Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.1.1. Sample Origin
2.1.2. Sample Acquisition and Concentration
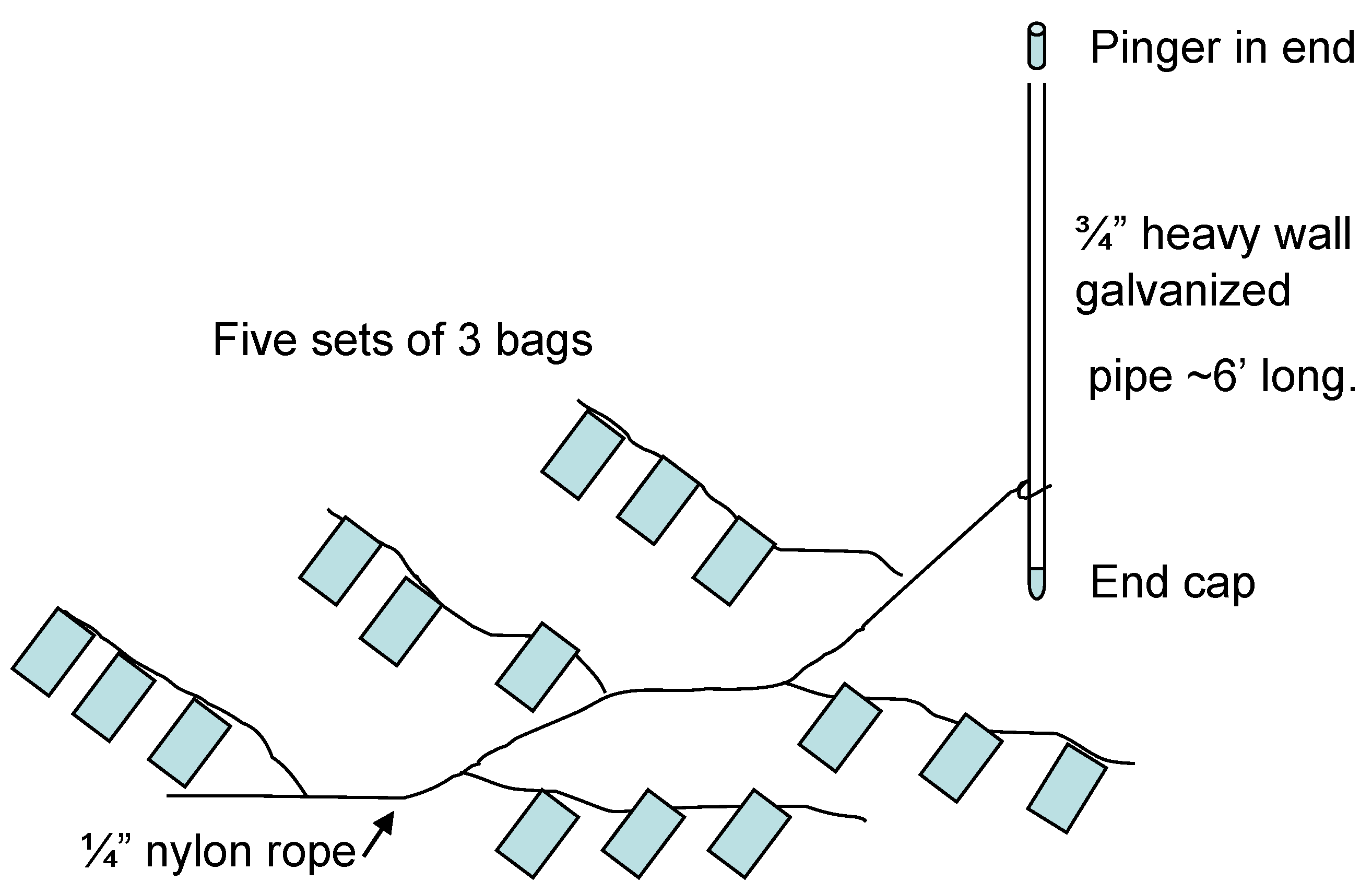
2.1.3. Preparation and Deployment of Bags Containing Concentrated Cyst Samples
2.1.4. Preparation of Permanent Microscope Slides for Start Values
2.1.5. Dry Weight Measurement
2.1.6. Sampling of Bags
2.1.7. Permanent Microscope Mounts from Sampling
2.1.8. Germination Experiments
2.1.9. Microscopy and Naming of Samples
2.2. Statistical Analyses
3. Results
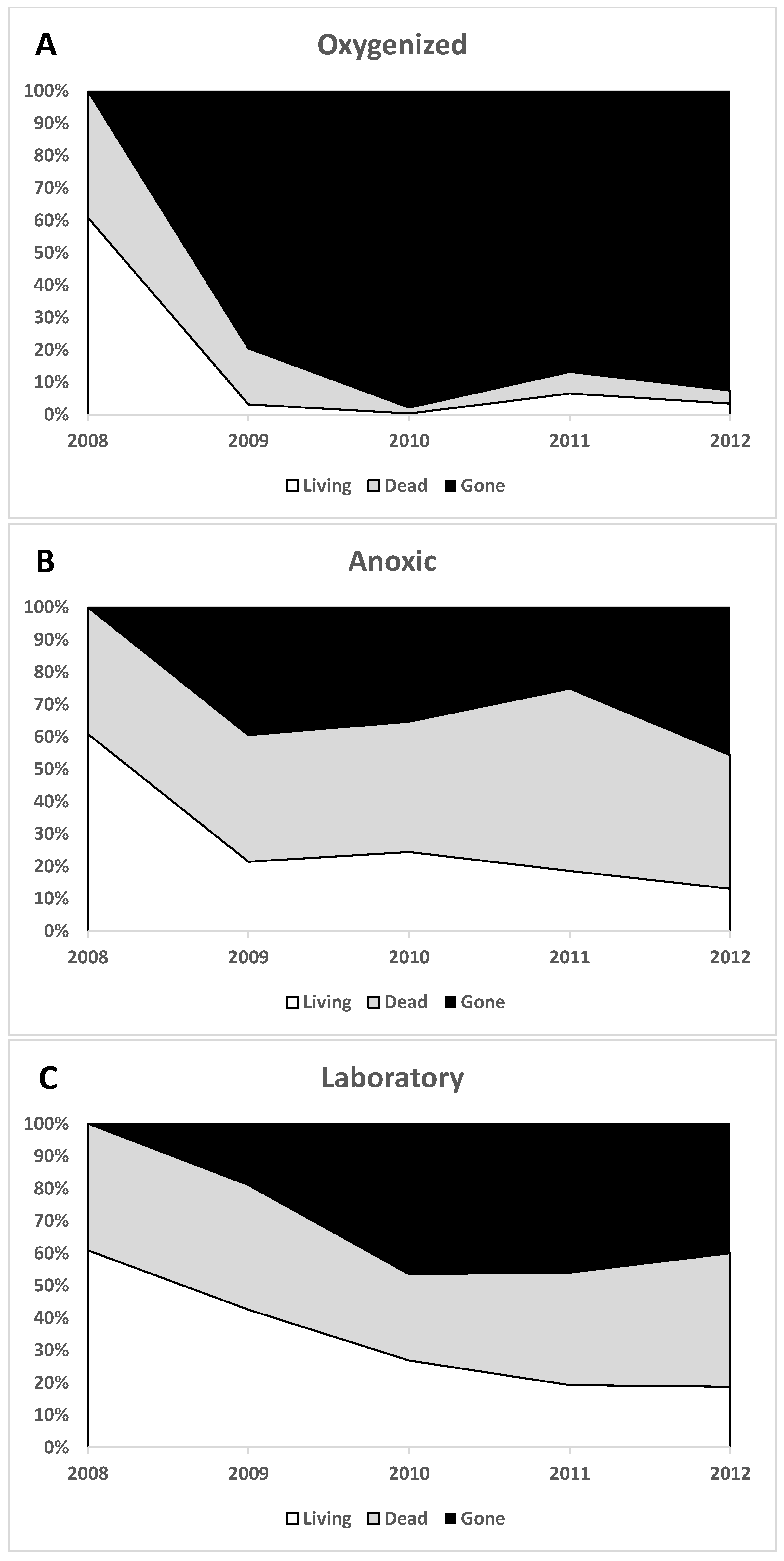
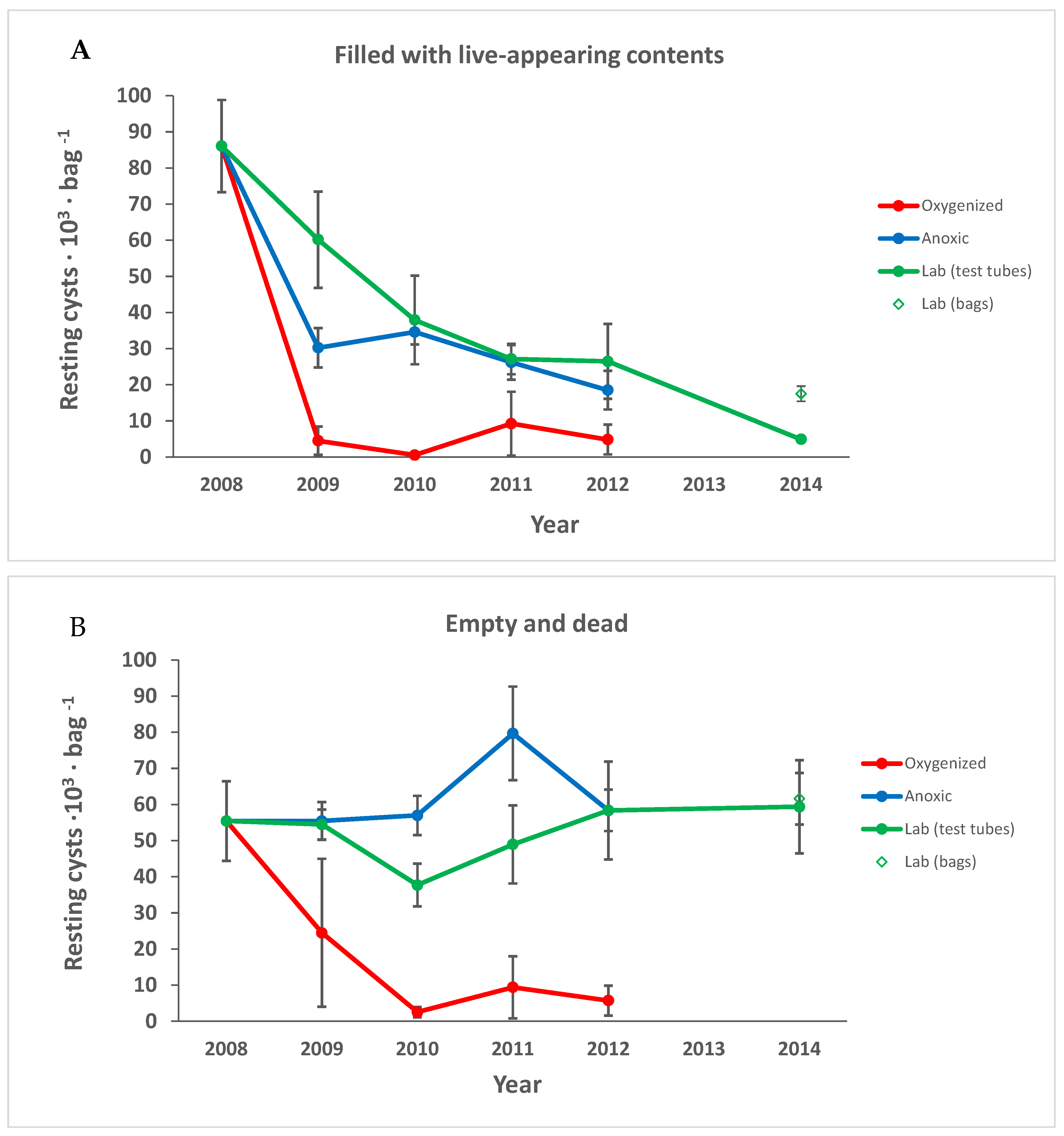
3.1. Cyst Numbers and Diversity
3.1.1. Preservation of Empty Cyst Walls
3.1.2. Variation
3.1.3. Macroenvironment Observations
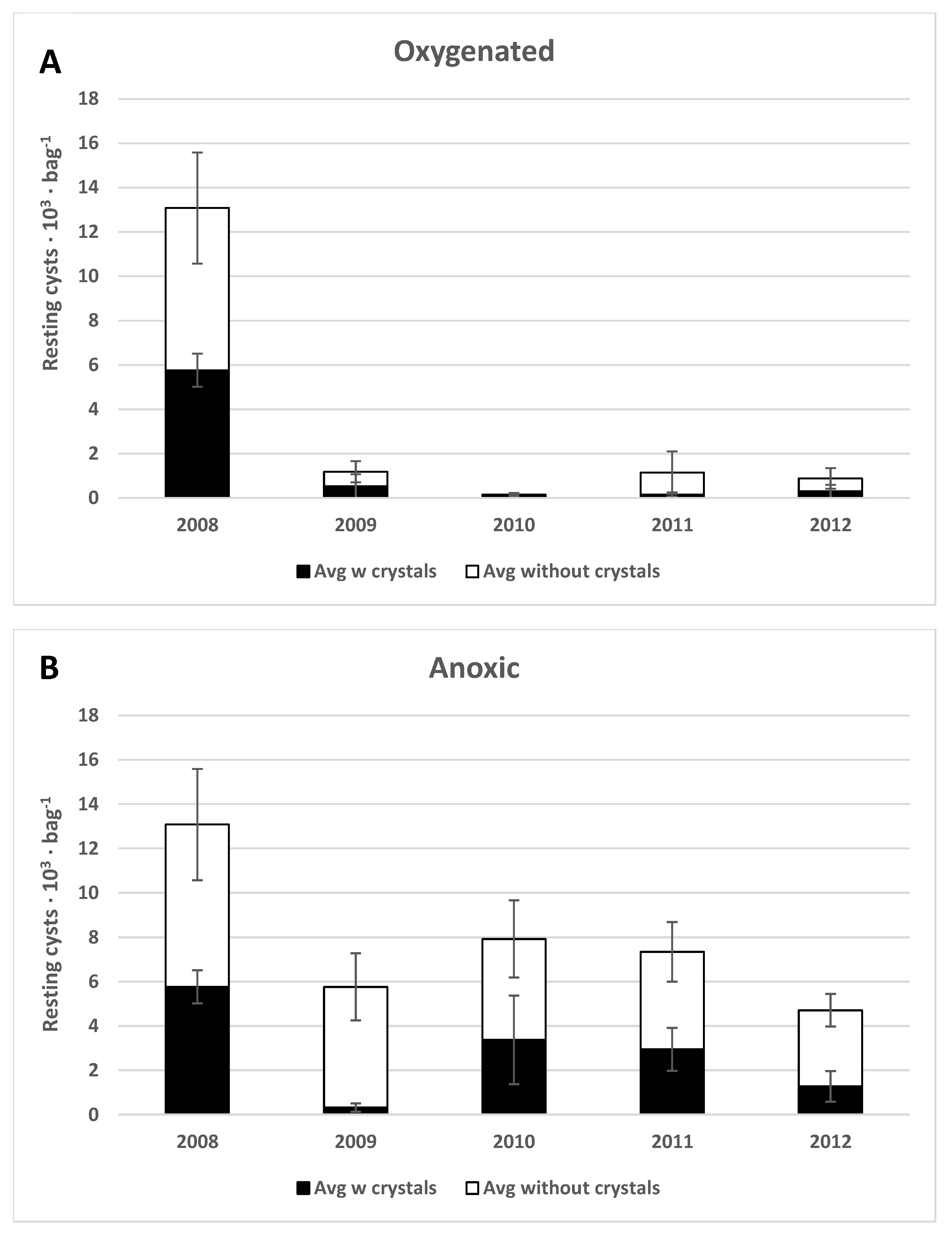
3.1.4. Species-Dependent Differences in Preservation
3.2. Gemination Experiments
3.3. Major Groups and Species of Specific Interest
3.3.1. Kryptoperidinium sp.
3.3.2. Diplopsalidaceae
3.3.3. Scrippsiella spp.
3.3.4. Pentapharsodinium dalei
3.3.5. Gonyaulacaceae
3.3.6. Protoperidinaceae
3.3.7. Gymnodiniales
3.4. Macrofauna
3.5. Meiofauna
4. Discussion
4.1. Preservation under Anoxic versus Oxygenated Conditions
4.2. Natural Variation in Dinoflagellate Cyst Beds
4.2.1. Variation between Seasons
4.2.2. How to Study the Same Living Sediment Sample over Time
4.2.3. Variation within Sites or Samples
4.3. Oxygen Availability and Organic Matter Degradation
4.3.1. Microbial Decomposition of Sediment
4.3.2. Bioturbation and Grazing
4.4. Dinoflagellates as Part of the Micro- and Meiofauna
4.5. Preservability of Living and Dead Dinoflagellate Cysts
4.6. Major Species/Groups and Species of Special Interest
4.6.1. Protoperidinum, Diplopsalids and Round Brown Cysts
4.6.2. Pentapharsodinium dalei
4.6.3. Scrippsiella spp.
4.6.4. The Gonyaulax Group
4.6.5. Margalefodinium polykrikoides
4.6.6. Peridinium quinquecorne
4.6.7. Kryptoperidinium sp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherr, E.B.; Sherr, B.F. Heterotrophic dinoflagellates: A significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 2007, 352, 187–197. [Google Scholar] [CrossRef]
- Jeong, H.J.; Du Yoo, Y.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef]
- Wang, D.-Z. Neurotoxins from Marine Dinoflagellates: A Brief Review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; Probyn, T.A. Suffocating phytoplankton, suffocating waters-red tides and anoxia. Front. Mar. Sci. 2016, 3, 186. [Google Scholar] [CrossRef]
- Dale, B. Dinoflagellate resting cysts: “benthic plankton”. In Survival Strategies of the Algae; Fryxell, G.A., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 69–136. [Google Scholar]
- Versteegh, G.J.M.; Blokker, P.; Bogus, K.A.; Harding, I.C.; Lewis, J.; Oltmanns, S.; Rochon, A.; Zonneveld, K.A.F. Infra red spectroscopy, flash pyrolysis, thermally assisted hydrolysis and methylation (THM) in the presence of tetramethylammonium hydroxide (TMAH) of cultured and sediment-derived Lingulodinium polyedrum (Dinoflagellata) cyst walls. Org. Geochem. 2012, 43, 92–102. [Google Scholar] [CrossRef]
- Bogus, K.; Mertens, K.N.; Lauwaert, J.; Harding, I.C.; Vrielinck, H.; Zonneveld, K.A.F.; Versteegh, G.J.M. Differences in the chemical composition of organic-walled dinoflagellate resting cysts from phototrophic and heterotrophic dinoflagellates. J. Phycol. 2014, 50, 254–266. [Google Scholar] [CrossRef]
