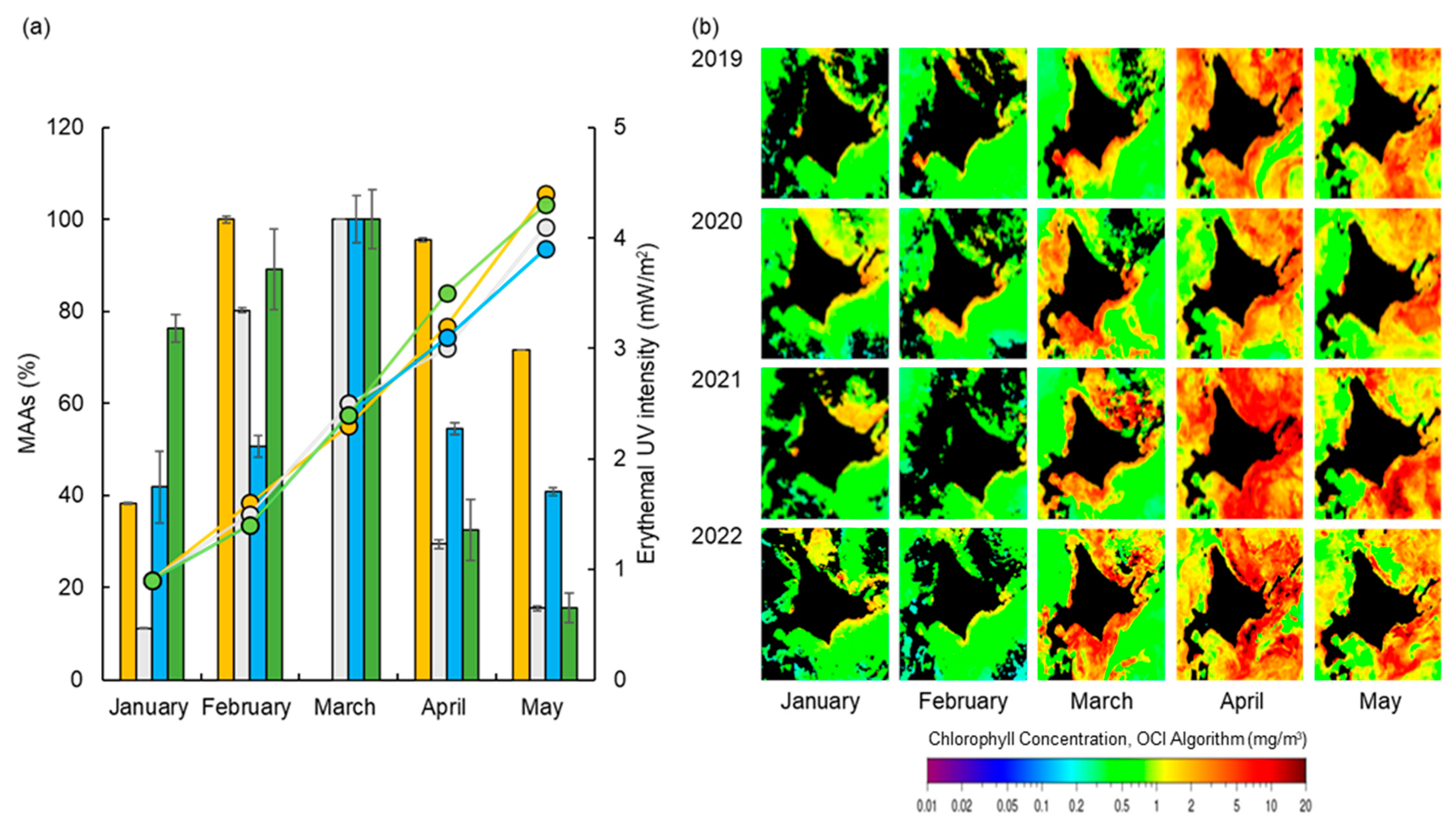
2.6. Compositional Comparison of Usujiri Dulse from 2019 to 2022
2.7. Effect of Extraction Volume on MAA Extraction
2.8. Effect of Extraction Time on MAA Extraction
2.9. Statistical Analysis
3. Results and Discussion
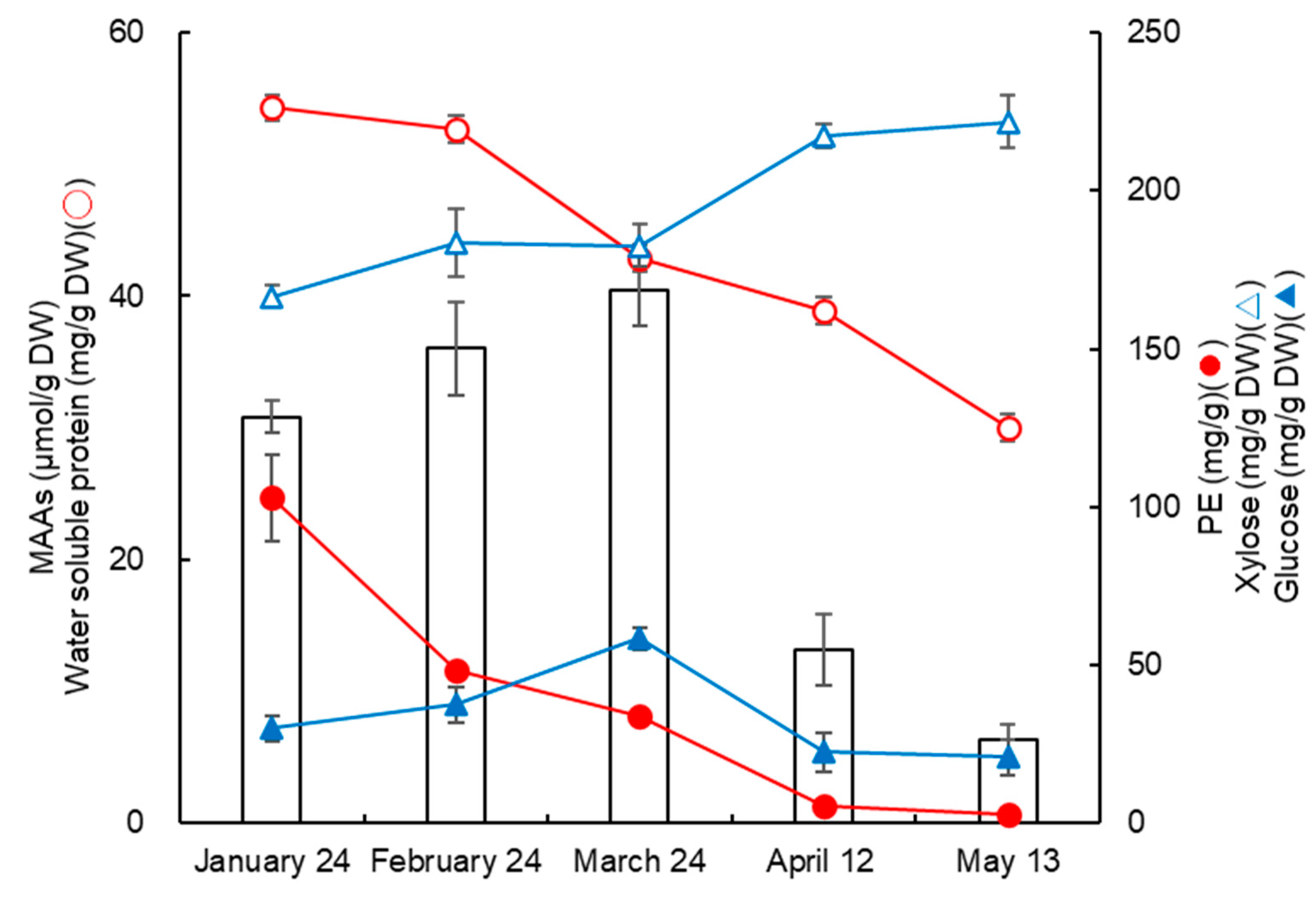
3.1. Monthly Variation in MAAs from Usujiri Dulse in 2022
3.2. Monthly Variation in MAA Contents
3.3. Changes in MAAs, Proteins, Saccharides, and Erythemal UV Intensity
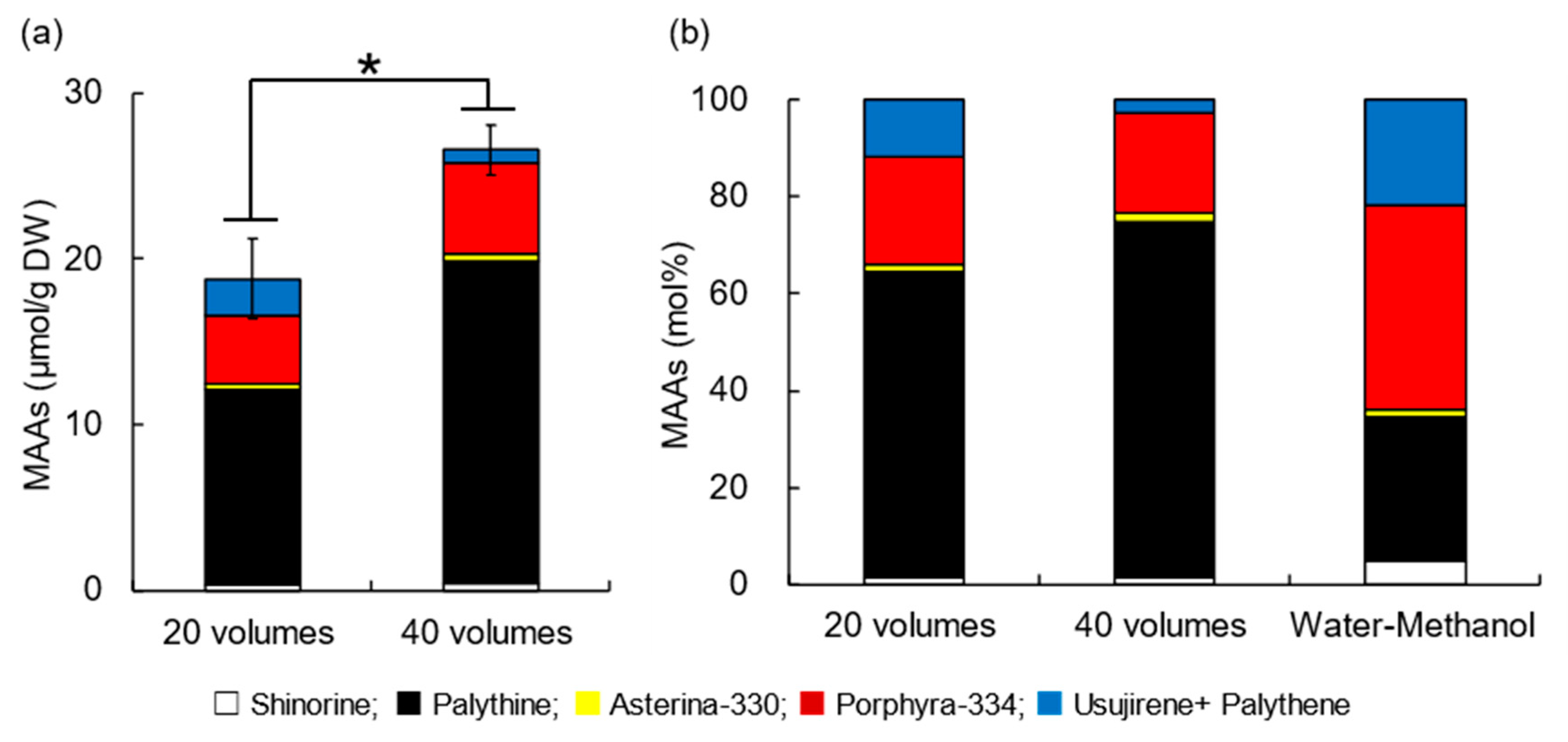
3.4. Effect of Extraction Volume on MAA Extraction from Dulse
3.5. Effect of Extraction Time on MAA Extraction from Dulse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential health and beauty ingredients. Mar. Drugs. 2017, 15, 326. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photo carcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotech. Advan. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi Hee, Y.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Sunscreens—What’s important to know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1119. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs. 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Tang, S.; Chen, Y.; Song, G.; Liu, X.; Shi, Y.; Xie, Q.; Chen, D. A Cocktail of Industrial Chemicals in Lipstick and Nail Polish: Profiles and Health Implications. Environ. Sci. Technol. Lett. 2021, 8, 760–765. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 11. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae Fucus vesiculosus with Antioxidant and Photo-Protecting Properties. Mar. Drugs. 2022, 20, 687. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the Southern Ocean. Mar. Drugs. 2018, 16, 336. