Abstract
Alkaline–surfactant–polymer (ASP) flooding by means of which alkali additives, surfactant and polymer are inserted as the same slug is one of the most favourable worldwide focuses of Chemical Enhanced Oil Recovery (cEOR) research and field trials, due to the individual synergy of the three chemical components. To develop efficient oil recovery chemicals, it is essential to fully understand the mechanism behind ASP flooding. Nonetheless, there are hardly any studies reporting a systematic characterization of the ASP process. Thus, the present paper focuses on modelling this process in a laboratory by the use of an anionic surfactant—sodium dodecyl sulphate (SDS) in alkaline–polymer media—which is composed of a commercial water-soluble polymer (Flopaam AN125SH®, SNF Floerger, Andrézieux-Bouthéon, France) and alkali compounds (NaOH and Na2CO3). The samples were characterized using rheometry, dynamic light scattering (DLS), infrared spectroscopy (IR) and measurement of inferfacial tension (IFT) between the samples and rapeseed oil. In accordance with the experimental results, surprisingly lower IFT values were recorded between the alkaline–polymer solutions and rapeseed oil than the samples which contained SDS. Increasing polymer and sodium chloride concentration caused a decrease (from 0.591 mN/m to 0.0486 mN/m) in IFT between the surfactant containing samples and rapeseed oil. The IR measurements confirmed that the surfactant was not detected in the oil phase in the absence of NaOH and Na2CO3. The effects of SDS on the viscosity of the mixtures were also investigated, as viscosity is a considerably important parameter in processes using polymers.
1. Introduction
Anticipated global energy requirements in the future have necessitated the demand for developing processes aiming to exploit energy carrier materials [1]. Alkaline surfactant polymer (ASP) flooding is a technology process which was developed from the basis of alkali flooding, surfactant flooding and polymer flooding. The main function of surfactants is the reduction of interfacial tension (IFT) between two immiscible phases (in this case, oil and water); the rule of alkali is to react with the crude oil’s acidic components, generating surfactants in situ (the common name of these surfactants is soap), while the polymer increases the viscosity of the injected fluid, providing mobility control [2,3]. ASP flooding makes use of these three types of chemicals: alkali, surfactant and polymer. This approach merges the macroscopic volumetric sweep efficiency increase from the polymer with the ability of detergents (to differentiate from an added synthetic surfactant, the in situ generated surfactant is generally called soap) to improve microscopic sweep efficiency [4,5]. Alkali additives form soaps through reaction with organic acid type compounds of the crude oil, which operates in synergy with the added surfactant to produce ultra-low IFT [6,7]. The ultra-low IFT is achieved by surfactant distribution between hydrophobic and aqueous phases, and surfactant disposition at interface of oil/water. The surfactant distribution is dependent on pH and ionic strength value [8].
Alkali is defined as a basic, ionic salt of an alkali metal or alkaline earth metal element. The roles of alkali in chemical flooding include promotion of crude oil emulsification, enhancing aqueous-phase ionic strength, and leading to optimization of phase behaviour of the injected surfactant. Furthermore, the presence of alkali reduces detergent adsorption by causing an increase in the stone surface’s negative density [9,10,11]. The most generally used alkaline agents are, e.g., sodium hydroxide (NaOH, or caustic soda), sodium carbonate (Na2CO3, or soda ash), sodium bicarbonate (NaHCO3) and sodium metaborate (NaBO2) [12,13].
Polymer flooding involves addition of a polymer to the liquid content of a waterflood, to lower its mobility. Polymer causes an increase in the viscosity of the aqueous phase, along with a reduction of water permeability. Mixing with a more viscous phase, the accumulated oil basis can be more easily exploited from the reservoir [14,15]. The polymer flooding was described to possess diverse advantages compared to waterflooding, covering improved mobility of the injected fluid, less water demand and reasonable pricing [16,17].
In ASP systems, different synergies and interactions were described; the addition of alkali compounds minimizes the adsorption (accumulation on solid surfaces) of both surfactant and polymer. The alkali also reacts with oil’s acidic components, generating soap. Soap has low optimum salinity; nonetheless, synthetic surfactants have comparatively higher optimum salinity. Consequently, the intermixture of soap and the synthetic surfactant has an extended variety of salinity in which the IFT is low. Other parameter to consider is that forms of emulsions advance the sweep efficiency. Polymer helps to stabilize emulsions because of its high viscosity to retard coalescence. The other rule of polymer is to increase the viscosity of the alkaline-polymer solution so developing sweep efficiency. However, alkali can reduce polymer viscosity owing to the higher salt concentration created by the alkali (this is an undesired effect) [18,19,20,21].
The successful use of the alkaline surfactant polymer flooding process in many fields has been reported [22,23,24,25,26,27,28].
In the literature, the use of injected surfactant compounds is also mentioned. It means that the polymer is mixed with the surfactant, the mixture will contain more detergent than alkali compounds can generate in situ. However, these injected surfactant containing systems are slightly characterized.
The aim of this study was to model the interactions between the compounds of ASP mixtures under laboratory circumstances, considering the use of sodium dodecylsulphate (SDS) as the initial surfactant compound. For the study, rapeseed oil was used, which also contained free acidic compounds which the sodium hydroxide can transform to surfactant-type compounds. The amount of sodium hydroxide and sodium carbonate were set based on a study which focused on the optimization process of an alkali–polymer flooding system [29].
The experimental results indicate that addition of SDS did not result in further interfacial tension decrease compared to the alkaline–polymer solutions; moreover, in the absence of NaOH and Na2CO3 the presence of the surfactant in the oil phase was not measured. The rheological properties of the prepared solutions were also determined, as one of the most important parameters during polymer-applying processes is viscosity.
