Using the Carnivorous Sponge Lycopodina hypogea as a Nonclassical Model for Understanding Apoptosis-Mediated Shape Homeostasis at the Organism Level
Abstract
1. Introduction
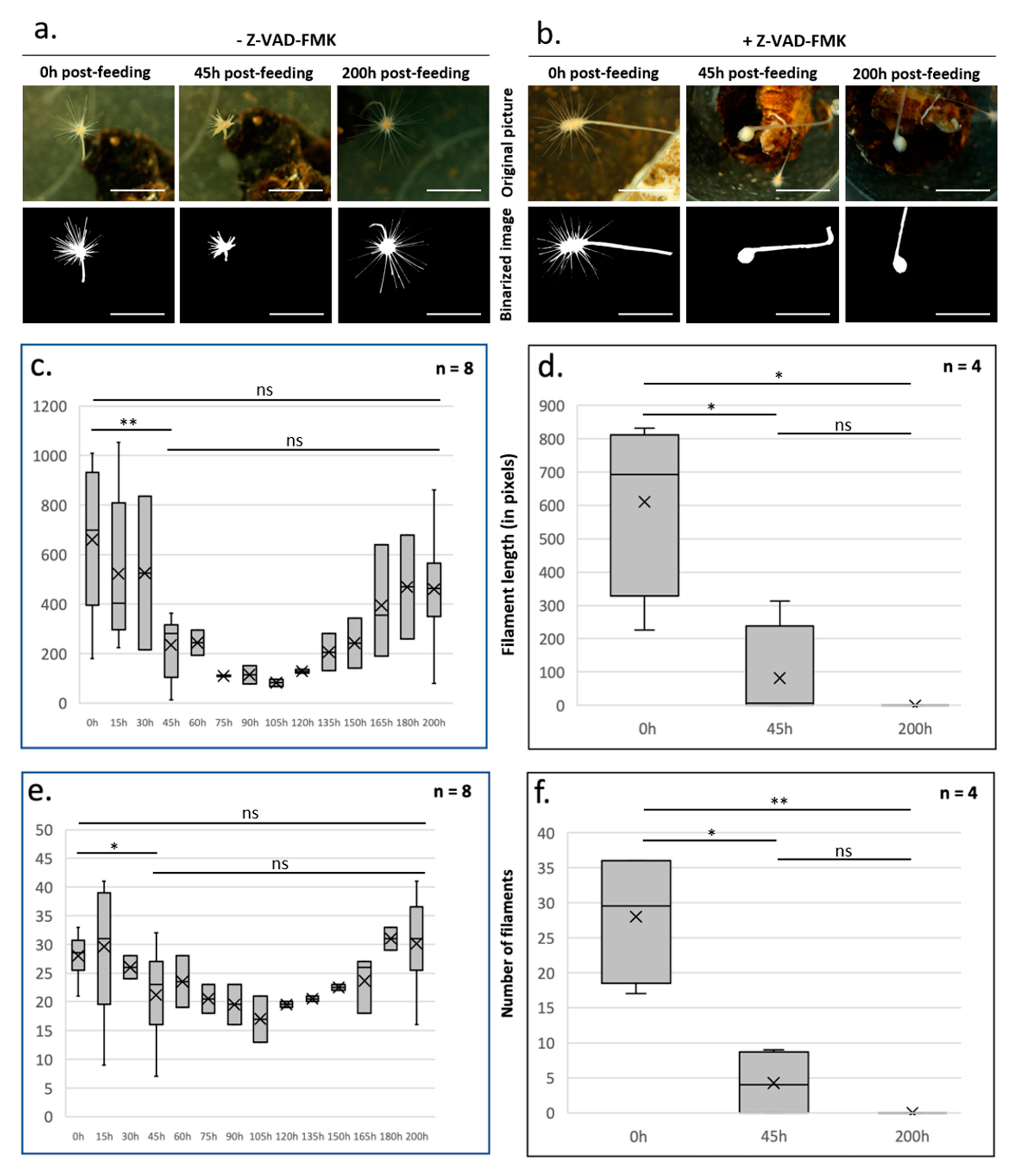
2. Using L. hypogea as a Model to Study Homeostasis at the Organism/Structure Level
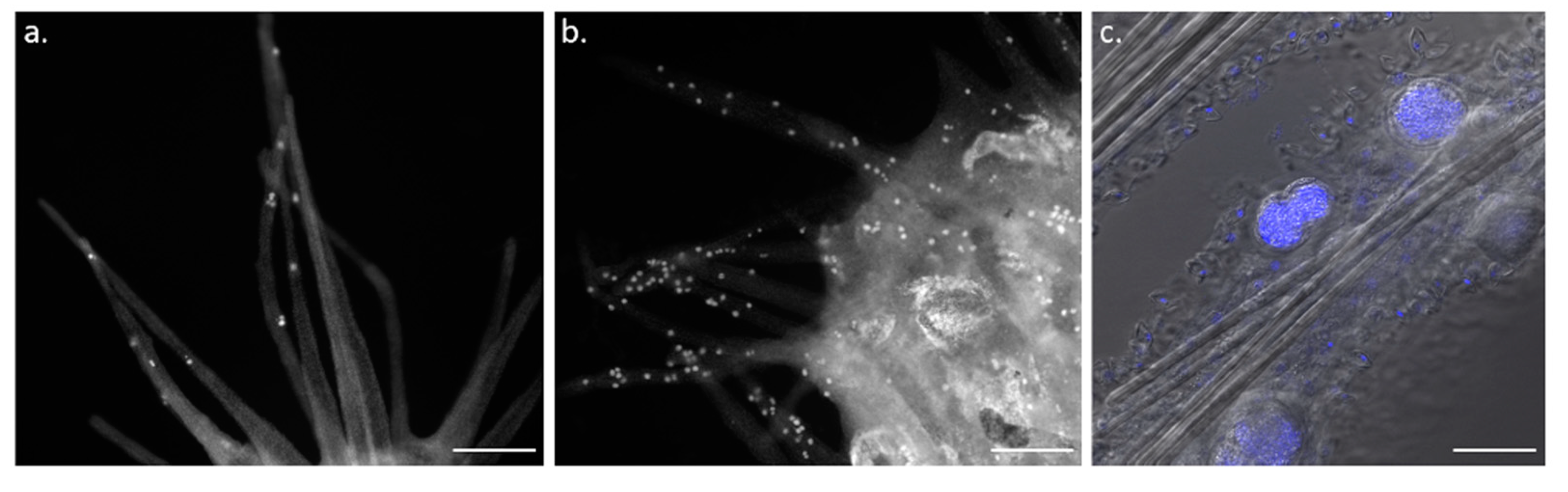
3. Cell Turnover and Adult Tissue Homeostasis in L. hypogea
4. Caspase-Dependent Apoptosis Controls Homeostatic Cell Turnover and Tissue Regeneration in L. hypogea
5. Homeostasis of Functional Structures
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bernard, C. Introduction à L’étude de la Médecine Expérimentale; J.B. Baillière et Fils, Librairie de l’Académie Impériale de Médecine: Paris, France, 1865. [Google Scholar]
- Cannon, W.B. The Wisdom of the Body; W. W. Norton&Co., Inc.: New York, NY, USA, 1932. [Google Scholar]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed Cell Death in Animal Development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Vacelet, J.; Boury-Esnault, N. Carnivorous Sponges. Nature 1995, 373, 333–335. [Google Scholar] [CrossRef]
- Le Goff, E.; Martinand-Mari, C.; Belkhir, K.; Vacelet, J.; Nidelet, S.; Godefroy, N.; Baghdiguian, S. Molecular Complexity and Gene Expression Controlling Cell Turnover during a Digestive Cycle of Carnivorous Sponge Lycopodina Hypogea. Cell Tissue Res. 2022, 388, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Memar, N.; Schiemann, S.; Hennig, C.; Findeis, D.; Conradt, B.; Schnabel, R. Twenty Million Years of Evolution: The Embryogenesis of Four Caenorhabditis Species Are Indistinguishable despite Extensive Genome Divergence. Dev. Biol. 2019, 447, 182–199. [Google Scholar] [CrossRef]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of Apoptosis-like Programmed Cell Death in Unicellular Protozoan Parasites. Parasit Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Kaczanowski, S. Symbiotic Origin of Apoptosis. In Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects; Kloc, M., Ed.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2020; pp. 253–280. ISBN 978-3-030-51849-3. [Google Scholar]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.A.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon Queenslandica Genome and the Evolution of Animal Complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef]
