1. Introduction
Seaweeds are a diverse group of organisms that are as important to our nearshore coastal marine world as land plants are to our terrestrial world. Seaweeds were the evolutionary precursors to land plants, and like land plants, they are critical primary producers, forming living links between the inorganic and organic worlds by using photosynthesis to convert CO
2 and nutrients into living biomass. These primary producers support other marine life by producing oxygen, contributing to marine food webs, and providing structures and habitats for fishes and invertebrates. Seaweeds are also an important resource for humans. Historically, coastal peoples have relied on seaweeds for food, minerals, medicine, insulation, fertilizer, and fodder.
Pyropia leucosticta (formerly
Porphyra leucosticta) belongs to the red algal order Bangiales. The red seaweed
Porphyra and other closely related genera such as
Pyropia, known collectively by the Japanese name nori, are of particular interest for their use in aquaculture and are the most economically valuable maricultured seaweeds in the world [
1]. These bladed Bangiales are highly valuable and extensively farmed in Southeast Asia, gaining popularity and acceptance as a sustainable and locally grown food ingredient, as they are high in health-beneficial substances, such as antioxidants, minerals, vitamins, and proteins. The cultivation of Atlantic
Porphyra species still faces several difficulties due to their complex heteromorphic life cycle, which is currently not fully understood.
P. leucosticta reproduces both sexually and asexually [
2]. The sexual life cycle alternates between the bladed gametophyte and the microscopic sporophyte (conchocelis). In nature, the conchocelis stage, difficult to detect due to its microscopic size, occurs in mollusk shells (mainly oysters) and releases conchospores upon maturation, which grow into blades. The sexual reproduction of nori species involves complex processes in accordance with environmental factors (mainly water temperature). Male gametes (sperm) form in the spermatium and can be found on the male section of the blade (monoecious) or on the male blade (dioecious). Once released, the sperm fertilizes the egg in the female carpogonia, and once the egg is fertilized, cell division occurs through mitosis, resulting in a zygotosporangium with mature zygotospores. These spores will be released into the environment, settle on suitable substrata (typically oysters or other shellfish shells), and develop into the sporophyte generation of nori known as the conchocelis, distinguishable as a red “fuzz” as it grows vegetatively. When triggered, the conchocelis will form filaments, indicating the presence of mature conchosporangium branches where meiosis occurs, resulting in four identical haploid spores. These conchospores will be released and settle on suitable substrata (usually rocks (epilithic) or other algae (epiphytic)), where they will germinate and grow into new haploid gametophytic male/female blades, thus completing the life history of nori [
1].
Asexual reproduction is common in nori. In the gametophyte phase, neutral spores, endospores, or archeospores (large spores, with only one produced per female cell) are produced in the carpogonium through mitosis and will develop into the gametophytic blade phase. Asexual reproduction has the advantage of maintaining the genetic identity of the phenotype, and, in addition, asexual reproduction through agamospores (another type of spore formed without fertilization through the mitotic cleavage of blade cells, able to germinate into conchocelis [
3]) has the added advantage of the conchocelis phase. The conchocelis phase is assumed to afford some advantages through different tolerances and the avoidance of competition, grazing, and intertidal stresses, since it develops on mussel shells. The major disadvantage of asexual reproduction is the lack of genetic diversity (no genetic recombination). In the event of ecological or biological stress, the organism will have less resilience [
1], but under laboratory conditions, this aspect can be controlled, with the impact being limited.
Conchocelis can also be cultured “freeliving” in laboratory settings [
1], but complex installations are required for the controlled mass release of conchospores (e.g., large bioreactors), since these are the spores that germinate into the edible thallus. Although this process is routinely used in Asian species with great success, this step is still considered a critical stage for cultivation, and the mass release of conchospores in European nori species still forms a bottleneck for large-scale production [
4]. To support large-scale cultivation, Green and Neefus (2015) successfully completed the life history of
P. leucosticta under laboratory-controlled conditions, but further work is still required to successfully control its conchocelis phase. The authors were able to successfully induce conchospore release under a wide range of factors but were not able to identify environmental conditions that would suppress release and allow the vegetative proliferation of the conchocelis phase [
5].