Other parameter to consider during this process is the formation of foam, which is an oil–water emulsion stabilized by surface active components, and may also contain polymer and some stabilizers [30].
2. Materials and Methods
Sodium dodecyl sulphate (C12H25NaO4S, SDS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium hydroxide (NaOH), sodium carbonate (Na2CO3) and sodium chloride (NaCl) were purchased from VWR Hungary (Debrecen, Hungary). Flopaam AN125SH® polymer (manufacturer: SNF Floerger, St. Etienne, France) and high erucic acid variety rapeseed oil were provided by MOL Hungarian Oil and Gas Company Plc. Flopaam AN125SH is a copolymer of polyacrylamide and sodium acrylamido tert butyl sulfuric acid, the polymer is approximately 25% hydrolysed and its molecular weight is approximately 8 million dalton. All chemicals were used without further purification. All solutions were prepared by MilliQ-MilliPore water (VWR Hungary).
2.1. Preparation of the Samples
The polymer solutions were prepared with 0.4 m/m% NaOH and 0.2 m/m% Na2CO3 content [31]. The polymer concentration was set in the samples to 0.75 g/L, 1.0 g/L and 2.0 g/L to optimize the mixture’s composition. The polymers were stirred for 24 h to get homogeneous solution, and SDS was dissolved in 5.0 g/L concentration in the polymer media. The ionic strength was increased in the samples by addition of sodium chloride in 0.1 M, 0.3 M and 0.5 M, respectively. The flow behaviour of the polymer solutions was also determined in 1.0 g/L (1000 ppm) polymer containing solutions.
The samples were characterized by using DLS, IR, rheometry and IFT measurement.
2.2. Characterization of SDS in Alkaline–Polymer Mixtures
The interfacial tension (IFT) values between the alkali compounds and polymer containing SDS samples and rapeseed oil were measured by using a Krűss spinning drop tensiometer (Krűss GmbH, Hamburg, Germany), which was maintained at a temperature of at 25 °C. A 4.0 μL sample was injected to the capillary. The IFT values were recorded at 4500 rpm angular velocity. One way to calculate the interfacial tension is the Vonnegut Equation (1), which is applicable if the elongation of the bubble stops when the centrifugal forces are balanced by the surface tension forces, supposing that the length of the bubble is comparable to the radius, which leads to a cylindrical shape.
In Equation (1), σ symbolizes interfacial tension, Δρ the density difference between the two immiscible phases, ω the angular velocity and R the cylinder radius [32].
The Young–Laplace Equation (2) [31] is the other equation to calculate interfacial tension. This rule can be applied to characterize spherical and non-spherical shapes in the absence as well as under the influence of an external field. Any non-spherical surface requires at least two orthogonal maximum curvature radii, and the pressure difference between two phases can be used to measure interfacial tension.
In Equation (2), σ is the interfacial tension, ΔP is the pressure difference between two phases, R is the orthogonal maximum curvature radii.
Exported files include pictures recorded during the measurement, characterizing the droplet by volume values (in case of Young–Laplace fitting) or fitted rectangle heights (in case of Vonnegut fitting).
The size of the micelles was measured by dynamic light scattering measurements (DLS) using a Horiba Sz-100 (HORIBA Jobin Yvon, Longjumeau, France) equipped with a diode pumped frequency doubled (532 nm, 10 mW) laser. The detection angle was 90° in every case and the temperature was 25 ± 0.1 °C. For the measurements, the viscosity of the solvent and the refractive index of the measured particles were the input parameters. To acquire the particle size distribution (dZ-average, nm), the software utilizes the cumulant method for calculation; afterwards, the histogram method is used to get the mean and the standard deviation of the distribution (d ± S.D. in nm).
The shear rheology was measured via a stress-controlled rheometer (Anton Paar Modular Compact Rheometer (MCR 302) fitted with double gap, roller geometry [33] (DG 26.7) and pressure cell [34] (Anton Paar Germany GmbH, Ostfildern, Germany). After loading the samples, to enable stress relaxation and temperature equilibration, a 5 min equilibration time was set. The system of the rheometer was thermostated by a Peltier cell, measurements were performed at 25 °C. Processing the rotational test measurements, the scale of shear rate (γ*) from 1 to 1000 1/s was set, shear stress (τ/Pa) and apparent viscosity (η/mPas) were registered under each shear rate. Each measurement run comprised 20 measurement points. For data collection the Anton Paar RheoCompass software (1.25) (Anton Paar Germany GmbH, Ostfildern, Germany) was used. The experimental data of flow curves were calculated based on the Herschel–Bulkley model, which is described in (3).
In the occurrence of a Newtonian fluid, the value of flow index (n) near to the unit and the yield stress (τ0) are calculated from the intersection of the extrapolated linear portion of the flow curves, and from the slope if this linear section the consistency index (k) can be derived. According to the flow index, the Herschel–Bulkley fluid behaves as shear-thinning, when n < 1, and in case of n > 1 the material is called shear-thickening [35].
2.3. Determination the Acid Value of Rapeseed Oil
The acid value is defined as the amount of sodium hydroxide that is necessary to neutralize the free fatty acids in 1.0 g oil. Acid number was determined using titration: 1.0 g of rapeseed oil was dissolved in 10 cm3 isopropanol. The determination was processed by titration of the oil sample in the presence of phenolphthalein, with 0.1039 mol/L sodium hydroxide [36]. The acid value of rapeseed oil was found to be 2.0–3.0 g/100 g NaOH.
2.4. Infrared Spectroscopic Measurements
The infrared spectroscopic measurements were carried out by using a JASCO FT/IR-4700 spectrophotometer equipped with a ZnSe ATR accessory and a DTGS detector. The spectra were recorded in the 4000–650 cm−1 wavenumber range with 4 cm−1 resolution, accumulating 256 scans.
