Abstract
Polyynes are alternations of single and triple bonds between carbon atoms, while cumulenes are successions of double bonds. Since the triple bond is the strongest bond between two carbon atoms, recent preoccupations included synthesizing and condensing cyclic polyynes and cumulenes and their clusters. Density functional theory calculations predicted stable monocyclic rings formation for a number of C atoms equal to or higher than 16. Alternative to the series of Carbon atoms are alternations of Boron and Nitrogen. Large rings (such as those of 24 atoms) can be crossed and thus small clusters can be formed. Patterns of three crosses seem to further stabilize the atomic ensemble. Clusters of and (96 atoms) as well as (104 atoms) have been designed, and their conformation has been studied here.
1. Introduction
It has been demonstrated that polyynes possess both metallic and semiconducting properties [1], being susceptible for valuable superconductivity [2], while a recent study [3] places its optical gap to below 1.6 eV. Properties of interest include tensile and chemical bond stiffness [4], the difference between lengths of single and triple bonds [5], resonant tunnel effect [6], and adjustable bandgap [7].
The recent rediscovery of polyyne molecules [8] is due to their unique features of single and triple bonds alternation. If a single C–C bond has an average distance of 1.53–1.54 Å and dissociation energy of 3.6–3.9 eV, then the presence of a double or of a triple bond updates these values to about 1.33 Å and 7.4 eV and to about 1.20 Å and 10 eV, respectively [9]. With an average decrease in distance of 22% relative to a single bond and of 13% relative to an aromatic bond, and an average increase in energy of 167% relative to a single bond and of 83% relative to an aromatic bond, this makes the triple bond between Carbon atoms very attractive for applications requiring dense materials [10] with very good compressibility [11]. Generally speaking, despite certain initial doubts [12], polyynes are the oligomeric cousins of carbene, being of scientific interest either as linear or cyclic complexes [13].
A polyyne-type () structure is favoured over a cumulene-type () structure [14,15]. Valuable theoretical [15,16,17,18], experimental [19,20,21,22] and review [23,24,25] studies were conducted to characterize this polymer. A chain with was firstly reportedly synthesized in 1995 [26], while an abundance of shorter chains () was reported much earlier [27]. Very good results were obtained in [28] for medium sized chains (72%, 36%, and 51% for n = 20, 24 and 28, respectively). In addition, of interest and under investigation is trapping of linear polyynes in nanotubes [29].
Cyclic polyynes ( and 18 in [30]; 24 in [31]) may provide further insight about stabilization of the polyynes. Moreover, old [32,33] and new [25,31] studies came to support the fact that certain rings are more stable than linear chains. Specifically, in [31], it is noted that rings are more likely to appear when and (). The tendency of the polyynes to form rings was observed at laser vaporization of graphite as secondary product in the synthesis of fullerenes in [34] (when ). Pieces of evidence that crosslinking of the chains stabilizes molecules as a cluster are [35,36,37].
Replacement of Carbon atoms with Boron and Nitrogen may produce significant changes to the molecular properties; for a full factorial study, consult [38].
Information regarding the stability of polyyne rings is essential in the identification of new materials with valuable properties. In this communication, a series of previous findings regarding the stabilization of polyyne rings is revised, along with some new findings regarding the replacement of Carbon atoms with alternating Boron and Nitrogen (called here {B,N}-congeners).
The study is motivated by the possibility to obtain more complex molecular architectures starting from polyynes [18,39,40,41].
2. Background and Supporting Data
Cyclic polyynes and cumulenes are of peculiar interest since their dimensionality is expanded from 1D to 2D, and they possess a very good symmetry (in convergence to ).
Before proceeding, it should be pointed out that the terminology ’in convergence’ is used here to reflect a state of facts: for small n, the stress over the strain due to bending of the molecule is high (bending angle is ). Due to this stress, the atoms have the tendency to jump up and down relative to a plane of a virtually perfect circular molecule (jumps can alternate regularly or not); hypothetically, each of these configurations defines a conformation, but since the differences are very small (so small that the molecular modeling programs detect breaking of the symmetry order from time to time), those configurations will not be considered conformations and polyyne molecule will be considered planar.