As
Porphyra is widely used in Asia as part of the human diet, it has been the subject of numerous studies performed in order to identify its functional properties, highlighting that these species contain many active principles [
6]. Most species have high levels of calcium, sodium, potassium, iron, and magnesium, as well as vitamins A, B12, C, and E.
Porphyra is one of the most protein-rich genera of macroalgae, with some species reaching ~25–47% protein in dry weight, which is more than protein-rich vegetables such as soybean. For many of these species, the make-up of these proteins is constituted by aspartic and glutamic acids, which have a strong effect on flavor development, since glutamic acid is the main component contributing to the umami taste [
7]. Another property of
Porphyra is the phycoerythrines specific to Rhodophyta, which has been shown in recent studies to have antioxidant properties [
8]. In addition, porphyran, an anionic sulfated polymer characteristic of
Porphyra species, has significant biological and pharmaceutical properties. More than 40% of the dry weight of
Porphyra is made up of porphyran, which is evaluated to have nutritional and health benefits [
9] and is considered to have a potential status as an antiviral agent [
10]. Several antioxidant molecules have been identified in the genus
Porphyra: histidine-related compounds, chlorophyll analogs, mycosporine-like amino acids, sulfated polysaccharides, and oligosaccharides. In particular, it has been suggested that the accumulation of UV-absorbing mycosporine-like amino acids, such as porphyra-344, provides photoprotection to
P. leucosticta, and thus, these compounds may function as biological antioxidants. This screening emphasized the great antioxidant potential of
P. leucosticta [
11].
P. leucosticta could be utilized as a sea vegetable, a source of pigments (namely, R-phycoerythrin, which is used as a fluorescent tag), a protein substitute for fish meal, and countless other applications [
5].
An ideal candidate for aquaculture would have several attributes, including a fast growth rate, a high capacity for nutrient accumulation, high protein content, extended seasonality, and a life history that allows for easy propagation. In addition, it should be native to the locality where it will be grown [
12]. These aspects are fully covered by
P. leucosticta, hence its selection for laboratory cultivation along the Romanian Black Sea coast. The seasonal fluctuations in environmental conditions on temperate intertidal rocky shores result in the necessity of organisms to withstand wide temperature, irradiance, and nutrient ranges. One adaptation of marine macrophytes to ensure survival in these highly dynamic and extreme habitats is a complex life cycle, with alterations in preferred conditions depending on the life history stage. Changing environmental conditions can be important triggers of life history events, such as initiating reproduction, and the temperature and photoperiod have been found to be especially important factors in the life history of bladed Bangiales [
4]. Before starting a large-scale culture, it is important to know the influence of environmental factors on
Porphyra/Pyropia species, as these are natural triggers of the reproductive process, for a more faithful simulation under laboratory-controlled conditions. It is also necessary to know the onset of the reproductive process in the natural environment in order to accurately determine the period when aquaculturists can collect wild fertile nori specimens to start the cultivation process.
For example, the results of a phenological survey of
Porphyra umbilicalis indicate that the season has the most influence on reproduction in this alga. Plants are reproductive from fall through early spring, when ample nutrients and cold temperatures support neutral spore production. During the summer months, depleted nutrients and high temperatures cause plants to die back and become mostly vegetative.
P. umbilicalis is highly exposed to incoming wave action, something that appears to benefit reproduction [
13]. This is also the case for
P. leucosticta, which prefers shallow exposed coastal areas, with an optimal reproductive period during winter.
P. leucosticta has a characteristic appearance during the reproductive period, with clearly differentiated thallus edges with an alternation of darker and lighter areas, indicating the so-called “
neutral spores-rich margins” [
13]. Specimens with this characteristic appearance can be found along the Romanian coast, usually between January and early April (depending on the water temperature). Royer (2011) demonstrated an interesting fact: the storage of reproductively mature
P. umbilicalis blades at −20 °C only showed a significant effect on the number of sporelings that germinated but did not divide. The small number of sporelings affected by this difference from controls suggested that it is not a negative indicator for the storage of frozen seed stock for aquaculture. This promising result indicates that short periods of freezing may have little effect on spore viability when reproductively mature
P. umbilicalis blades are frozen for storage [
13]. This aspect should also be verified for other nori species. For the same species,
P. umbilicalis, Gavrielidis and Neefus (2016) established that maximum growth rates (>9% day
−1) were observed when blades were grown at 10 to 15 °C with at least 12 h of light in the day and ≥110 μmol photons m
−2 s
−1. At the same time, the authors demonstrated that growing
P. umbilicalis under low light (≤60 μmol photons m
−2 s
−1) and day-neutral/long-day conditions will result in higher pigment content and higher protein content, making the blades more suitable for either an aquaculture feed substitute or a human food product [
12].