3. Results and Discussion
3.1. Measurement of Interfacial Tension (IFT) Values of the Surfactant–, Polymer–, and Alkaline–Surfactant–Polymer Mixtures
First, the IFT values were determined during a comprehensive process to investigate the effects of the polymer, alkali compounds and the ionic strength of the media on IFT. The values were measured by using a spinning drop tensiometer between the prepared samples and the rapeseed oil, whose acid value was 2–3 mg/100 g NaOH. The interfacial tension of aqueous and NaCl containing SDS solutions were measured in the presence of the alkali additives. As a reference measurement, the interfacial tension was also recorded between milliQ water and rapeseed oil, and this value was determined as 10.769 ± 0.53 mN/m. The amount of the alkali additives (CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%) were set according to published field experiment studies [29].
The aqueous 5.0 g/L SDS solution possessed a considerably higher IFT value (0.591 ± 0.0025 mN/m); it indicates the presence of NaCl to decrease (0.083–0.099 mN/m) the IFT value between SDS and rapeseed oil. This reliance on the ionic strength reveals that the adsorbed amount of surfactant depends on the ionic strength value. By increasing the ionic strength, the charge of the head groups is screened, facilitating more surfactant molecules to adsorb to the droplet surface, further decreasing interfacial tension [37].
During the optimalization of the solutions’s composition, the polymer concentration was increased in the presence of different media. The IFT values between the prepared aqueous and alkali containing polymer solutions and rapeseed oil indicate how the polymer media is expected to affect the properties of SDS. The IFT values of the aqueous and alkaline–polymer solutions are listed in Table 1.

Table 1.
Recorded IFT values between the aqueous and alkaline–polymer solutions and rapeseed oil (CSDS = 0 g/L, CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%).
According to the measured values, the IFT between the aqueous (alkali-free) polymer solutions and rapeseed oil slightly increased by the increase of the sodium chloride concentration in the solutions (Panel#2), but the measured values were higher than 10 mN/m in all three cases. Addition of the alkali compounds caused a considerable decrease (0.0247–0.0484 mN/m) in the IFT values of the polymer solutions (comparison of Panel#4 with Panel#3–5).
This decrease is caused by the alkali additives, which formed soap with the fatty acids in the oil during a saponification-type reaction. The IFT was measured between milliQ water and rapeseed oil, and this value (10.769 ± 0.534 mN/m) confirmed the effect of the saponification process in decreasing IFT between the current investigated alkaline–polymer mixtures and rapeseed oil. The increasing ionic strength (cNaCl) caused an IFT reduction in the case of polymer-free samples (Panel#1), the effect of which was not observable in the case of polymer containing samples (Panel#3–5). The IFT values of the alkaline–polymer solutions, depending on the polymer concentration, are illustrated in Figure 1.
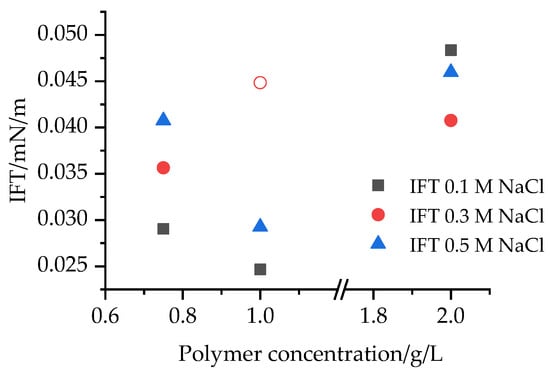
Figure 1.
IFT values of the alkaline–polymer solutions depending on the polymer concentration.
Considering the effects of the salt concentration on the IFT values, it can be concluded that in 0.75 g/L polymer containing alkaline–polymer solutions, the IFT values increased with the increasing sodium chloride concentration. Increasing the polymer concentration the effects of the metal salts on the IFT was determined to be unambiguous; among the 1.0 g/L polymer containing solutions the lowest IFT value was recorded in presence of 0.1 M NaCl, while among the 2.0 g/L polymer containing solutions in presence of 0.3 M NaCl. These results indicate that increasing the polymer concentration of the aqueous polymer solutions (which caused higher viscosity) caused the IFT values to decrease. However, increasing the polymer concentration increased the viscosity of the solutions, but did not increase the salt resistance. It also can be observed that among the alkaline–polymer solutions, the lowest IFT value was measured in the 1.0 g/L Flopaam AN125SH and 0.1 mol/L NaCl containing sample.
Considering the effects of the polymer concentration on the IFT values confirms the previously discussed results, as the lowest IFT value was measured in case of the 1.0 g/L polymer and 0.1 mol/L sodium chloride containing sample, but the effect of the polymer concentration was found to be unambiguous; in presence of 0.1 M and 0.5 M NaCl the lowest IFT value was measured in case of the 1.0 g/L concentrated polymer solution, while in presence of 0.3 M NaCl the lowest IFT was recorded measuring the 2.0 g/L polymer solution. As a reference measurement, the IFT between the alkaline solvents and rapeseed oil was also determined. The solvents decreased the IFT more than the aqueous polymer solutions, but less than mixing them with the polymer. These values confirm the importance of the alkali compounds in reducing IFT through a saponific-type reaction, and also the viscosity increasing role of the polymer in decreasing the IFT.
The abovementioned alkaline–polymer solutions were further investigated as the media of SDS surfactant. The IFT values between the prepared alkaline–polymer–surfactant mixtures and rapeseed oil were also measured (Figure 2).