- Simion, P.; Philippe, H.; Baurain, D.; Jager, M.; Richter, D.J.; Di Franco, A.; Roure, B.; Satoh, N.; Quéinnec, É.; Ereskovsky, A.; et al. A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals. Curr. Biol. 2017, 27, 958–967. [Google Scholar] [CrossRef]
- Martinand-Mari, C.; Vacelet, J.; Nickel, M.; Wörheide, G.; Mangeat, P.; Baghdiguian, S. Cell Death and Renewal during Prey Capture and Digestion in the Carnivorous Sponge Asbestopluma Hypogea (Porifera: Poecilosclerida). J. Exp. Biol. 2012, 215, 3937–3943. [Google Scholar] [CrossRef]
- Melnikov, N.P.; Bolshakov, F.V.; Frolova, V.S.; Skorentseva, K.V.; Ereskovsky, A.V.; Saidova, A.A.; Lavrov, A.I. Tissue Homeostasis in Sponges: Quantitative Analysis of Cell Proliferation and Apoptosis. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 360–381. [Google Scholar] [CrossRef]
- Funayama, N. The Stem Cell System in Demosponges: Suggested Involvement of Two Types of Cells: Archeocytes (Active Stem Cells) and Choanocytes (Food-Entrapping Flagellated Cells). Dev. Genes Evol. 2013, 223, 23–38. [Google Scholar] [CrossRef]
- Chautan, M.; Chazal, G.; Cecconi, F.; Gruss, P.; Golstein, P. Interdigital Cell Death Can Occur through a Necrotic and Caspase-Independent Pathway. Curr. Biol. 1999, 9, 967–970. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Johnson, A.F.; Wilde, S.; Sands, J.S.; Monteiro, M.P.; LaRock, C.N. Group A Streptococcus Induces GSDMA-Dependent Pyroptosis in Keratinocytes. Nature 2022, 605, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Armenta, D.A.; Laqtom, N.N.; Alchemy, G.; Dong, W.; Morrow, D.; Poltorack, C.D.; Nathanson, D.A.; Abu-Remaileh, M.; Dixon, S.J. Ferroptosis Inhibition by Lysosome-Dependent Catabolism of Extracellular Protein. Cell Chem. Biol. 2022, 29, 1588-1600.e7. [Google Scholar] [CrossRef] [PubMed]
- Godefroy, N.; Le Goff, E.; Martinand-Mari, C.; Belkhir, K.; Vacelet, J.; Baghdiguian, S. Sponge Digestive System Diversity and Evolution: Filter Feeding to Carnivory. Cell Tissue Res. 2019, 377, 341–351. [Google Scholar] [CrossRef]
- Duffy, D.J. Instructive Reconstruction: A New Role for Apoptosis in Pattern Formation: Instructive Apoptotic Patterning Establishes de Novo Tissue Generation via the Apoptosis Linked Production of Morphogenic Signals. Bioessays 2012, 34, 561–564. [Google Scholar] [CrossRef]