Regarding the light influence, it has been shown that many red algae can be described as subtidal algae, areas where blue and green light prevails, so the specific photopigment of red algae allows efficient absorption. In addition, several red algal species’ growth rates and photosynthesis depend on the light quality during the culture period and on the pigment composition under these conditions. In conclusion, light characteristics (spectral quality, quantity, and duration) have a profound influence on plant and seaweed metabolism and development. Wu (2016) performed some experiments on
Pyropia haitanensis and showed that fluorescent tubes, along with blue and green light, were more efficient in promoting algal growth than thalli grown under red lighting. Similarly, some studies have shown that blue and green light could play an advantageous role in red algal growth and development [
14].
These are extremely important aspects for establishing a large-scale cultivation protocol, and all of the conditions stated above must be tested and applied particularly to
P. leucosticta from the Romanian Black Sea coast, since each species has its own ecological valences. The present study aims to highlight the importance of asexual reproduction for the red alga
P. leucosticta collected from the Romanian Black Sea coast in obtaining new specimens under laboratory-controlled conditions by manipulating its reproductive stages. At the same time, a short review of the main existing studies addressing this topic was carried out, and the results of this study are presented in the frame of the most important results obtained by other researchers. Along the Romanian coast of the Black Sea, there is no tradition regarding either the ex situ or in situ cultivation of macroalgae for subsequent capitalization. In this regard, this study established the basic conditions for cultivating
P. leucosticta under laboratory-controlled conditions, wishing to support future Romanian seaweed growers; since most studies regarding the culture of
Porphyra/Pyropia species refer to the sexual reproduction of the species (the conchocelis phase) and fewer are related to asexual reproduction, particularly along the Romanian Black Sea coast, these differentiated aspects have not been addressed. In this study, we tested a simple methodology that is easier to apply in comparison with the complicated conchocelis stage method and allowed new specimens to be obtained under controlled conditions without high costs and complicated infrastructure. In a previous study [