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; van Sluys, M.A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Karentz, D.; Mceuen, E.S.; Land, M.C.; Dunlap, W.C. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: Potential protection from ultraviolet exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Conde, F.; Churio, M.; Previtali, C. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2020, 56, 139–144. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. chemical and ecological aspects. Mar. Drugs. 2011, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.; Otero, P.; Murray, P.; Saha, S.K. Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential. Sustainability 2023, 15, 3792. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-Like amino acid profiles of wild-Harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient extraction and antioxidant capacity of mycosporine-like amino acids from red alga dulse Palmaria palmata in Japan. Mar. Drugs. 2020, 18, 502. [Google Scholar] [CrossRef]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Yamamoto, R.; Mune Mune, M.A.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan). Phycology 2023, 3, 127–137. [Google Scholar] [CrossRef]
- Skriptsova, V.S.; Suzuki, M.; Semenchenko, A.A. Morphological variation in Northwest Pacific Devaleraea mollis and description of D. inkyuleei sp. Nov. (Palmariaceae, Rhodophyta). Phycologia 2022, 61, 606–615. [Google Scholar] [CrossRef]
- Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs. 2022, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar. Drugs. 2016, 14, 32. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kishimura, H.; Kinoshita, Y.; Saburi, W.; Kumagai, Y.; Yasui, H.; Ojima, T. Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem. 2019, 82, 117–122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kumagai, Y.; Yamamoto, Y.; Yasui, H.; Kishimura, H. Identification of a key enzyme for the hydrolysis of β-(1-3)-xylosyl linkage in red alga dulse xylooligosaccharide from Bifidobacterium adolescentis. Mar. Drugs. 2020, 18, 174. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton. Arch. Hydrobiol. 2002, 154, 691–703. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, isolation and characterization of mycosporine-like amino acids from four species of red macroalgae. Mar. Drugs. 2021, 19, 615. [Google Scholar] [CrossRef]
- Beer, S. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Kudo, I.; Yoshimura, T.; Lee, C.; Yanada, M.; Maita, Y. Nutrient Regeneration at Bottom after a Massive Spring Bloom in a Subarctic Coastal Environment, Funka Bay, Japan. J. Oceanogr. 2007, 63, 791–801. [Google Scholar] [CrossRef]
- Isada, T.; Hirawake, T.; Nakada, S.; Kobayashi, T.; Sasaki, K.; Tanaka, Y.; Watanabe, S.; Suzuki, K.; Saitoh, S.I. Influence of hydrography on the spatiotemporal variability of phytoplankton assemblages and primary productivity in Funka Bay and the Tsugaru Strait. Estuar. Coast. Shelf Sci. 2017, 188, 199–211. [Google Scholar] [CrossRef]
- Ekman, P.; Yu, S.; Pedersen, M. Effects of altered salinity, darkness and algal nutrient status on floridoside and starch content, α-galactosidase activity and agar yield of cultivated Gracilaria sordida. Br. Phycol. J. 1991, 26, 123–131. [Google Scholar] [CrossRef]
- Kudo, I.; Matsunaga, K. Environmental factors affecting the occurrence and production of the spring phytoplankton bloom in funka bay, Japan. J. Oceanogr. 1999, 55, 505–513. [Google Scholar] [CrossRef]