- Vriz, S.; Reiter, S.; Galliot, B. Cell Death. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 108, pp. 121–151. ISBN 978-0-12-391498-9. [Google Scholar]
- Huh, J.R.; Guo, M.; Hay, B.A. Compensatory Proliferation Induced by Cell Death in the Drosophila Wing Disc Requires Activity of the Apical Cell Death Caspase Dronc in a Nonapoptotic Role. Curr. Biol. 2004, 14, 1262–1266. [Google Scholar] [CrossRef]
- Pellettieri, J.; Sánchez Alvarado, A. Cell Turnover and Adult Tissue Homeostasis: From Humans to Planarians. Annu. Rev. Genet. 2007, 41, 83–105. [Google Scholar] [CrossRef]
- Askew, D.S.; Ashmun, R.A.; Simmons, B.C.; Cleveland, J.L. Constitutive C-Myc Expression in an IL-3-Dependent Myeloid Cell Line Suppresses Cell Cycle Arrest and Accelerates Apoptosis. Oncogene 1991, 6, 1915–1922. [Google Scholar]
- Evan, G.I.; Wyllie, A.H.; Gilbert, C.S.; Littlewood, T.D.; Land, H.; Brooks, M.; Waters, C.M.; Penn, L.Z.; Hancock, D.C. Induction of Apoptosis in Fibroblasts by C-Myc Protein. Cell 1992, 69, 119–128. [Google Scholar] [CrossRef]
- Bao, L.; Shi, B.; Shi, Y.-B. Intestinal Homeostasis: A Communication between Life and Death. Cell Biosci. 2020, 10, 66. [Google Scholar] [CrossRef]
- Riesgo, A.; Taylor, C.; Leys, S.P. Reproduction in a Carnivorous Sponge: The Significance of the Absence of an Aquiferous System to the Sponge Body Plan. Evol. Dev. 2007, 9, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Vacelet, J.; Boury-Esnault, N.; Le Goff, E.; Ereskovsky, A. Spermatogenesis in the Carnivorous Sponge Lycopodina Hypogea (Porifera, Demospongiae). Zoomorphology 2022, 141, 1–17. [Google Scholar] [CrossRef]
- Orton, J.H. Sea-Temperature, Breeding and Distribution in Marine Animals. J. Mar. Biol. Assoc. UK 1920, 12, 339–366. [Google Scholar] [CrossRef]
- Vacelet, J. Deep-Sea Sponge in a Mediterranean Cave. In Deap-Sea and Extreme Shallow Water Habitats: Affinities and Adaptations; Uiblein, F., Ott, J., Stachowitsch, M., Eds.; Austrian Academy of Sciences: Vienna, Austria, 1996; pp. 299–312. [Google Scholar]
- Boruczkowski, T.; Boruczkowska, H.; Drożdż, W.; Miszczak, M.; Leszczyński, W. Use of ImageJ Software for Assessment of Mechanical Damage to Starch Granules. Processes 2022, 10, 630. [Google Scholar] [CrossRef]