Crosses of polyynes and cumulenes can be the pillars of 3D architectures. According to [42], a more appropriate term would be [n]catenanes, where n is the number of macrocycles.
While molecular modeling may provide insights of the conformations and equilibriums at the atomic and molecular levels, regression analysis may cause an oversight of the whole process of formation and stabilization.
At first glance (see Figure 1), the association between Energy (E) and number of atoms (n) seems perfectly linear, with no other information to discover. Figure 1 was made using HF/STO-3G model data. One can observe almost a perfect association. Nevertheless, the intercept has no physical meaning despite of the fact that is statistically significant (Student’s ; probability to be 0: ). However, this is due to the fact that the small variations (providing essential information about the molecular stability) are buried beneath big variations—adding a pair of atoms to the molecule adds a large amount of energy, much larger than the variation due to the increase or decrease in its stability.
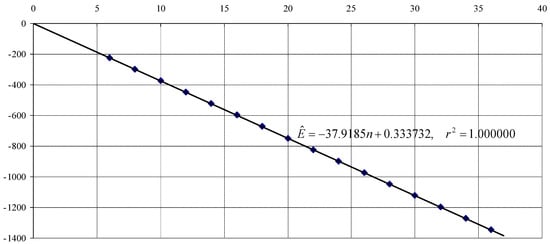
Figure 1.
Energy (in a.u.) of polyyne as function of n.
Some representative models were selected (see relative residual error in column in Table 1, models for molecule, for the magnitude of estimation error).

Table 1.
Experimental vs. calculated (at different theory levels) values for energy and distance between atoms at molecule.
Due to the simplicity of the composition (all Carbon atoms for cyclic polyynes; Carbon and Hydrogen atoms for linear polyynes, Boron, Carbon, and Nitrogen for {B,N}-congeners of cyclic polyynes appearing later in this study), the increasing complexity of the basis function, and sometimes of the theory level as well, does not provide further insights nor increase the accuracy of the modeling. One reference example for this is the calculations for a much well studied system, molecule (Table 1).
Somebody may argue that DFT BP 6-311G* (BP 5 (36) in Table 1) is not enough. Let us take for instance [45] comment, which found B3LYP inappropriate for some calculations; their main argument lies in a table pretty much alike our Table 1; anyway, since the method should be always a subject to change and improvement we should point our opinion that not always bigger is the better (“The B97XD functional with at least 6-311G(d) basis set” is suggested in [45]). To prove the contrary, in Table 1, the results for B97X-D were added as well.
3. Modeling
In order to reveal smaller amounts of variations, the average energy per atom () has been calculated (see Table 2). Furthermore, average energy per atom (or per bond) and bond lengths calculations for the formation of the polyyne rings (for , even number) were conducted at different theory levels (including Hartree–Fock [46,47,48,49] and Becke–Perdew [50,51]), and three sets of results derived employing three methods were selected for discussion in this paper (see Table 2).

Table 2.
Average energy per atom (, a.u.) and distances between atoms (, Å) for a single monocyclic ring polyyne (), calculated at three different theory levels.
Inspecting Table 2, one can see that it is in perfect agreement with the fact that, for , the cumulene configuration is preferred (see the entry for in Table 2). Hartree–Fock (HF) level of theory (methods M1 and M2 in Table 2) is the first (and the most simplistic) level of theory involving Schrädinger’s equation (the wave function of the quantum-mechanical system [52]). For this reason is not only scholastic, but also informative to reveal tendencies in stability (see BH&H functional in [53]), to inspect the dependencies using data retrieved from the modelling at this level of theory. Thus, in order to reveal the evolution of the stability, the data from Hartree–Fock () levels of theory ( and in Table 2) was plotted against the number of atoms (n) in Figure 2. Both dependencies show an increase in stability for with the increase of n. However, the increase is decaying with the increase of n and it reaches about 95% of it for . Furthermore, stability increases in a decaying manner, while decay is most likely hyperbolic (see Equations (1) and (2)).
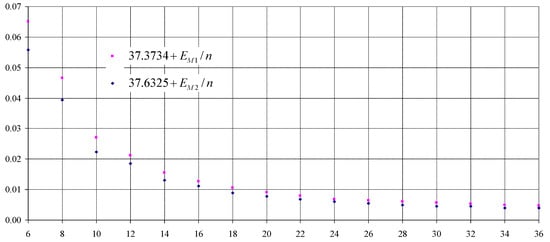
Figure 2.