15], intermediate results obtained after only 3 months of
P. leucosticta laboratory culture were presented in a general manner, so the current study aims to present the final results while also providing additional information and the main conclusions of the experiment and highlighting future research directions.
3. Results
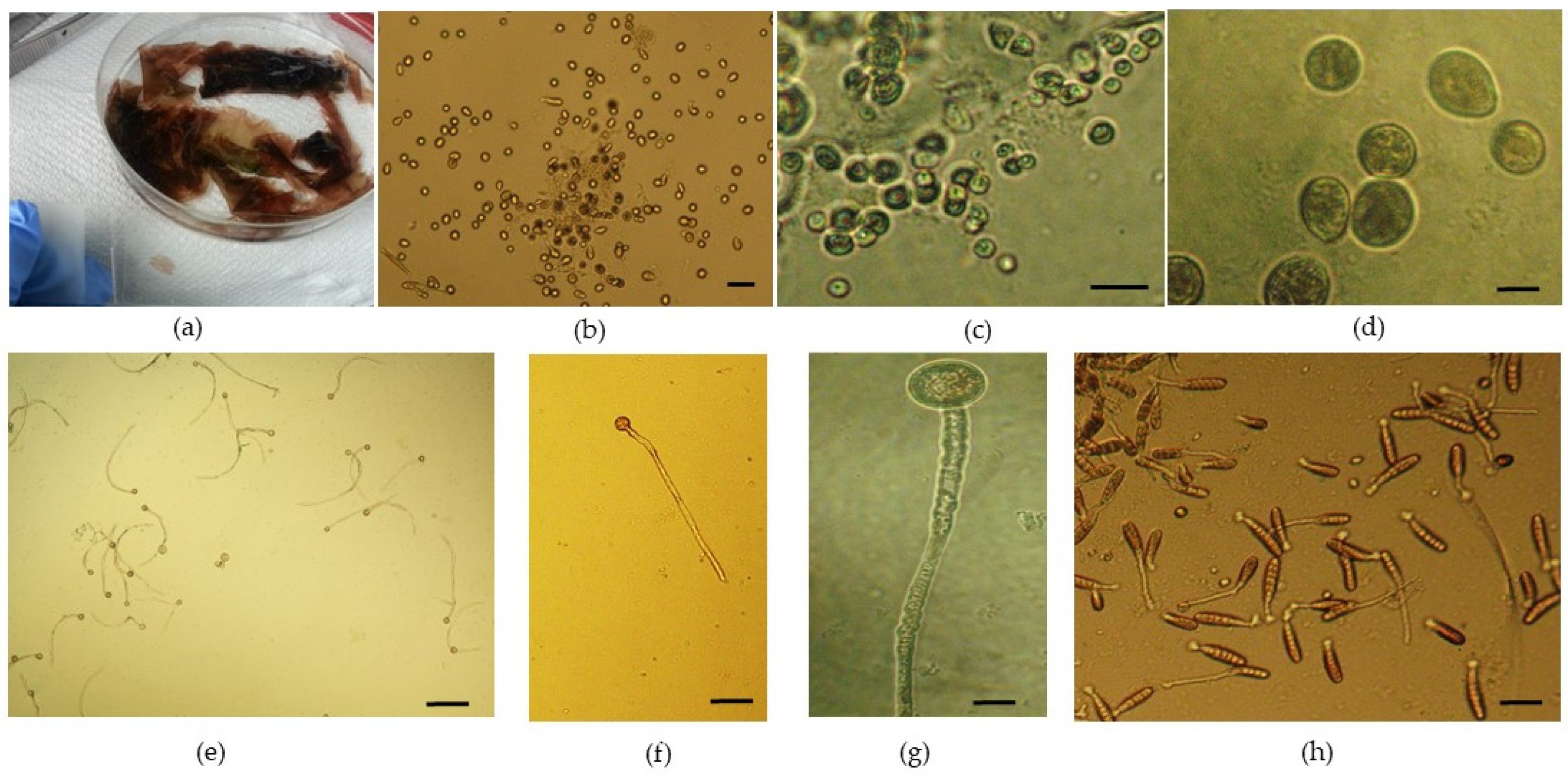
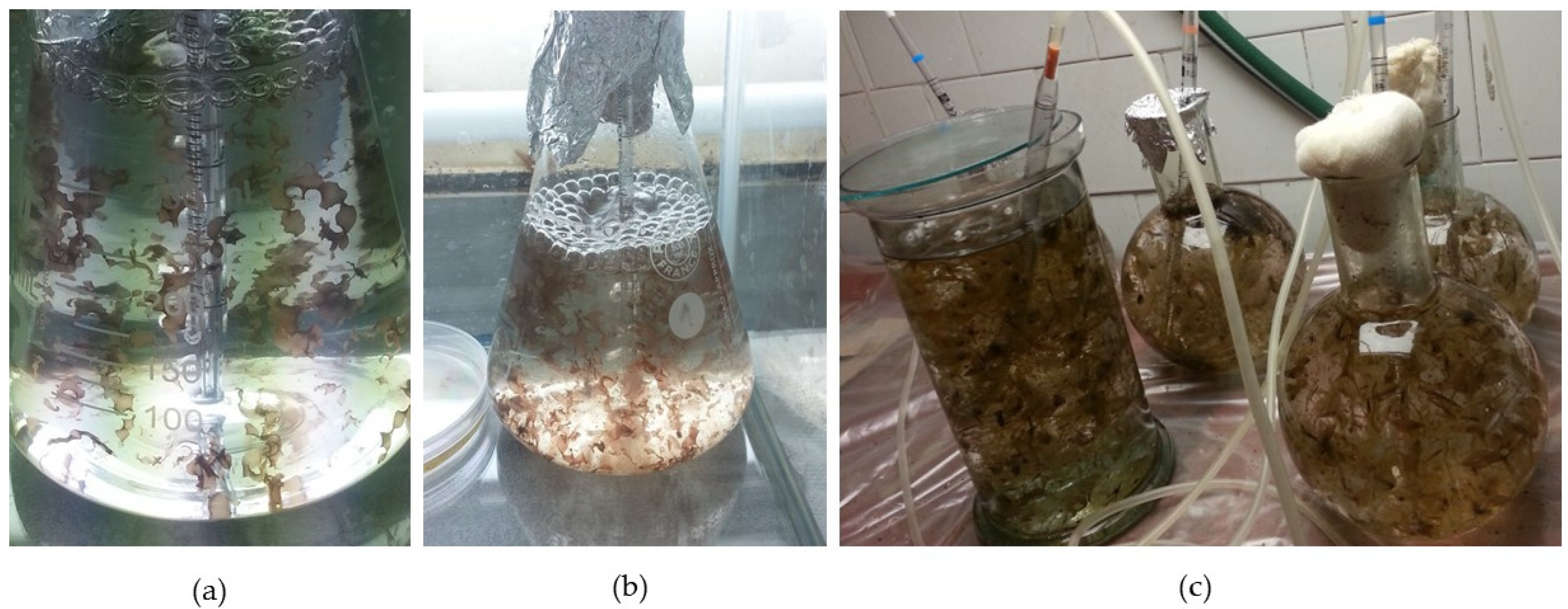
Spore germination, blade growth, and further development were followed for 5.5 months, from February to mid-July. After handling the reproductive material (
Figure 1a), as described in the “Materials and Methods” section (
Section 2.2), the evolution of juvenile blades was carefully monitored. The released reproductive cells were initially round (
Figure 1b–d). The evolution of newly formed blades obtained exclusively under laboratory-controlled conditions started after only one week, when germinated spores (with a germ tube and cell division taking place—
Figure 1e–g) were noticed in Petri dishes. After 2 weeks, neutral-spore-germinated thalli with single-row cell division were observed (