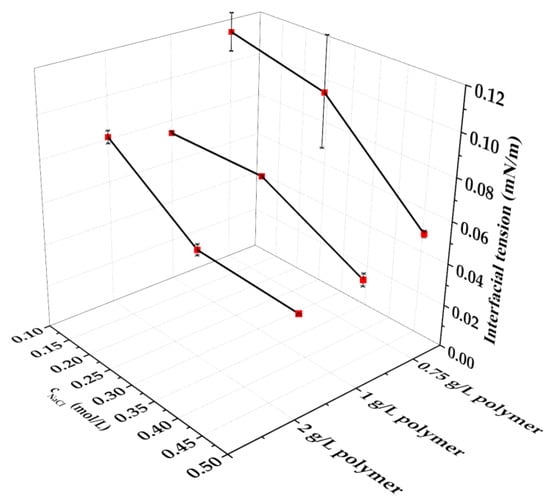
Figure 2.
IFT values recorded between the alkaline–polymer SDS solutions and rapeseed oil (CSDS = 5.0 g/L, CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%), error bars represent standard deviation.
It can be observed from the figure that the increasing polymer concentration results slightly lower IFT while the values increased as a result of increasing NaCl concentration. Considering the effects of the sodium chloride concentration on the IFT of the alkaline–polymer containing SDS solutions the lowest IFT value was recorded in the 1.0 g/L polymer containing sample, while in presence of 0.3 M and 0.5 M sodium chloride the lowest IFT values were recorded in 2.0 g/L polymer containing solutions. In the polymer containing SDS solutions, the increasing sodium chloride concentration caused the measure of decreasing IFT values.
The lowest IFT value was recorded between the 2.0 g/L Flopaam AN125SH and 0.5 mol/L containing sodium chloride containing SDS solution and rapeseed oil. Considering the effects of the polymer concentration on the IFT values, it can be observed that the lowest IFT values were recorded in presence of 0.5 mol/L at all the investigated (0.75–2.0 g/L) polymer concentrations, and the decrease of the IFT was observed to be monotonous by decreasing the sodium chloride concentration.
Strikingly, between the alkaline–surfactant–polymer solutions and rapeseed oil, higher IFT values were recorded (from 0.0486 mN/m to 0.114 mN/m) than in the alkaline–polymer solutions (from 0.0247 mN/m to 0.0460 mN/m). To elucidate this phenomenon, the interactions between the injected samples and the rapeseed oil were also characterized.
The interactions between the surfactant and the rapeseed oil were also determined based on the results of the interfacial tension measurements carried out by using the spinning drop tensiometer, and these interactions are demonstrated by photos recorded by the spinning drop instrument. Figure 3 demonstrates the oil droplet in presence of the 5.0 g/L SDS aqueous (alkali-free) solution.
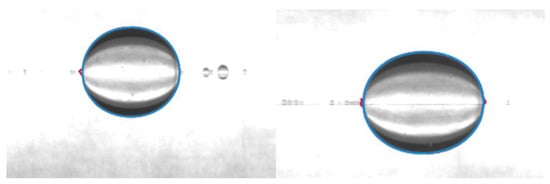
Figure 3.
Exported spinning drop instrument picture of the structure of the rapeseed oil droplet in presence of 5.0 g/L aqueous SDS solution after 1.0 min and 15.0 min (droplet volume = 0.35 μL).
As represented in the tensiometer picture, the surfactant positioned around the oil droplet, and the SDS did not dissolve into the inner phase of the hydrophobic phase.
The interactions between the rapeseed oil and the alkali containing SDS solution are shown in Figure 4.

Figure 4.
Exported spinning drop instrument picture of the structure of the rapeseed oil droplet in presence of 0.4 m/m% NaOH, 0.2 m/m% Na2CO3 and 0.5 mol/L NaCl containing 5.0 g/L aqueous SDS solution after 1.0 min and 15.0 min (droplet height = 0.46 mm).
Both the shape of the droplet and the pattern of the picture were different compared to the measurement between the shape of the oil droplet in presence of the aqueous surfactant solution. Considering the shape, a spheric droplet could not be identified. The surfactant solution was observed in the inner phase of the rapeseed oil from the beginning of the measurement. Comparing the results with the previously demonstrated ones, it can be observed that the presence of the alkali components and sodium chloride led to the formation of a surfactant–salt composite, which induced the dissolution of the surfactant into the oil phase. This phenomenon can be considered as a quick procession, as the surfactant was detected inside the rapeseed oil droplet during the first minute of the measurement.
The picture about the rapeseed oil droplet in presence of an alkaline–polymer–surfactant solution is shown in Figure 5.

Figure 5.
Exported spinning drop instrument picture of the structure of the rapeseed oil droplet in presence of 2.0 g/L Flopaam AN125SH, 0.4 m/m% NaOH, 0.2 m/m% Na2CO3 and 0.3 mol/L NaCl containing 5.0 g/L aqueous SDS after 1.0 min and 15.0 min (droplet height = 0.41 mm).
Comparing the results shown in Figure 5 with the previously demonstrated ones, it can be observed that during the first minute of the measurement the oil droplet could be detected to encircle the rapeseed oil droplet. This is a considerable difference compared to the polymer non-containing SDS solution, where the surfactant phase was detected inside the droplet during the whole measurement. However, when observing the mixture at the end of the measurement, the same pattern was observed; as was shown in Figure 3, the surfactant was located inside of the rapeseed oil droplet. Comparing Figure 4 and Figure 5 indicated that the polymer had an inhibition on the dissolution of the surfactant phase into the rapeseed oil droplet. However, this phenomenon was also observed in the presence of the polymer by the end of the measurement.