- Mertens, K.N.; Gu, H.; Takano, Y.; Price, A.M.; Pospelova, V.; Bogus, K.; Versteegh, G.J.M.; Marret, F.; Turner, R.E.; Rabalais, N.N.; et al. The cyst-theca relationship of the dinoflagellate cyst Trinovantedinium pallidifulvum, with erection of Protoperidinium lousianensis sp. nov. and their phylogenetic position within the Conica group. Palynology 2017, 41, 183–202. [Google Scholar] [CrossRef]
- Persson, A.; Godhe, A.; Karlson, B. Dinoflagellate Cysts in Recent Sediments from the West Coast of Sweden. Bot. Mar. 2000, 43, 69–79. [Google Scholar] [CrossRef]
- Head, M.J. Modern dinoflagellate cysts and their biological affinities. In Palynology: Principles and Applications; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; pp. 1197–1248. [Google Scholar]
- Brosnahan, M.L.; Kulis, D.M.; Solow, A.R.; Erdner, D.L.; Percy, L.; Lewis, J.; Anderson, D.M. Outbreeding lethality between toxic Group I and nontoxic Group III Alexandrium tamarense spp. isolates: Predominance of heterotypic encystment and implications for mating interactions and biogeography. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 175–189. [Google Scholar] [CrossRef]
- López-Cortés, D.J.; Núñez-Vázquez, E.J.; Dorantes-Aranda, J.J.; Band-Schmidt, C.J.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Leyva-Valencia, I.; Fernández-Herrera, L.J. The State of Knowledge of Harmful Algal Blooms of Margalefidinium polykrikoides (a.k.a. Cochlodinium polykrikoides) in Latin America. Front. Mar. Sci. 2019, 6, 463. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Marret, F.; Versteegh, G.J.; Bogus, K.; Bonnet, S.; Bouimetarhan, I.; Crouch, E.; de Vernal, A.; Elshanawany, R.; Edwards, L.; et al. Atlas of modern dinoflagellate cyst distribution based on 2405 data points. Rev. Palaeobot. Palynol. 2013, 191, 1–197. [Google Scholar] [CrossRef]
- Jacobson, D.M.; Anderson, D.M. Thecate Heterophic Dinoflagellates: Feeding Behavior and Mechanisms. J. Phycol. 1986, 22, 249–258. [Google Scholar] [CrossRef]
- Schnepf, E.; Elbrächter, M. Nutritional strategies in dinoflagellates: A review with emphasis on cell biological aspects. Eur. J. Protistol. 1992, 28, 3–24. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Hoppenrath, M.; Saldarriaga, J.F. Dinoflagellate diversity and distribution. In Protist Diversity and Geographical Distribution; Foissner, W., Hawksworth, D.L., Eds.; Topics in Biodiversity and Conservation; Springer: Dordrecht, The Netherlands, 2007; Volume 8. [Google Scholar] [CrossRef]
- Sun, J.; Guo, S. Dinoflagellate Heterotrophy. Shengtai Xuebao Acta Ecol. Sin. 2011, 31, 6270–6286. [Google Scholar]
- Dodge, J.D. The functional and phylogenetic significance of dinoflagellate eyespots. BioSystems 1983, 16, 259–267. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y. Technical Guide for Modern Dinoflagellate Cyst Study; WESTPAC-HAB/WESTPAC/IOC: Tokyo, Japan, 2000; 29p. [Google Scholar]
- Lundholm, N.; Ribeiro, S.; Andersen, T.J.; Koch, T.; Godhe, A.; Ekelund, F.; Ellegaard, M. Buried alive—Germination of up to a century-old marine protist resting stages. Phycologia 2011, 50, 629–640. [Google Scholar] [CrossRef]