- Martin, T.N.; Fipke, G.M.; Winck, J.E.M.; Marchese, J.A. ImageJ Software as an Alternative Method for Estimating Leaf Area in Oats. Acta Agronómica 2020, 69, 162–169. [Google Scholar] [CrossRef]
- Miura, K.; Sladoje, N. Bioimage Data Analysis Workflows—Advanced Components and Methods; Springer Nature: Berlin/Heidelberg, Germany, 2022; ISBN 978-3-030-76394-7. [Google Scholar]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, 57648. [Google Scholar] [CrossRef]
- Yasmin, N.Z.; Tohir, R.S.; Prajitno, P.; Soejoko, D.S. Quantification of Pericardial and Epicardial Fat Using ImageJ. J. Phys. Conf. Ser. 2021, 2019, 012078. [Google Scholar] [CrossRef]
- Le Goff, E.; Martinand-Mari, C.; Martin, M.; Feuillard, J.; Boublik, Y.; Godefroy, N.; Mangeat, P.; Baghdiguian, S.; Cavalli, G. Enhancer of Zeste Acts as a Major Developmental Regulator of Ciona Intestinalis Embryogenesis. Biol. Open 2015, 4, 1109–1121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghdiguian, S.; Le Goff, E.; Paradis, L.; Vacelet, J.; Godefroy, N. Using the Carnivorous Sponge Lycopodina hypogea as a Nonclassical Model for Understanding Apoptosis-Mediated Shape Homeostasis at the Organism Level. Foundations 2023, 3, 220-230. https://doi.org/10.3390/foundations3020018
Baghdiguian S, Le Goff E, Paradis L, Vacelet J, Godefroy N. Using the Carnivorous Sponge Lycopodina hypogea as a Nonclassical Model for Understanding Apoptosis-Mediated Shape Homeostasis at the Organism Level. Foundations. 2023; 3(2):220-230. https://doi.org/10.3390/foundations3020018
Chicago/Turabian StyleBaghdiguian, Stephen, Emilie Le Goff, Laure Paradis, Jean Vacelet, and Nelly Godefroy. 2023. "Using the Carnivorous Sponge Lycopodina hypogea as a Nonclassical Model for Understanding Apoptosis-Mediated Shape Homeostasis at the Organism Level" Foundations 3, no. 2: 220-230. https://doi.org/10.3390/foundations3020018
APA StyleBaghdiguian, S., Le Goff, E., Paradis, L., Vacelet, J., & Godefroy, N. (2023). Using the Carnivorous Sponge Lycopodina hypogea as a Nonclassical Model for Understanding Apoptosis-Mediated Shape Homeostasis at the Organism Level. Foundations, 3(2), 220-230. https://doi.org/10.3390/foundations3020018