To further confirm the above conclusions, emulsions were prepared and the oil phases were characterized using FT-IR spectroscopy. Aqueous and alkali containing surfactant solutions were prepared, the alkaline media contained 4.0 m/m% NaOH, 2.0 m/m% Na2CO3 and 0.1 M NaCl. The solutions were mixed with the rapeseed oil in 1:1 volume ratio and stirred at 1500 rpm for 2 min. After phase separation, the infrared spectra of oil phases were recorded.
The infrared spectra of the surfactant and rapeseed oil are shown in Figure 6.
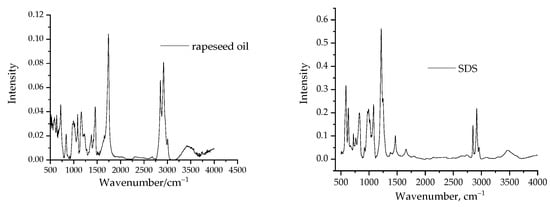
Figure 6.
Infrared spectra of the pure rapeseed oil and sodium dodecylsulphate.
These spectra were used as reference measurements to investigate the presence of the surfactant in the separated oil phases. The infrared spectra of the rapeseed oil after mixing with surfactant solutions are shown in Figure 7.
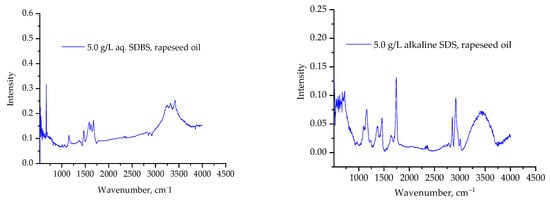
Figure 7.
Infrared spectra of rapeseed oil phases mixed with aqueous and alkaline containing sodium dodecylsulphate solutions.
The spectra of rapeseed oil contained largely very weak absorption bands with characteristic peak at 672 cm−1, and some hardly detectable bands at 1485 cm−1 and 1741 cm−1, respectively. Comparing these spectra (Figure 6) with that of the oil phase mixed with aqueous SDS solution (Figure 7), it can be concluded that the surfactant did not dissolve in the oil phase under these conditions; in Figure 7 only the abovementioned bands of the rapeseed oil can be detected. A close inspection of each spectra led us to conclude that the infrared spectra did not show the presence of SDS in the oil phase in absence of alkali compounds. Comparing the spectra with the oil phase mixed with an alkali additive containing SDS solution (Figure 7), the signals of rapeseed oil are present in the spectra, but the other new signs are related to the presence of SDS in the oil phase: −CH2− groups (1463 cm−1, 2819 cm−1), methyl groups (2929 cm−1, 2857 cm−1) and sulphate group (1231 cm−1 and 1380 cm−1), respectively.
3.2. Measurement of Micelle Size of the SDS Solutions
The critical micelle concentration (CMC) of sodium dodecylsulphate was determined in numerous studies, among the experimental techniques including using tensiometry. Depending on the surfactant’s concentration, the surface tension decreased from 55.0 to 30.0 mN/m in deionized water, and the CMC was determined as a value of 8.03 mmol/L, which is equal to 2.32 g/L [38]. As the prepared samples contained sodium dodecylsulphate in 5.0 g/L concentration, it is ensured that the characterisation of the polymer–surfactant mixtures indicates the micellar properties of the surfactant.
Dynamic light scattering (DLS) is an appropriate method for determination of micelle size values in surfactant solutions [39,40], the micelle size of the SDS in the samples prepared with different media was determined by DLS measurements. The results were calculated based on three independent measurements, and each measurement comprised at least 15 measurement runs. SDS was characterized with different experimental methods and was described to form micelles between 3.5 and 4.0 nm in diameter [41,42]. In the present study the SDS in 5.0 g/L concentrated solution was measured to form 4.55 nm diameter micelles.
The change of the micelle size values depending on the polymer, and sodium chloride concentration is illustrated in Figure 8.
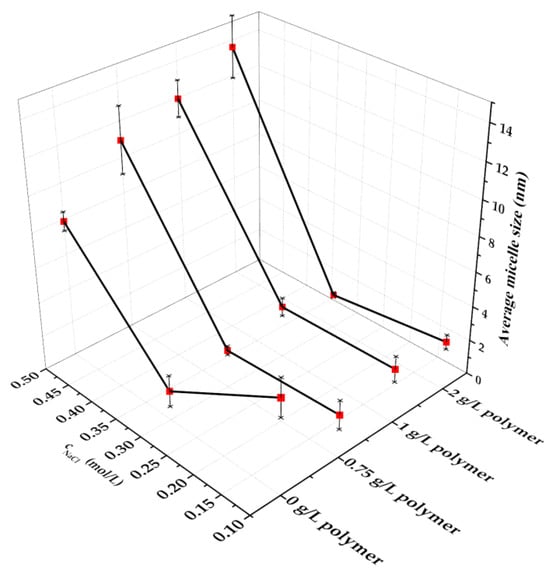
Figure 8.
Micelle size values of sodium dodecylsulphate measured in different sodium chloride- and polymer containing media (CSDS = 5.0 g/L, CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%), error bars represent standard deviation.
According to the registered micelle size values, it can be concluded that measuring in a certain salt containing media, the micelle size values slightly increased by increasing the polymer concentration. Considering the effects of the salt’s presence on the micelle size values determined in the alkaline–polymer containing surfactant solutions, unambiguous effects were observed; increasing the salt concentration from 0.1 M to 0.3 M the micelle size values showed a slight decrease, further increasing the salt concentration the micelle size values considerably increased. Measuring at 2.0 g/L polymer concentration, the micelle size values increased by increasing the salt concentration. This trend confirms the phenomenon that in surfactant–polymer mixtures micellar growth is promoted by the reduced electrostatic repulsion between the charged surfactant headgroups which follows salt addition [43].