- Persson, A.; Rosenberg, R. Impact of grazing and bioturbation of marine benthic deposit feeders on dinoflagellate cysts. Harmful Algae 2003, 2, 43–50. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B.C.; Wikfors, G.H.; Quilliam, M. Grazing on toxic Alexandrium fundyense resting cysts and vegetative cells by the eastern oyster (Crassostrea virginica). Harmful Algae 2006, 5, 678–684. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B.C.; Dixon, M.S.; Wikfors, G.H. The Eastern mudsnail, Ilyanassa obsoleta, actively forages for, consumes, and digests cysts of the dinoflagellate, Scrippsiella lachrymosa. Malacologia 2008, 50, 341–345. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B. Grazing on a natural assemblage of ciliate and dinoflagellate cysts by the eastern oyster Crassostrea virginica. Aquat. Biol. 2009, 6, 227–233. [Google Scholar] [CrossRef][Green Version]
- Smith, B.C.; Persson, A.; Wikfors, G.H. A particle separator used to concentrate dinoflagellate cysts from sediment. Limnol. Oceanogr. Methods 2009, 7, 521–526. [Google Scholar] [CrossRef]
- Anderson, D.M.; Taylor, C.D.; Armbrust, E.V. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 1987, 32, 340–351. [Google Scholar] [CrossRef]
- Arndt, S.; Jørgensen, B.B.; LaRowe, D.E.; Middelburg, J.J.; Pancost, R.D.; Regnier, P. Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth-Sci. Rev. 2013, 123, 53–86. [Google Scholar] [CrossRef]
- Graue, J.; Kleindienst, S.; Lueders, T.; Cypionka, H.; Engelen, B. Identifying fermenting bacteria in anoxic tidal-flat sediments by a combination of microcalorimetry and ribosome-based stable-isotope probing. FEMS Microbiol. Ecol. 2012, 81, 78–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Montresor, M.; Zingone, A.; Sarno, D. Dinoflagellate cyst production at a coastal Mediterranean site. J. Plankton Res. 1998, 20, 2291–2312. [Google Scholar] [CrossRef]
- Shin, H.H.; Jung, S.W.; Jang, M.C.; Kim, Y.O. Effect of pH on the morphology and viability of Scrippsiella trochoidea cysts in the hypoxic zone of a eutrophied area. Harmful Algae 2013, 28, 37–45. [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Kim, Y.O.; Jung, S.W.; Han, M.S.; Lim, W.A.; Yoon, Y.H. Morphological features and viability of Scrippsiella trochoidea cysts isolated from fecal pellets of the polychaete Capitella sp. Harmful Algae 2014, 37, 47–52. [Google Scholar] [CrossRef]
- Head, M.J.; Lewis, J.; de Vernal, A. The cyst of the calcareous dinoflagellate Scrippsiella trifida: Resolving the fossil record of its organic wall with that of Alexandrium tamarense. J. Paleontol. 2006, 80, 1–18. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y.; Anderson, D.M. Methods for Modern Dinoflagellate Cyst Studies. In Red Tides: Biology, Environmental Science and Toxicology; Oxford University Press: Oxford, UK, 1989; pp. 461–479. [Google Scholar]
- Dale, B. Collection, preparation and identification of dinoflagellate resting cysts. In Toxic Dinoflagellate Blooms, Proceedings of the Second International Conference on Toxic Dinoflagellate Blooms, Key Biscayne, FL, USA, 31 October–5 November 1978; Taylor, D.L., Seliger, H.H., Eds.; Elsevier/North-Holland: New York, NY, USA, 1979; pp. 443–452. [Google Scholar]
- Zonneveld, K.A.F.; Versteegh, G.J.M.; de Lange, G.J. Preservation of organic-walled dinoflagellate cysts in different oxygen regimens: A 10,000 year natural experiment. Mar. Micropaleontol. 1997, 29, 393–405. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Bockelmann, F.; Holzwarth, U. Selective preservation of organic-walled dinoflagellate cysts as a tool to quantify past net primary production and bottom water oxygen concentrations. Mar. Geol. 2007, 237, 109–126. [Google Scholar] [CrossRef]