Figure 1h).
Cellular division on multiple planes started immediately after this process (
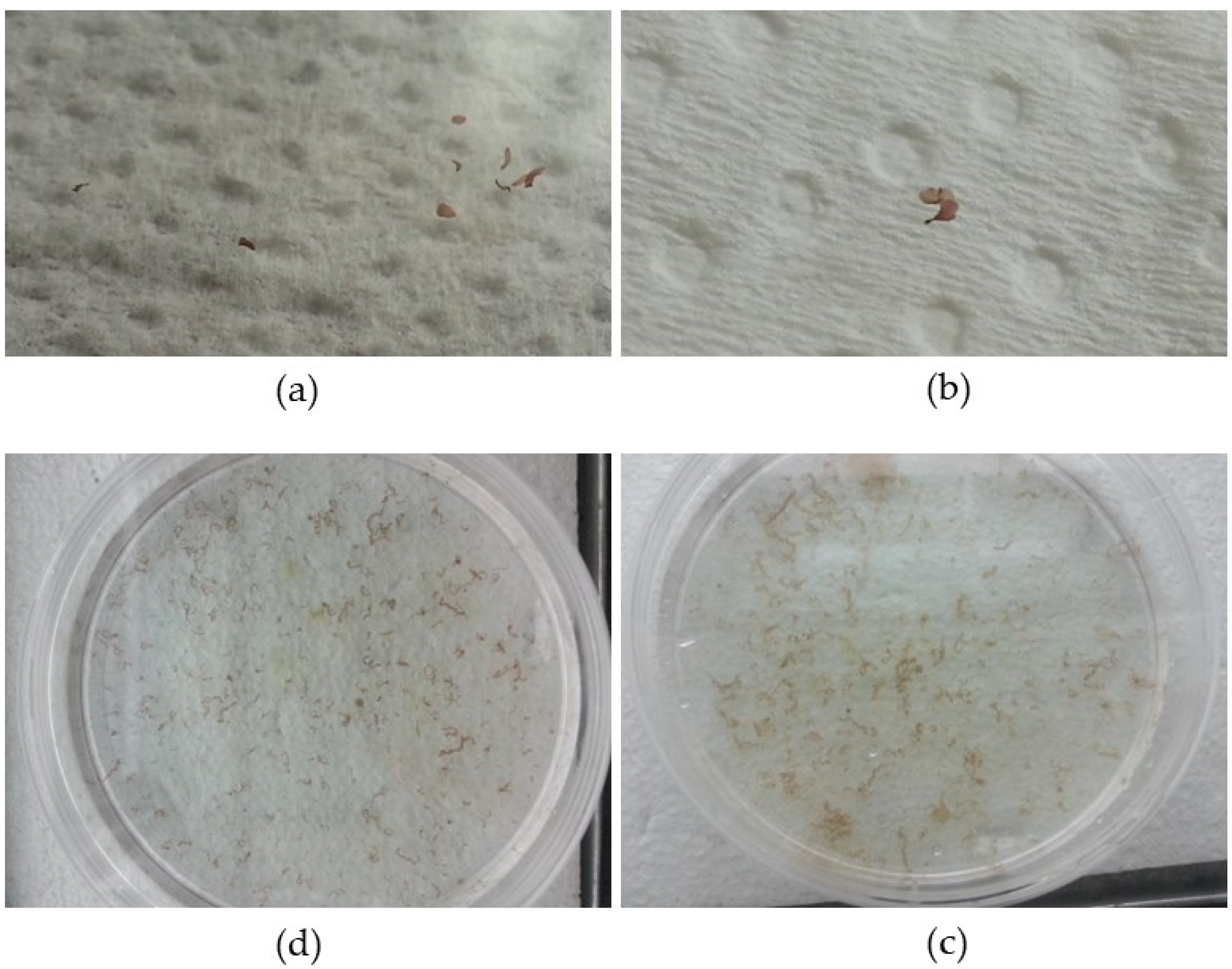
Figure 2a–d). After 4 weeks of controlled culture, the macroscopic phase began (
Figure 3a,b).
Although with reduced dimensions of up to approx. 5 mm, young foliose blades started to become macroscopically visible (
Figure 3a,b). At these reduced dimensions, they were still growing while attached to microscopic slides or the bottom of Petri dishes (
Figure 3c,d). As they grew, some of the specimens detached, because the microscope slides, having no asperities, do not ensure good adhesion, and it was necessary to transfer them to Erlenmeyer containers. The delay in transferring them to larger containers suitable for their new dimensions caused the twisting of the newly formed specimens and a drastic reduction in the growth rate.
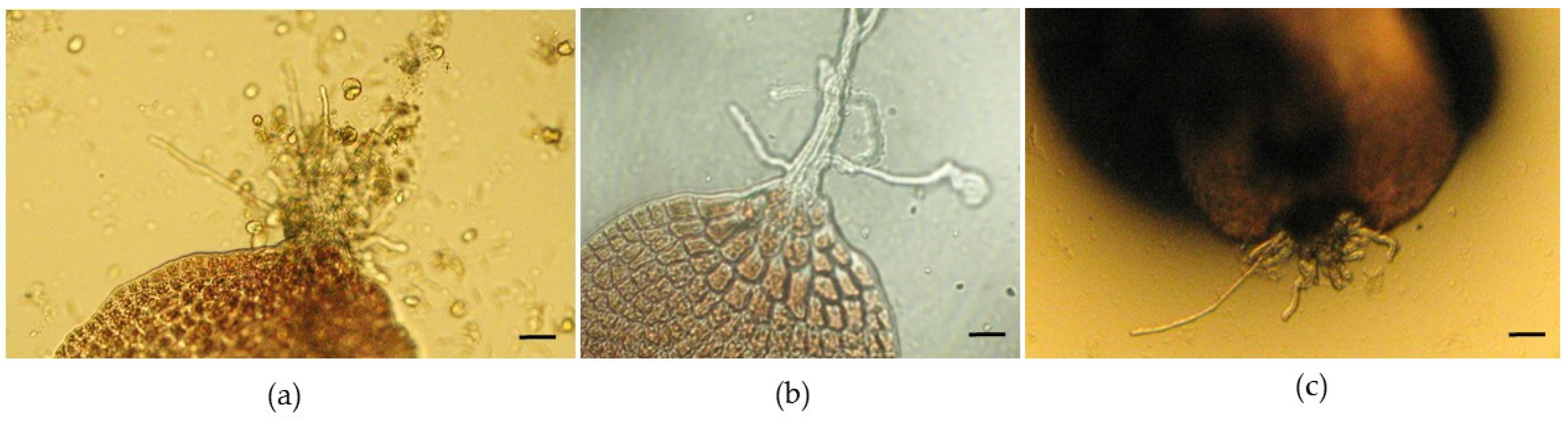
After approximately 6 weeks, a maximum of 30 neutral-spore-germinated thalli were counted in one of the Petri dishes (the largest was approximately 10 mm). After about 4 weeks, some specimens presented some rhizoids, indicating that holdfasts had developed (
Figure 4a–c).