3.3. Rheological Properties of the Samples
Processing the nonlinear fit of the shear stress and viscosity, depending on the shear rate according to the Herschel–Bulkley model provides the rheological parameters of the prepared samples: consistency index (k/mPas), yield stress (τ0/Pa), flow index (n) and zero shear viscosity (η0/mPas).
The flow curves of the SDS solutions in different media, the aqueous- and alkali containing polymer solutions, and the SDS solutions in presence of alkaline–polymer containing carrier were also recorded.
The flow curves of 2.0 g/L polymer containing solutions in different media are illustrated in Figure 9.
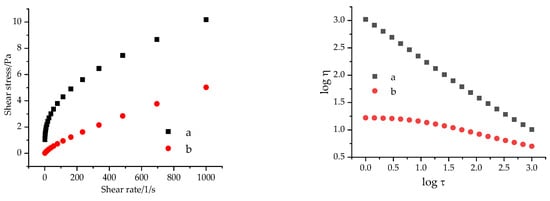
Figure 9.
Flow curves of 2.0 g/L Flopaam AN 125SH in aqueous solution (a) and in presence of 0.4 m/m% NaOH, 0.2 m/m% Na2CO3 and 0.5 mol/L NaCl (b).
In the presence of the alkali compounds and sodium chloride, the flow curve becomes approximately linear, which represents Newtonian flow behaviour; the polymer solution does not possess a coherent structure.
The SDS containing samples in the presence of the alkali and the polymer possessed a pseudoplastic flow behaviour, with τ0 = 0 yield stress and n < 1.0 flow number values. The flow curves of the polymer non-containing samples were determined to be approximately linear, and these solutions possessed n = 0.98 and n = 0.99 flow index values, which indicates a Newtonian flow behaviour.
The rheological parameters of the surfactant solutions in presence of the alkali compounds and sodium chloride are listed in Table 2.

Table 2.
Rheological parameters of the alkali and sodium chloride containing SDS solutions (CSDS = 5.0 g/L, CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%).
The viscosity of the samples is represented by two parameters: consistency index and zero shear viscosity. About the viscosity of the SDS in the alkaline stock solutions, it can be stated that the increasing ionic strength did not measurably affect the viscosity of the 5.0 g/L SDS containing solutions. Moreover, in the surfactant solutions the consistency index and the zero shear viscosity values were determined to be the same values.
The effects of the presence of the alkali on the rheological behaviour of the Flopaam AN125SH polymer was investigated, the rheological parameters of the aqueous- and alkali containing polymer solutions determined in media with different ionic strengths are represented in Table 3.

Table 3.
Rheological parameters of the aqueous and alkaline–polymer solutions.
In the aqueous polymer solutions, a considerable increase can be determined in both the consistency index value and the zero shear viscosity by increasing the polymer concentration (Panel#1).
The presence of the alkali compounds (NaOH and Na2CO3) and sodium chloride (NaCl) caused a considerable change in the rheological behaviour of the Flopaam AN125SH polymer, a significant decrease can be calculated in both the consistency index and zero shear viscosity values compared to the aqueous solutions (Panel#2–Panel#4). In the presence of a certain ionic strength increasing the polymer concentration an increase can be measured in the polymer’s viscosity. The effects of the solvent’s ionic strength are less considerable in lower polymer containing solutions; the 0.75 g/L Flopaam AN125SH solution possessed 5.93 mPas consistency index in presence of 0.1 M NaCl (Panel#2) and 4.94 mPas in presence of 0.5 M (Panel#4), while the consistency index of 2.0 g/L Flopaam AN125SH containing sample in presence of 0.1 M was determined 36.9 mPas (Panel#2) and in presence of 0.5 M NaCl 24.44 mPas (Panel#4).
The alkaline containing polymer solutions were used as the solvents of sodium dodecylsulphate to prepare the model alkaline–surfactant–polymer solutions. The rheological parameters of the prepared alkaline–surfactant–polymer solutions are listed in Table 4.

Table 4.
Rheological parameters of the alkaline- and sodium chloride containing SDS–polymer solutions (CSDS = 5.0 g/L, CNaOH = 0.4 m/m%, CNa2CO3 = 0.2 m/m%).
The polymer containing surfactant solutions were determined to possess a pseudoplastic flow behaviour, with τ0 = 0 yield stress and n < 1.0 flow index values (Panel#1–Panel#3). Comparing the viscosity values, it can be observed that the dissolution of the high amount of surfactant in the polymer containing media did not increase either the consistency index or the zero shear viscosity compared to the surfactant non-containing alkaline–polymer solutions (Table 4, Panel#2–Panel#4), and this decrease is more significant in higher polymer containing solutions. Considering the polymer concentration in the SDS samples in presence of a certain ionic strength both the consistency index and the zero shear viscosity values were determined to increase by the increase of the polymer concentration. Similarly to the surfactant non-containing solutions, in a solution with a certain ionic strength, the 2.0 g/L polymer concentration caused the most significant increase in viscosity; the 1.0 g/L polymer and 5.0 g/L SDS containing sample possessed 9.6 mPas consistency index in presence of 0.1 M NaCl (Panel#1), while this value was measured 36.3 mPas in 2.0 g/L polymer containing sample in the solution with the same ionic strength (Panel#1).
4. Conclusions
The present study describes a comprehensive investigation of sodium-dodecylsulphate (SDS) dissolved in polymer (Flopaam AN125SH) and alkali additives (0.2 wt.%NaOH and 0.4 wt.% Na2CO3) containing media with increasing (0.1–0.5 M) ionic strength. These experiments consider the SDS as an injected surfactant component of the alkaline–surfactant–polymer (ASP) system.