- Versteegh, G.J.M.; Zonneveld, K.A.F. Use of selective degradation to separate preservation from productivity. Geology 2002, 30, 615–618. [Google Scholar] [CrossRef]
- Kremp, A.; Shull, D.; Anderson, D. Effects of deposit-feeder gut passage and fecal pellet encapsulation on germination of dinoflagellate resting cysts. Mar. Ecol. Prog. Ser. 2003, 263, 65–73. [Google Scholar] [CrossRef]
- Montresor, M.; Nuzzo, L.; Mazzocchi, M.G. Viability of dinoflagellate cysts after the passage through the copepod gut. J. Exp. Mar. Biol. Ecol. 2003, 287, 209–221. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B.C. Consumption of Scrippsiella lachrymosa Resting Cysts by the Eastern Oyster (Crassostrea virginica). J. Shellfish Res. 2009, 28, 221–225. [Google Scholar] [CrossRef]
- Zeppilli, D.; Sarrazin, J.; Leduc, D.; Arbizu, P.M.; Fontaneto, D.; Fontanier, C.; Gooday, A.J.; Kristensen, R.M.; Ivanenko, V.; Sørensen, M.; et al. Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar. Biodivers. 2015, 45, 505–535. [Google Scholar] [CrossRef]
- Zeppilli, D.; Leduc, D.; Fontanier, C.; Fontaneto, D.; Fuchs, S.; Gooday, A.J.; Goineau, A.; Ingels, J.; Ivanenko, V.N.; Kristensen, R.M.; et al. Characteristics of meiofauna in extreme marine ecosystems: A review. Mar. Biodivers. 2017, 48, 35–71. [Google Scholar] [CrossRef]
- Persson, A. Proliferation of cryptic protists and germination of resting stages from untreated sediment samples with emphasis on dinoflagellates. Ophelia 2001, 55, 151–166. [Google Scholar] [CrossRef]
- Fenchel, T.; Finlay, B.J. The Ubiquity of Small Species: Patterns of Local and Global Diversity. BioScience 2004, 54, 777–784. [Google Scholar] [CrossRef]
- Kodrans-Nsiah, M.; de Lange, G.J.; Zonneveld, K.A. A natural exposure experiment on short-term species-selective aerobic degradation of dinoflagellate cysts. Rev. Palaeobot. Palynol. 2008, 152, 32–39. [Google Scholar] [CrossRef]
- Gray, D.D.; Zonneveld, K.A.; Versteegh, G.J. Species-specific sensitivity of dinoflagellate cysts to aerobic degradation: A five-year natural exposure experiment. Rev. Palaeobot. Palynol. 2017, 247, 175–187. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Gray, D.D.; Kuhn, G.; Versteegh, G.J.M. Postdepositional aerobic and anaerobic particulate organic matter degradation succession reflected by dinoflagellate cysts: The Madeira Abyssal Plain revisited. Mar. Geol. 2018, 408, 87–109. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Yang, L.; Mou, S.; Li, H.; Zhang, Z.; Jiao, N.; Zhang, Y. Terrestrial input of herbicides has significant impacts on phytoplankton and bacterioplankton communities in coastal waters. Limnol. Oceanogr. 2021, 66, 4028–4045. [Google Scholar] [CrossRef]
- Hennekam, R.; van der Bolt, B.; van Nes, E.H.; de Lange, G.J.; Scheffer, M.; Reichart, G.-J. Early-warning signals for marine anoxic events. Geophys. Res. Lett. 2020, 47, e2020GL089183. [Google Scholar] [CrossRef]
- Smayda, T.J. Turbulence, watermass stratification and harmful algal blooms: An alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 2002, 1, 95–112. [Google Scholar] [CrossRef]
- AECOM. Monitoring Survey of the Morris Cove Borrow Pit, September–October 2011. DAMOS Contribution No. 190; U.S. Army Corps of Engineers, New England District: Concord, MA, USA, 2012; 103p.