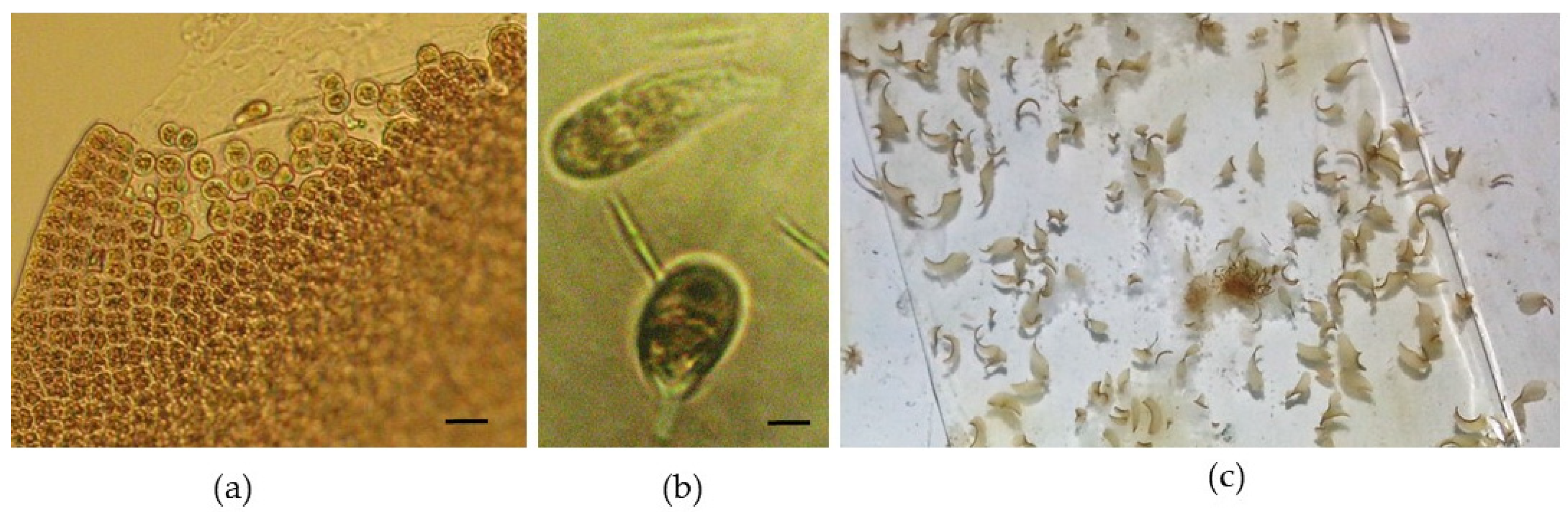
A temperature above 15 °C triggered the entry of newly formed blades into an early reproductive period, which led to spore release (
Figure 5a,b). The evolution of the spores followed the same pattern as the adult reproductive material, and after 10 days, these new thalli became macroscopically visible (
Figure 5c). Although Abdel-Rahman (2015) mentions that neutral-spore production was higher at 20 °C than at other temperatures [
19], for
P. leucosticta, an increase in temperature was considered to be a stress factor, hence this biological response.
At the end of the experiment, the maximum dimensions were 80–90 mm (
Figure 6a–c). During the release of neutral spores, some filamentous conchocelis were also generated, but at low quantities.
During five and a half months of the experiment, an upward trend of specimens’ growth was noticed (considering the blade’s average length, expressed in mm) (
Figure 7).
P. leucosticta maintained positive growth during the culture period for the majority of the thalli developed under experimental conditions.