The results indicate that processing experiments using an ASP method considering the addition of an injected surfactant component to the system, a higher initial polymer concentration is expected to be preferred. However, addition of surfactant into an alkaline polymer solution is not expected to further decrease IFT in basic media. In an alkali containing medium, the measured IFT values were significantly decreased (0.1287 ± 0.02141 mN/m) compared to IFT value recorded between milliQ water and rapeseed oil (10.769 ± 0.53 mN/m). This is obviously due to the saponification reaction of crude oil’s acidic components, generating surfactants in situ. Furthermore, with increasing ionic strength, the measured IFT values were further decreased because of the decreasing charge repulsion of the surfactant’s head groups. As a result, the measured micelle sizes were also increased with the ionic strength and the lower solubility of the surfactants facilitating more surfactant molecules to adsorb to the droplet surface, further decreasing interfacial tension.
The addition of SDS (5 g/L) further reduced the IFT values, but only to a small extent. The increasing ionic strength reduces the solvation of the anionic macromolecule chains, which was clearly seen from the significant decrease in the viscosity. The size of the micelles increases with both polymer concentration and NaCl content, indicating increasingly poor solvation and increasing aggregation.
Consequently, the surfactant molecules enriched the oil/water interface and showed a surface tension-reducing effect.
Author Contributions
Conceptualization, P.S. and L.J.; methodology, C.B. and Á.Á.; investigation, B.K. and L.J.; resources, L.J. and P.S.; writing—original draft preparation, C.B., writing—review and editing, L.J. and P.S.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support provided by the Ministry of Human Resources Hungary (Grant No. GINOP-2.3.4-15-2020-00006) is gratefully acknowledged. This paper was also supported by the UNKP-23-5 New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund as well as by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| ASP | flooding–Alkaline–surfactant–polymer flooding |
| CMC | critical micelle concentration |
| DLS | dynamic light scattering |
| EOR | enanced oil recovery |
| IFT | interfacial tension |
| SDS | sodium dodecylsulphate |
| σ | interfacial tension |
| ω | angular velocity |
| ρ | density |
| τ | shear stress |
| τ0 | yield stress |
| γ* | shear rate |
| k | consistency index |
| n | flow number |
| η0 | zero shear viscosity |
References
- Lu, S.; Li, R.F.; Miller, C.A.; Hirasaki, G.J. Alkaline/surfactant/polymer processes: Wide range of conditions for good recovery. Soc. Pet. Eng. J. 2010, 15, 282–293. [Google Scholar] [CrossRef]
- Sheng, J.J. Status of surfactant EOR technology. Petroleum 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Wang, B.; Wu, T.; Li, Y.; Sun, D.; Yang, M.; Gao, Y.; Lu, F.; Li, X. The effects of oil displacement agents on the stability of water produced from ASP (alkaline surfactant/polymer) flooding. Colloids. Surf. A 2011, 79, 121–126. [Google Scholar] [CrossRef]
- Johnson, C.E., Jr. Status of caustic and emulsion methods. J. Pet. Technol. 1976, 28, 85–92. [Google Scholar] [CrossRef]
- Ehrlich, R.; Wygal, R.J. Interaction of crude oil and rock properties with the recovery of oil by caustic waterflooding. Soc. Pet. Eng. J. 1977, 17, 263–279. [Google Scholar] [CrossRef]
- Al-Sahhaf, T.; Ahmed, A.S.; Elkamel, A. Producing ultralow interfacial tension at the oil/water interface. J. Pet. Sci. Technol. 2002, 20, 773–788. [Google Scholar] [CrossRef]
- Gao, S.; Li, H.; Li, H. Laboratory investigation of combination of alkali/surfactant/ polymer technology for Daqing EOR. SPE Reserv. Eng. 1995, 10, 194–197. [Google Scholar]
- Rudin, J.; Bernard, C.; Wasan, D.T. Effect of added surfactant on interfacial tension and spontaneous emulsification in alkali/acidic oil systems. Ind. Eng. Chem. Res. 1994, 33, 1150–1158. [Google Scholar] [CrossRef]
- Ehrlich, R.; Hasiba, H.H.; Raimondi, P. Alkaline waterflooding for wettability alteration-evaluating a potential field application. J. Pet. Technol. 1974, 26, 1335–1343. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent advances in surfactant EOR. Soc. Pet. Eng. J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Sheng, J.J. A comprehensive review of alkaline–surfactant–polymer (ASP) flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Sydansk, R.D. Elevated-temperature caustic/sandstone interaction: Implications for improving oil recovery. Soc. Pet. Eng. J. 1982, 22, 453–462. [Google Scholar] [CrossRef]
- Hirasaki, G.; Zhang, D.L. Surface chemistry of oil recovery from fractured, oilwet, carbonate formations. Soc. Pet. Eng. J. 2004, 9, 151–162. [Google Scholar]
- Khayet, M. Solar desalination by membrane distillation: Dispersion in energy consumption analysis and water production costs (a review). Desalination 2013, 308, 89–101. [Google Scholar] [CrossRef]