- Persson, A. On the Ecology of Cyst-Producing Dinoflagellates on the Swedish West Coast. Ph.D. Thesis, Department of Marine Botany, Botanical Institute, Göteborg University, Göteborg, Sweden, 2001; 122p. [Google Scholar]
- Anderson, D.M.; Chisholm, S.W.; Watras, C.J. Importance of life cycle events in the population dynamics of Gonyaulax tamarensis. Mar. Biol. 1983, 76, 179–189. [Google Scholar] [CrossRef]
- Anderson, D.M.; Keafer, B.A.; Kleindinst, J.L.; McGillicuddy, D.J.; Martin, J.L.; Norton, K.; Pilskaln, C.H.; Smith, J.L.; Sherwood, C.R.; Butman, B. Alexandrium fundyense cysts in the Gulf of Maine: Long-term time series of abundance and distribution, and linkages to past and future blooms. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 103, 6–26. [Google Scholar] [CrossRef]
- Erard, L.-D.E.; Desbruyeres, E.; Olu, K.; Erard-Le Denn, E.; Desbruyeres, E.; Olu, K. Alexandrium minutum: Resting cyst distribution in the sediments collected along the Brittany coast, France. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 3, pp. 109–114. [Google Scholar]
- Lee, M.-H.; Lee, J.-B.; Lee, J.-A.; Park, J.-G. Community Structure of Flagellates and Dynamics of Resting Cysts in Kamak Bay, Korea. Algae 1999, 14, 255–266. [Google Scholar]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R.I. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Gao, H.; You, S.; Lei, X.; Xiao, Y.; Gu, H.; Tong, M. The impact of biotic and abiotic factors on the distribution of surface sediment dinoflagellate cyst assemblages on the Nanji Island in the East China Sea. Acta Oceanol. Sin. 2019, 38, 160–171. [Google Scholar] [CrossRef]
- Miyazono, A.; Nagai, S.; Kudo, I.; Tanizawa, K. Viability of Alexandrium tamarense cysts in the sediment of Funka Bay, Hokkaido, Japan: Over a hundred year survival times for cysts. Harmful Algae 2012, 16, 81–88. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fukuyo, Y.; Matsuoka, K. Cyst methodologies. In Manual on Harmful Marine Microalgae; UNESCO Publishing: Paris, France, 2003; pp. 165–189. [Google Scholar]
- Feifel, K.M.; Fletcher, S.J.; Watson, L.R.; Moore, S.K.; Lessard, E.J. Alexandrium and Scrippsiella cyst viability and cytoplasmic fullness in a 60-cm sediment core from Sequim Bay, WA. Harmful Algae 2015, 47, 56–65. [Google Scholar] [CrossRef]
- Ribeiro, S.; Berge, T.; Lundholm, N.; Andersen, T.J.; Abrantes, F.; Ellegaard, M. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat. Commun. 2011, 2, 311. [Google Scholar] [CrossRef]
- Keafer, B.A.; Buesseler, K.O.; Anderson, D.M. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar. Micropaleontol. 1992, 20, 147–161. [Google Scholar] [CrossRef]
- Lewis, J.; Harris, A.S.D.; Jones, K.J.; Edmonds, R.L. Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. J. Plankton Res. 1999, 21, 343–354. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Versteegh, G.J.M.; Kasten, S.; Eglinton, T.I.; Emeis, K.-C.; Huguet, C.; Koch, B.P.; de Lange, G.J.; de Leeuw, J.W.; Middelburg, J.J.; et al. Selective preservation of organic matter in marine environments; Processes and impact on the sedimentary record. Biogeosciences 2010, 7, 483–511. [Google Scholar] [CrossRef]
- Bogus, K.A.; Zonneveld, K.A.F.; Fischer, D.; Kasten, S.; Bohrmann, G.; Versteegh, G.J.M. The effect of meter-scale lateral oxygen gradients at the sediment-water interface on selected organic matter based alteration, productivity and temperature proxies. Biogeosciences 2012, 9, 1553–1570. [Google Scholar] [CrossRef]
- Middelburg, J.J. Reviews and syntheses: To the bottom of carbon processing at the seafloor. Biogeosciences 2018, 15, 413–427. [Google Scholar] [CrossRef]
- Middelburg, J.J. Marine Carbon Biogeochemistry; Springer Briefs in Earth System Sciences; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Persson, A. Possible predation of cysts—A gap in the knowledge of dinoflagellate ecology? J. Plankton Res. 2000, 22, 803–809. [Google Scholar] [CrossRef][Green Version]
- Herman, P.M.J.; Middelburg, J.J.; van de Koppel, J.; Heip, C.H.R. Ecology of Estuarine Macrobenthos. Adv. Ecol. Res. 1999, 29, 195–240. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Warwick, R.M. Meiofauna Techniques. In Methods for the Study of Marine Benthos; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 253–284. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Radzikowski, J. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. J. Plankton Res. 2013, 35, 707–723. [Google Scholar] [CrossRef]
- Belmonte, G.; Rubino, F. Cysts and Resting Eggs from Marine Zooplankton: Dimension of the Phenomenon, Physiology of Rest, and Ecological and Biogeographic Implications. In Dormancy in Aquatic Organisms. Theory, Human Use and Modeling; Springer: Cham, Switzerland, 2019; pp. 71–94. [Google Scholar] [CrossRef]
- Binder, B.J.; Anderson, D.M. Biochemical composition and metabolic activity of Scrippsiella trochoidea (Dinophyceae) resting cysts. J. Phycol. 1990, 26, 289–298. [Google Scholar] [CrossRef]
- Heip, C.; Vincx, M.; Vranken, G.; Vranken, S. The Systematics and Ecology of Free-living Marine Nematodes. Helminthol. Abstr. 1982, 51, 1–37. [Google Scholar]
- Siano, R.; Montresor, M. Morphology, ultrastructure and feeding behaviour of Protoperidinium vorax sp. nov. (Dinophyceae, Peridiniales). Eur. J. Phycol. 2005, 40, 221–232. [Google Scholar] [CrossRef]
- Gribble, K.E. The Ecology, Life History, and Phylogeny of the Marine Thecate Heterotrophic Dinoflagellates Protoperidinium and Diplopsalidaceae (Dinophyceae); Massachusetts Institute of Technology: Cambridge, MA, USA; Woods Hole Oceanographic Institution: Falmouth, MA, USA, 2006. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Hou, Y.; Miranda, L.; Bhattacharya, D. Development of a Dinoflagellate-Oriented PCR Primer Set Leads to Detection of Picoplanktonic Dinoflagellates from Long Island Sound. Appl. Environ. Microbiol. 2006, 72, 5626–5630. [Google Scholar] [CrossRef]