4. Discussion
Our study demonstrated that the discharge of neutral spores capable of generating new thalli is massive under favorable conditions, namely, if adequate temperature and light regimes are ensured. The optimal development of foliose blades will be achieved with a constant temperature regime, as fluctuations are extremely harmful; in other words, large temperature changes are unfavorable compared to a constant maximum temperature. In our experiment, when the temperature reached 17 °C, the new specimens formed exclusively in controlled culture entered an early reproductive stage, leading to the massive release of neutral spores, with the formation of new thalli. These were considered stress conditions, and in this case, the tissue that housed the spores disintegrated, while the rest of the blade continued its evolution. However, these thalli appeared depigmented, which can be a problem if the intention is to harvest the biomass for further capitalization. The biological material could have a lower biochemical composition, which is not suitable for the extraction of various compounds.
There are also studies that indicate the growth of
P. leucosticta even at temperatures of 20 °C, but the final conclusions stated that temperatures above 15 °C may be suboptimal [
5]. Green and Neefus (2015) mentioned that
P. leucosticta blades grown under day-neutral (12:12 light–dark) and long-day (16:8 light–dark) conditions had higher growth rates compared with those grown under short-day (8:16 light–dark) conditions. However, blades grown under short-day conditions had higher phycobilin content than blades grown under day-neutral or long-day conditions. The authors mentioned that newly formed blades may effectively dilute the photosynthetic pigment concentration as they expand rapidly. For example, if the intention is to use
P. leucosticta as a sea vegetable, production should focus on producing highly pigmented biomass, so the optimal conditions would range from 10 to 15 °C, from 30 to 110 µmol photons m
−2 s
−1, and more than 12 h of light per day [
5].
Although these biochemical analyses were not performed on the newly formed blades, since the purpose of our study was only to initiate the cultivation process of this species, our data may support this hypothesis, taking into account that the blades appeared depigmented towards the end of the experiment, with a possible cause being a reduced amount of phycoerythrin. Although only standard light conditions (day-neutral) were used, in the future, other light regimes will be tested, particularly short and long days, to see the influence of light on the biochemical composition of P. leucosticta.
Nianci and co-workers (2019) mentioned that large numbers of neutral spores could serve as “seeds” that can be used directly for seedling cultivation [
20]. Our study confirms this theory for the species
P. leucosticta, collected from the Romanian coast. The experiment was based entirely on the asexual reproduction of the species. Although carried out on a small scale, it led to the development of numerous neutral-spore-germinated thalli and opens new paths for the development of macroalgal cultures along the Romanian coast, a field that has not been intensely explored to date but in which there is a major interest. The method used in this study has been tested worldwide on various species of
Porphyra. Compared with the traditional aquaculture mode based on sexual reproduction via shell conchocelis cultivation, this method requires minimal investments in instruments and space for efficient sectioning and is easy for farmers to adopt [
20]. Moreover, the whole propagation cycle took only 7 days, which is dramatically faster than conchospore-dependent seedling production. Our results support Nianci’s (2019) theory (although referring to a different species—
Pyropia yezoensis) and suggest that the neutral-spore-based method could be considered as an alternative
P. leucosticta cultivation methodology, with important technical support for innovation in Romanian Black Sea macroalgal aquaculture. The main conclusions of all of these studies were that asexually generated blades from neutral spores grow more quickly than those generated from conchospores. Considering that after only 5.5 months, specimens of even 90 mm in length were obtained, we confirm this aspect.
For the large-scale expansion of cultures, it would be interesting to test the introduction of specific ropes into the culture vessels, before the release of neutral spores, as substrates for adhesion. The method has already been tested and provided favorable results in other countries. In this regard, Nianci and co-workers (2019) mentioned that to test the adhesion capability, seedling ropes were incubated in the neutral-spore culture, and they found that 63.5% of the neutral spores could successfully attach to the seedling ropes after 24 h, and 81.3% attached after 48 h [
20]. Moreover, Blouin and co-workers (2007) [
21] highlighted that nets seeded with asexually derived spores have several advantages. The use of neutral spores, as opposed to conchospores, eliminates the need to complete the sexual life history of
Porphyra. This means that the considerable time, expense, and infrastructure necessary to maintain conchocelis cultures can be avoided. Further experiments are also necessary to investigate the importance of the initial spore density for maximal germination.