| MAAs | Collection Date (2022) | ||||
|---|---|---|---|---|---|
| (µmol/g DW) | 24 January | 24 February | 24 March | 12 April | 13 May |
| Shinorine | 1.14 ± 0.07 a | 1.16 ± 0.09 a | 1.04 ± 0.40 a | 0.41 ± 0.17 b | 0.23 ± 0.13 b |
| Palythine | 9.71 ± 0.16 a | 10.3 ± 0.9 a | 12.0 ± 4.2 a | 4.01 ± 1.09 b | 2.17 ± 0.58 b |
| Asterina-330 | 0.47 ± 0.03 b | 0.60 ± 0.05 a | 0.58 ± 0.21 ab | 0.22 ± 0.02 c | 0.11 ± 0.01 c |
| Porphyra-334 | 13.2 ± 0.6 b | 17.0 ± 1.5 ab | 17.8 ± 7.3 a | 4.59 ± 0.52 c | 1.98 ± 0.23 c |
| Usujirene + Palythene | 6.32 ± 0.27 ab | 6.94 ± 1.14 a | 8.97 ± 4.05 a | 3.91 ± 0.41 bc | 1.80 ± 0.21 c |
| Total | 30.8 ± 1.2 b | 36.0 ± 3.5 ab | 40.4 ± 2.6 a | 13.1 ± 2.7 c | 6.29 ± 1.27 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, R.; Takizawa, K.; Miyabe, Y.; Mune Mune, M.A.; Kishimura, H.; Kumagai, Y. Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction. Phycology 2023, 3, 394-404. https://doi.org/10.3390/phycology3030026
Yamamoto R, Takizawa K, Miyabe Y, Mune Mune MA, Kishimura H, Kumagai Y. Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction. Phycology. 2023; 3(3):394-404. https://doi.org/10.3390/phycology3030026
Chicago/Turabian StyleYamamoto, Ryuya, Koki Takizawa, Yoshikatsu Miyabe, Martin Alain Mune Mune, Hideki Kishimura, and Yuya Kumagai. 2023. "Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction" Phycology 3, no. 3: 394-404. https://doi.org/10.3390/phycology3030026
APA StyleYamamoto, R., Takizawa, K., Miyabe, Y., Mune Mune, M. A., Kishimura, H., & Kumagai, Y. (2023). Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction. Phycology, 3(3), 394-404. https://doi.org/10.3390/phycology3030026