- Fenchel, T. Cosmopolitan microbes and their “cryptic” species. Aquat. Microb. Ecol. 2005, 41, 49–54. [Google Scholar] [CrossRef][Green Version]
- Martin, J.L.; LeGresley, M.M.; Hanke, A.R. Thirty years—Alexandrium fundyense cyst, bloom dynamics and shellfish toxicity in the Bay of Fundy, eastern Canada. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 103, 27–39. [Google Scholar] [CrossRef]
- Kawami, H.; Iwataki, M.; Matsuoka, K. A new diplopsalid species Oblea acanthocysta sp. nov. (Peridiniales, Dinophyceae). Plankton Benthos Res. 2006, 1, 183–190. [Google Scholar] [CrossRef][Green Version]
- Penaud, A.; Hardy, W.; Lambert, W.; Marret, F.; Masure, E.; Servais, T.; Siano, R.; Wary, M.; Mertens, K.N. Dinoflagellate Fossils: Geological and Biological Applications. Revue Micropaléontol. 2018, 61, 235–254. [Google Scholar] [CrossRef]
- Lewis, J.; Dodge, J.D.; Tett, P. Cyst-theca relationships in some Protoperidinium species (Peridiniales) from Scottish sea lochs. J. Micropalaeontol. 1984, 3, 25–34. [Google Scholar] [CrossRef][Green Version]
- Mertens, K.N.; Yamaguchi, A.; Takano, Y.; Pospelova, V.; Head, M.J.; Radi, T.; Pieńkowski, A.J.; de Vernal, A.; Kawami, H.; Matsuoka, K. A new heterotrophic dinoflagellate from the north-eastern pacific, Protoperidinium fukuyoi: Cyst-theca relationship, phylogeny, distribution and ecology. J. Eukaryot. Microbiol. 2013, 60, 545–563. [Google Scholar] [CrossRef]
- Ribeiro, S.; Lundholm, N.; Amorim, A.; Ellegaard, M. Protoperidinium minutum (Dinophyceae) from Portugal: Cyst-theca relationship and phylogenetic position on the basis of single-cell SSU and LSU rDNA sequencing. Phycologia 2010, 49, 48–63. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Dale, B. The cyst-motile stage relationships of Protoperidinium monospinum (Paulsen) Zonneveld et Dale comb.nov. and Gonyaulax verior (Dinophyta, Dinophyceae) from the Oslo Fjord (Norway). Phycologia 1994, 33, 359–368. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hoppenrath, M.; Pospelova, V.; Horiguchi, T.; Leander, B.S. Molecular phylogeny of the marine sand-dwelling dinoflagellate Herdmania litoralis and an emended description of the closely related planktonic genus Archaeperidinium Jörgensen. Eur. J. Phycol. 2011, 46, 98–112. [Google Scholar] [CrossRef]
- Mertens, K.N.; Yamaguchi, A.; Kawami, H.; Ribeiro, S.; Leander, B.S.; Price, A.M.; Pospelova, V.; Ellegaard, M.; Matsuoka, K. Archaeperidinium saanichi sp. nov.: A new species based on morphological variation of cyst and theca within the Archaeperidinium minutum Jörgensen 1912 species complex. Mar. Micropaleontol. 2012, 96–97, 48–62. [Google Scholar] [CrossRef]
- Liu, T.; Mertens, K.N.; Ribeiro, S.; Ellegaard, M.; Matsuoka, K.; Gu, H. Cyst-theca relationships and phylogenetic positions of Peridiniales (Dinophyceae) with two anterior intercalary plates, with description of Archaeperidinium bailongense sp. nov. and Protoperidinium fuzhouense sp. nov. Phycol. Res. 2015, 63, 134–151. [Google Scholar] [CrossRef]
- Kawami, H.; Matsuoka, K. A new cyst-theca relationship for Protoperidinium parthenopes Zingone and Montresor 1988 (Peridiniales, Dinophyceae). Palynology 2009, 33, 11–18. [Google Scholar] [CrossRef]
- Rubino, F.; Belmonte, M.; Galil, B.S. Plankton resting stages in recent sediments of Haifa port, Israel (Eastern Mediterranean)—Distribution, viability and potential environmental consequences. Mar. Pollut. Bull. 2017, 116, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.N.; Takano, Y.; Gu, H.; Yamaguchi, A.; Pospelova, V.; Ellegaard, M.; Matsuoka, K. Cyst-theca relationship of a new dinoflagellate with a spiny round brown cyst, Protoperidinium lewisiae sp. nov., and its comparison to the cyst of Oblea acanthocysta. Phycol. Res. 2015, 63, 110–124. [Google Scholar] [CrossRef]
- Matsuoka, K.; Head, M.J. Clarifying cyst-motile stage relationships in dinoflagellates. In Biological and Geological Perspectives of Dinoflagellates; Geological Society of London: London, UK, 2013; pp. 325–350. [Google Scholar] [CrossRef]
- Radi, T.; Bonnet, S.; Cormier, M.-A.; de Vernal, A.; Durantou, L.; Faubert, É.; Head, M.J.; Henry, M.; Pospelova, V.; Rochon, A.; et al. Operational taxonomy and (paleo-)autecology of round, brown, spiny dinoflagellate cysts from the Quaternary of high northern latitudes. Mar. Micropaleontol. 2013, 98, 41–57. [Google Scholar] [CrossRef]
- Lundholm, N.; Nielsen, L.R.; Ribeiro, S.; Ellegaard, M. Microsatellite markers for the palaeo-temperature indicator Pentapharsodinium dalei (Dinophyceae). J. Appl. Phycol. 2013, 26, 417–420. [Google Scholar] [CrossRef]
- de Vernal, A.; Hillaire-Marcel, C.; Rochon, A.; Fréchette, B.; Henry, M.; Solignac, S.; Bonnet, S. Dinocyst-based reconstructions of sea ice cover concentration during the Holocene in the Arctic Ocean, the northern North Atlantic Ocean and its adjacent seas. Quat. Sci. Rev. 2013, 79, 111–121. [Google Scholar] [CrossRef]
- Ribeiro, S.; Berge, T.; Lundholm, N.; Ellegaard, M. Hundred Years of Environmental Change and Phytoplankton Ecophysiological Variability Archived in Coastal Sediments. PLoS ONE 2013, 8, e61184. [Google Scholar] [CrossRef]
- Lundholm, N.; Ribeiro, S.; Godhe, A.; Rostgaard Nielsen, L.; Ellegaard, M. Exploring the impact of multidecadal environmental changes on the population genetic structure of a marine primary producer. Ecol. Evol. 2017, 7, 3132–3142. [Google Scholar] [CrossRef]
- Wall, D.; Guillard, R.R.L.; Dale, B.; Swift, E.; Watabe, N. Calcitic resting cysts in Peridinium trochoideum (Stein) Limmermann, an autotrophic marine dinoflagellate. Phycologica 1970, 9, 151–156. [Google Scholar] [CrossRef]