- Riley, B.N.; Doe, P.H. Polymer flooding review. J. Pet. Technol. 1987, 39, 1503–1507. [Google Scholar]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for enhanced oil recovery technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Weiss, W.W.; Baldwin, R.W. Planning and implementing a large-scale polymer flood. J. Pet. Technol. 1985, 37, 720–730. [Google Scholar] [CrossRef]
- Abass, A.O. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar]
- Sheng, J.J. Critical review of alkaline–polymer flooding. J. Petrol. Explor. Prod. Technol. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Sheng, D.C.; Yang, P.H.; Liu, Y.L. Effect of alkali-polymerinteraction on the solution properties. Petrol. Explor. Develop. 1994, 21, 81–85. [Google Scholar]
- Kazempour, M.; Sundstrom, E.A.; Alvarado, V. Effect of alkalinity on oil recovery during polymer floods in Sandstone. SPE Reserv. Eval. Eng. 2012, 15, 195–209. [Google Scholar] [CrossRef]
- Edinga, K.J.; McCaffery, F.G.; Wytrychowski, I.M. Cessford basal Colorado A reservoir caustic flood evaluation. J. Pet. Technol. 1980, 32, 2103–2110. [Google Scholar] [CrossRef]
- Manji, K.H.; Stasiuk, B.W. Design considerations for Dome’s David alkali/polymer flood. J. Can. Pet. Technol. 1988, 27, 48–54. [Google Scholar] [CrossRef]
- Chen, Z.Y. Experimental study of AP flooding in Yangshamu Guan II upper group. Oil Gas Recover. Technol. 1994, 1, 33–38. [Google Scholar]
- Xu, W.D.; Sun, L.; Pu, W.F.; Zhao, J.Z.; Xin, J. Effect of GH on AP solution flooding. J. S. Petrol. Univ. Sci. Technol. Ed. 2008, 30, 151–153. [Google Scholar]
- Mayer, E.H.; Berg, R.L.; Carmichael, J.D.; Weinbrandt, R.M. Alkaline injection for enhanced oil recovery-a status report. J. Petrol. Technol. 1983, 35, 209–221. [Google Scholar] [CrossRef]
- Leonard, J. Annual Production report: Steam dominates enhanced oil recovery. Oil Gas J. 1982, 80, 152–159. [Google Scholar]
- Chang, L.; Zhang, Z.Q.; Wang, Q.M.; Xu, Z.S.; Guo, Z.D.; Sun, G.Q. Advances in polymer flooding and alkaline/surfactant/polymer processes as developed and applied in the People’s Republic of China. J. Pet. Technol. 2006, 58, 84–89. [Google Scholar] [CrossRef]
- Yongge, W.; Mingzhe, D.; Ezeddin, S. Study of Alkaline/Polymer Flooding for Heavy-Oil Recovery Using Channeled Sandpacks. SPE Res. Eval. Eng. 2011, 14, 310–319. [Google Scholar]
- Srivastava, A.; Qiao, W.; Wu, Y.; Li, X.; Bao, L.; Liu, C. Effects of silica nanoparticles and polymers on foam stability with sodium dodecylbenzene sulfonate in water–liquid paraffin oil emulsions at high temperatures. J. Mol. Liq. 2017, 241, 1069–1078. [Google Scholar] [CrossRef]
- Princen, H.M.; Zia, Y.Z.; Mason, S.G. Measurement of Interfacial Tension from the Shape of a Rotating Drop. J. Colloid. Interface Sci. 1967, 23, 99–107. [Google Scholar] [CrossRef]
- Viades-Trejo, J.; Gracia-Fadrique, J. Spinning drop method From Young–Laplace to Vonnegut. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 549–552. [Google Scholar] [CrossRef]
- Behzadfar, E.; Hatzikiriakos, S.G. Rheology of bitumen: Effects of temperature, pressure, CO2 concentration and shear rate. Fuel 2014, 116, 78–87. [Google Scholar]
- Aho, J.; Syrjälä, S. On the measurement and modeling of viscosity of polymers at low temperatures. Polym. Test 2008, 27, 35–40. [Google Scholar] [CrossRef]
- Burgos, G.R.; Alexandrou, A.N. On the determination of yield surfaces in Herschel–Bulkley fluids. J. Rheol. 1999, 43, 463–483. [Google Scholar] [CrossRef]
- Kardash, E.; Tur’yan, Y.I. Acid value determination in vegetable oils by indirect titration in aqueous-alcohol media. Croat. Chem. Acta 2005, 78, 99–103. [Google Scholar]
- Dickhout, J.M.; Virga, E.; Lammertnink, R.G.H.; de Vos, W.M. Surfactant specific ionic strength effects on membrane fouling during produced water treatment. J. Colloid Interface Sci. 2019, 556, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Fluksman, A.; Benny, O. A robust method for critical micelle concentration determination using coumarin-6 as a fluorescent probe. Anal. Methods 2019, 11, 3810–3818. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ohno, A.; Esumi, K. Mixed micellar properties of cationic trimeric-type quaternary ammonium salts and anionic sodium n-octyl sulfate surfactants. J. Colloid Interface Sci. 2004, 272, 191–196. [Google Scholar] [CrossRef]
- Kaushik, P.; Vaidya, S.; Ahmad, T.; Ganguli, A.K. Optimizing the hydrodynamic radii and polydispersity of reverse micelles in the Triton X-100/water/cyclohexane system using dynamic light scattering and other studies. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 162–166. [Google Scholar] [CrossRef]
- Chun, B.J.; Choi, J.I.; Jang, S.S. Molecular dynamics simulation study of sodium dodecyl sulfate micelle: Water penetration and sodium dodecyl sulfate dissociation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 474, 36–43. [Google Scholar] [CrossRef]
- Mirgorod, Y.; Chekadanov, A.; Dolenko, T. Structure of micelles of sodium dodecyl sulphate in water: X-ray and dynamic light scattering study. Chem. J. Mold. 2019, 14, 107–119. [Google Scholar] [CrossRef]
- Nillson, S.; Thuresson, K.; Hansson, P.; Lindman, B. Mixed solutions of surfactant and hydrophobically modified Polymer. Controlling viscosity with micellar size. J. Phys. Chem. B 1998, 102, 7099–7105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).