- Bolch, C.J.; Hallegraeff, G.M. Dinoflagellate Cysts in Recent Marine Sediments from Tasmania, Australia. Bot. Mar. 1990, 33, 173–192. [Google Scholar] [CrossRef]
- Xiaoping, G.; Dodge, J.D.; Lewis, J. An ultrastructural study of planozygotes and encystment of a marine dinoflagellate, Scrippsiella sp. Br. Phycol. J. 1989, 24, 153–165. [Google Scholar] [CrossRef]
- Rochon, A.; Lewis, J.; Ellegaard, M.; Harding, I.C. The Gonyaulax spinifera (Dinophyceae) “complex”: Perpetuating the paradox? Rev. Palaeobot. Palynol. 2009, 155, 52–60. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.-S.; Matsuoka, K.; Kim, S.-Y.; Shin, H.H. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J. Phycol. 2015, 51, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.N.; Gu, H.; Gurdebeke, P.R.; Takano, Y.; Clarke, D.; Aydin, H.; Li, Z.; Pospelova, V.; Shin, H.H.; Li, Z.; et al. A review of rare, poorly known, and morphologically problematic extant marine organic-walled dinoflagellate cyst taxa of the orders Gymnodiniales and Peridiniales from the Northern Hemisphere. Mar. Micropaleontol. 2020, 159, 101773. [Google Scholar] [CrossRef]
- Mudie, P.J.; Marret, F.; Mertens, K.N.; Shumilovskikh, L.; Leroy, S.A.G. Atlas of modern dinoflagellate cyst distributions in the Black Sea Corridor: From Aegean to Aral Seas, including Marmara, Black, Azov and Caspian Seas. Mar. Micropaleontol. 2017, 134, 1–152. [Google Scholar] [CrossRef]
- Faust, M.A.; Gulledge, R.A. Identifying Harmful Marine Dinoflagellates; National Museum of Natural History: Washington, DC, USA, 2002. [Google Scholar]
- Iwataki, M.; Takayama, H.; Takahashi, K.; Matsuoka, K. Taxonomy and distribution of the unarmored dinoflagellates Cochlodinium polykrikoides and C. fulvescens. In Marine Protists: Diversity and Dynamics; Springer: Tokyo, Japan, 2015; pp. 551–565. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae 2009, 8, 454–462. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 2010, 406, 19–31. [Google Scholar] [CrossRef]
- Jung, S.W.; Kang, D.; Kim, H.-J.; Shin, H.H.; Park, J.S.; Park, S.Y.; Lee, T.-K. Mapping distribution of cysts of recent dinoflagellate and Cochlodinium polykrikoides using next-generation sequencing and morphological approaches in South Sea, Korea. Sci. Rep. 2018, 8, 7011. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Muñetón-Gómez, M.D.S. Bloom of Peridinium quinquecorne abé in la Ensenada de la Paz, Gulf of California (July 2003). Acta Bot. Mex. 2008, 83, 33–47. [Google Scholar] [CrossRef]
- Rubino, F.; Belmonte, M.; Boero, F. Benthic recruitment for planktonic dinoflagellates: An experimental approach. Biol. Mar. Mediterr. 2009, 16, 158–161. [Google Scholar]
- Satta, T.C.; Anglès, S.; Garcés, E.; Lugliè, A.; Padedda, B.M.; Sechi, N. Dinoflagellate cysts in recent sediments from two semi-enclosed areas of the Western Mediterranean Sea subject to high human impact. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 256–267. [Google Scholar] [CrossRef]
- Yamada, N.; Sym, S.D.; Horiguchi, T. Identification of highly divergent diatom-derived chloroplasts in dinoflagellates, including a description of Durinskia kwazulunatalensis sp. nov. (Peridiniales, Dinophyceae). Mol. Biol. Evol. 2017, 34, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, M.; Tillmann, U.; Elbrächter, M.; Kusber, W.-H.; Hoppenrath, M. Glenodinium triquetrum Ehrenb. Is a species not of Heterocapsa F.Stein but of Kryptoperidinium Er.Lindem. (Kryptoperidiniaceae, Peridiniales). Phytotaxa 2019, 391, 155–158. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Del Carmen Merino-Virgilio, F.; Huerta-Quintanilla, D.A.; Gárate-Lizárraga, I.; Steidinge, K.A.; Aguilar-Trujillo, A.C.; Herrera-Silveira, J.A.; Espinosa-Matías, S.; Martínez-Mena, A. A Kryptoperidiniaceae species (Dinophyceae: Peridiniales) blooming in coastal Yucatan waters, Gulf of Mexico. Protistology 2020, 14, 58–69. [Google Scholar] [CrossRef]
- Imanian, B.; Keeling, P.J. The dinoflagellates Durinskia baltica and Kryptoperidinium foliaceum retain functionally overlapping mitochondria from two evolutionarily distinct lineages. BMC Evol. Biol. 2007, 7, 172. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Bravo, I.; Fraga, S.; Garcés, E.; Llaveria, G. The Life History and Cell Cycle of Kryptoperidinium foliaceum, A Dinoflagellate with Two Eukaryotic Nuclei. Protist 2009, 160, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Imanian, B.; Pombert, J.-F.; Keeling, P.J. The complete plastid genomes of the two “Dinotoms” Durinskia baltica and Kryptoperidinium foliaceum. PLoS ONE 2010, 5, e10711. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Howe, C.J. Integration of plastids with their hosts: Lessons learned from dinoflagellates. Proc. Natl. Acad. Sci. USA 2015, 112, 10247–10254. [Google Scholar] [CrossRef]
- Gottschling, M.; Čalasan, A.; Kretschmann, J.; Gu, H. Two new generic names for dinophytes harbouring a diatom as an endosymbiont, Blixaea and Unruhdinium (Kryptoperidiniaceae, Peridiniales). Phytotaxa 2017, 306, 296. [Google Scholar] [CrossRef]
- Yamada, N.; Bolton, J.J.; Trobajo, R.; Mann, D.G.; Dąbek, P.; Witkowski, A.; Onuma, R.; Horiguchi, T.; Kroth, P.G. Discovery of a kleptoplastic ‘dinotom’ dinoflagellate and the unique nuclear dynamics of converting kleptoplastids to permanent plastids. Sci. Rep. 2019, 9, 10474. [Google Scholar] [CrossRef]
- Dodge, J.D.; Crawford, R.M. Observations on the Fine Structure of the Eyespot and Associated Organelles in the Dinoflagellate Glenodinium Foliaceum. J. Cell Sci. 1969, 5, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, G. Reflective Properties of Different Eyespot Types in Dinoflagellates. Protist 1999, 150, 311–323. [Google Scholar] [CrossRef]
- Saburova, M.; Polikarpov, I.; AlYamani, F. First record of Kryptoperidinium foliaceum (Dinophyceae: Peridiniales) from a hypersaline environment in Kuwait, northwestern Arabian Gulf. Mar. Biodiv. Rec. 2012, 5, e104. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Calado, A.J. Süßwasserflora von Mitteleuropa, Bd. 6—Freshwater Flora of Central Europe, Vol. 6: Dinophyceae; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]







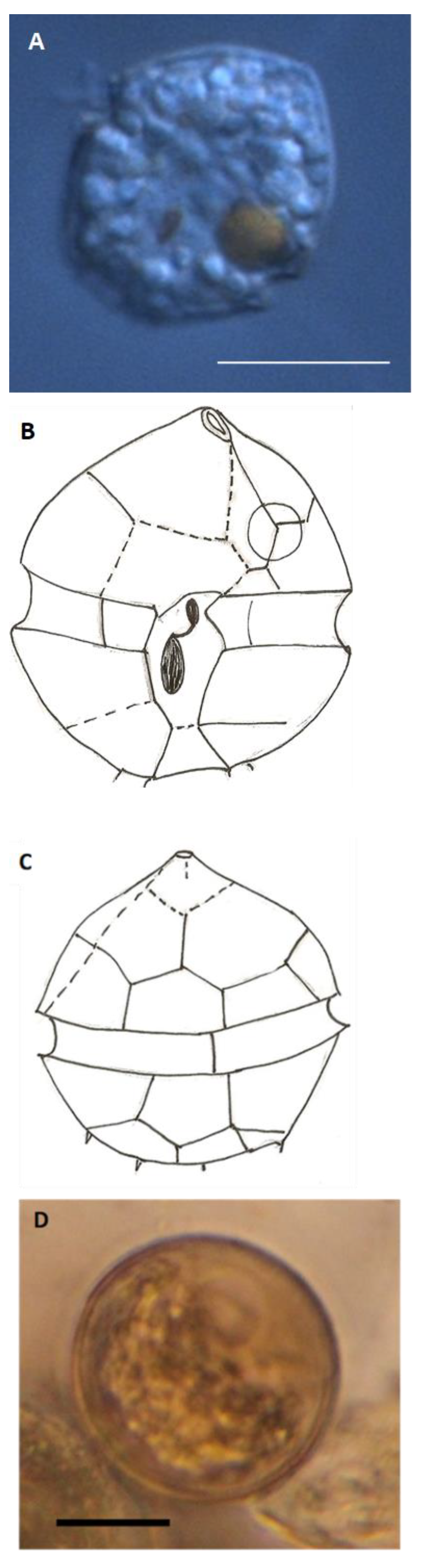
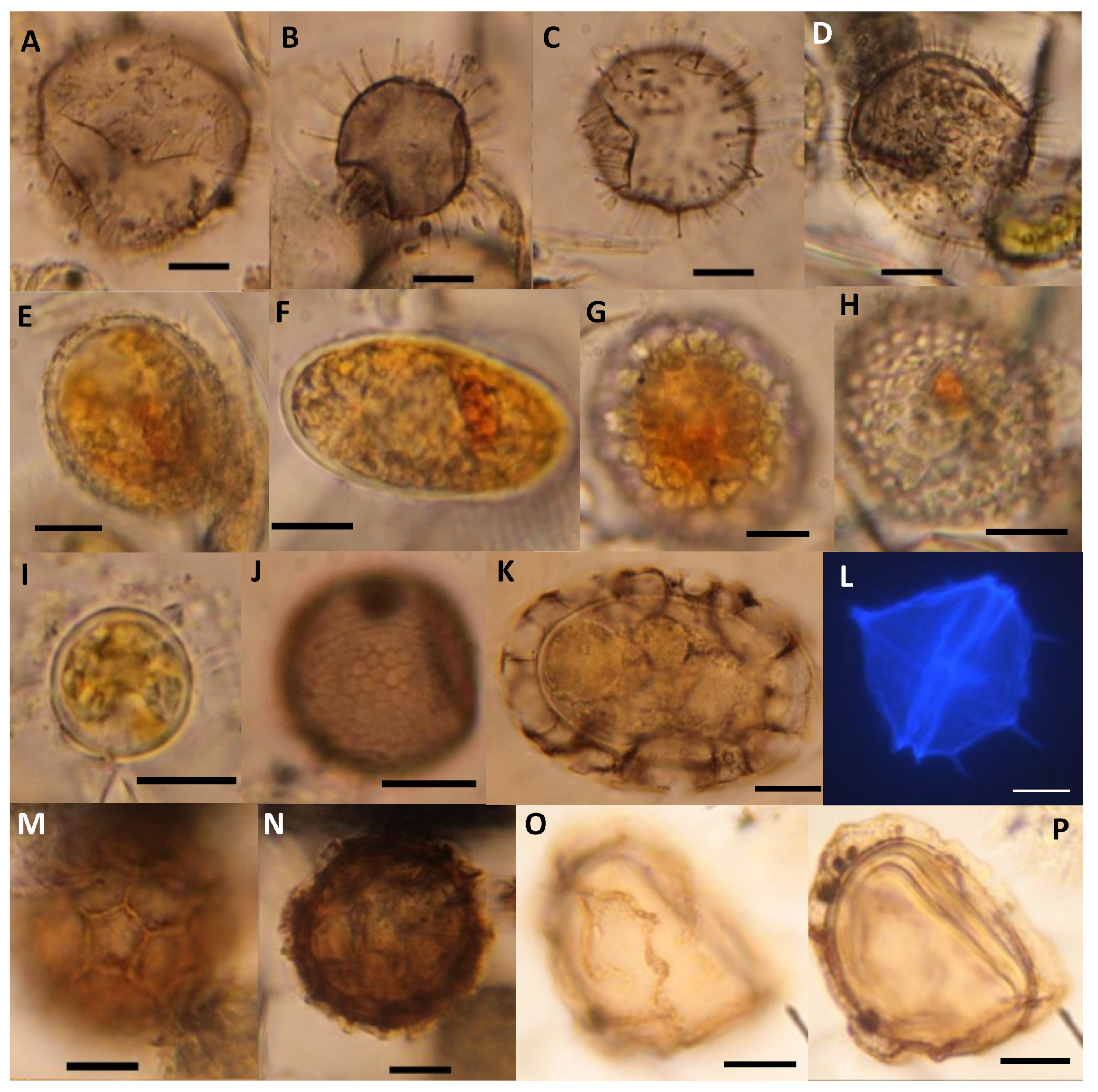
| Group | Proportion | Resting Cysts in Permanent Mounts | Vegetative Cells in Slurry Cultures |
|---|---|---|---|
| Gonyaulacales | |||
| U | <1% | Alexandrium spp. | Alexandrium spp. |
| F | <1% | Ataxiodinium choane | Unknown, likely autotrophic |
| F | <1% | Bitectatodinium tepikiense | Gonyaulax sp. |
| U | <1% | Gonyaulax verior | Gonyaulax verior |
| F | <1% | Lingulodinium polyedrum | Not seen (has characteristic shape) |
| F | <1% | Nematosphaeropsis labyrinthus | Gonyaulax spinifera |
| F | 25% ± 3% | Pentapharsodinium dalei | Pentapharsodinium dalei |
| F | <1% | Protoceratium reticulatum | Protoceratium reticulatum |
| F | <1% | Pyrophacus steinii | Not seen (has characteristic shape) |
| F | <1% | Spiniferites bentori (=Gonyaulax digitalis) | Gonyaulax digitalis |
| F | <1% | Spiniferites bulloideus (=Gonyaulax scrippsae) | Gonyaulax spinifera/Gonyaulax sp. |
| F | <1% | Spiniferites cf. furca | Gonyaulax spinifera |
| F | <1% | Spiniferites cf. membranaceum | Gonyaulax spinifera |
| F | <1% | Spiniferites mirabilis | Gonyaulax spinifera |
| F | <1% | Spiniferites elongatus | Gonyaulax spinifera |
| F | <2% | Spiniferites spp. unidentified | Gonyaulax spinifera |
| Gymnodiniales | |||
| F | <1% | Gymnodinium microreticulatum/nolleri spp. | Gymnodinium sp. |
| F | 4% ± 1% | Polykrikos kofoidii | Polykrikos |
| F | <1% | Polykrikos other spp. | Polykrikos |
| F | <1% | Margalefodinium polykrikoides | Margalefodinium polykrikoides |
| Peridiniales | |||
| F | <2% | Diplopelta symmetrica | Diplopsalis group |
| F | <2% | Diplopelta parva | Diplopsalis group |
| F | <1% | Diplopelta latipeltata | Diplopsalis group |
| F | <2% | Diplopsalis lenticula | Diplopsalis group |
| F | <1% | Diplopsalis orbicularis | Diplopsalis group |
| F | <2% | Diplopsalis group, unidentified | Diplopsalis group |
| F | <1% | Echinidinium with thin processes | Diplopsalis group |
| F | <2% | Echinidium cf. aceulatum | Diplopsalis group |
| F | <1% | Echinidinium, spp. with capitate processes | Diplopsalis group |
| F | <1% | Echinidinium, spp. with acuminate processes | Diplopsalis group |
| F | 3% ± 1% | Echinidinium/Islandinium spp., other | Diplopsalis group |
| F | <2% | Islandinium sp. | Diplopsalis group |
| F | <1% | Operculadinium israelianum cf. | Diplopsalis group |
| F | <1% | Protoperidinium americanum | Dinoshaped heterotroph |
| F | <1% | Protoperidinium cf. americanum, other spp. | Dinoshaped heterotroph |
| F | <1% | Protoperidinium claudicans | Protoperidinium sp. |
| F | <1% | Protoperidinium stellatum | Protoperidinium sp. with small antapical horns |
| F | <1% | Protoperidinium conicoides | Elongate rounded heterotroph; swimming wiggly |
| F | <2% | Protoperidinium conicum | Protoperidinium sp. with small antapical horns |
| F | <1% | Protoperidinium leonis | Protoperidinium sp. with small antapical horns |
| F | <1% | Archaeperidinium minutum | Dropshaped heterotroph, cf. P. minutum |
| F | <1% | Protoperidinium nudum | Dropshaped heterotroph, round with pointed apex |
| F | <1% | Protoperidinium oblongum group | Protoperidinium sp., large |
| F | <1% | Protoperidinium pentagonum | Protoperidinium sp. with small antapical horns |
| F | <1% | Protoperidinium spp. unidentified | Protoperidinium sp. |
| F | <1% | Square brown cyst with membrane | Unknown, likely Protoperidinium sp. |
| U | <1% | Scrippsiella cf. crystallina | Scrippsiella sp. |
| U | <1% | Scrippsiella trochoidea | Scrippsiella sp. |
| U | <3% | Scrippsiella spp. without crystals, elongate | Scrippsiella sp. |
| U | <2% | Scrippsiella sp., spherical | Scrippsiella sp. |
| U | <1% | Scrippsiella without and with dissolving crystals, spherical | Scrippsiella sp. |
| U | <1% | Scrippsiella spp. other | Scrippsiella sp. |
| Unidentified dinoflagellate cysts | |||
| F | <1% | Brown with buds/dents/spikes, different spp. | Unknown, likely heterotrophic |
| F | <1% | Unidentified brown spp. | Unknown, likely heterotrophic |
| F | <1% | Colorless spiny spp. | Unknown, likely autotrophic |
| U | 3% ± 4% | Colorless spp. With rugged surface/mucus | Unknown, likely autotrophic |
| U | 4% ± 4% | Colorless smooth spp. | Many different autotrophic |
| F | 11% ± 2% | Round brown smooth spp. | Different Diplopsalis and Protoperidinium spp. |
| G | 15% ± 4% | Round gray smooth | Kryptoperidinium sp. ? |
| Number of Germinated Dinoflagellate Species/Groups in Slurry Cultures | 2009 | 2010 | 2011 | 2012 | 2014 |
|---|---|---|---|---|---|
| Morris cove (anoxic) | 13 | 17 | 13 | 10 | |
| New Haven Harbor (oxygenated) | 5 | 3 | 6 | ||
| Laboratory (test tubes) | 14 | 16 | 19 | 1 | 1 |
| Laboratory, extra sediment (anoxic) | 19 | ||||
| Laboratory, bags in sediment (anoxic) | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persson, A.; Smith, B.C. Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments. Phycology 2022, 2, 384-418. https://doi.org/10.3390/phycology2040022
Persson A, Smith BC. Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments. Phycology. 2022; 2(4):384-418. https://doi.org/10.3390/phycology2040022
Chicago/Turabian StylePersson, Agneta, and Barry C. Smith. 2022. "Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments" Phycology 2, no. 4: 384-418. https://doi.org/10.3390/phycology2040022
APA StylePersson, A., & Smith, B. C. (2022). Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments. Phycology, 2(4), 384-418. https://doi.org/10.3390/phycology2040022