Average energy per atom (, from M1, and M2, in a.u.) as function of number of atoms (n, even numbers from 6 to 36).
Two equations with a great likelihood were identified for HF energy per atom (Equations (1) and (2)). Both equations reveal an asymptotic convergence to infinity as well as a vertical asymptote in . What is intriguing is the appearance of the vertical asymptote, and it can be associated with the nonexistence in normal conditions of cyclopentadienyl as neutral molecule, only as an anion [54], stabilized typically by the presence of an electron transferring element such as is Fe [55,56]. It should be noticed that there is a bump in symmetry for (see Table 2, entry for ). Employing a significantly different theory level with a notable increase in the number of orbital functions as well (M3 is a Density Functional Theory (DFT) method and is chosen as being the most accurate alternative for ; see Table 1), polyyne is no longer preferred over cumulene almost anywhere, and modeling reveals an alternation between these two configurations, cumulenes being preferred when n is a multiple of two but not a multiple of four (, with a symmetry) and polyynes when n is a multiple of four (, with a symmetry).
When , we obtain equations for polyynes and when for cumulenes.
3.1. Unusual Regression for Polyyne/Cumulene Energy (Equations (3) and (4))
If one constructs a regression with as function () of n for polyynes (), it will get with a high confidence that in , (see Equation (3)), just like in Equation (1) and (2). Thus, to solve the regression equation for polyynes, it is necessary to estimate and parameters from . Following the same reasoning, to solve the regression equation for cumulenes, it is necessary to estimate and parameters from . With increase of the number of atoms (n) the change in the energy per atom () must (and it, as Figure 2, reveals as well) become smaller, so, in convergence (), both formulas should express the same. Thus, . Let us consider a series of values (such as is in Table 2) associated with and corresponding to the increasing values of n for which the regression analysis has revealed that the model estimating values in the series have an alternating pattern, such as is the one provided in Equations (3) and (4):
and
In order to find the values of the parameters of the regression equations under a constraint: (let us set ), the following sum must be minimized:
where , , and substitutions are used for simplification here and from hereon.
The result is a linear and homogenous system with three equations and three variables and the variances are to be obtained by inversing the Fisher Information Matrix [57]:
from which is
and and are subsequently immediate (from first two equations of the system).
3.2. Further Deriving of Regression Equations
All obtained Equations (1)–(4) are statistically significant. It is in fact surprising how accurate the obtained models are (the precision digit, given for a 5% risk of being in error is the 6th digit everywhere), considering the small size of the data analyzed. It is interesting how the average energy per atom has a vertical asymptote at for the polyynes (see Equation (3)) and a vertical asymptote at for the cumulenes (see Equation (4)), and this behavior warrants further investigation.
The distances between atoms (columns and in Table 2) are in any circumstance, independently of the model, at least smaller, stronger than the aromatic bond (1.385 Å). Expected to be a more accurate model, BP/6-311G* reveals (Equations (5)–(7)) a convergence of the cumulene bond length to the average of the polyyne bond length ().
Equations (5)–(7) were obtained following the same regression strategy as for Equations (3) and (4) (see Section 3.1). Consistent with the energy assessment (about 5% of the stabilizing energy lost due to bending, as seen above), the polyyne bonds lengths are about 2% different than the limit values (single bond is about 2.7% longer, triple bond is about 1.3% shorter, as seen by the data in Table 2 and Equations (5) and (6)), making it a very good candidate for stabilization by condensation.
4. Constructing Molecular Rings
4.1. Constructing a Polyyne Ring
A polyyne ring is characterized by the alternating of single and triple bonds which corresponds to an even-number sided polygon. A good starting geometry places the nucleus of atoms on a circle of a certain radius (r) at alternating distances corresponding to single () and triple () bonds lengths. Assuming that AB is the triple bond (of length ), BC is the single bond (of length ) and OA is the radius (r) of the circumscribed circle (see Figure 3), then using AOC = AOB + BOC and expressing the angles (AOB = 2arcsin(), BOC = 2arcsin() and AOC = ), the result is a rather difficult one to be resolved directly with equation:
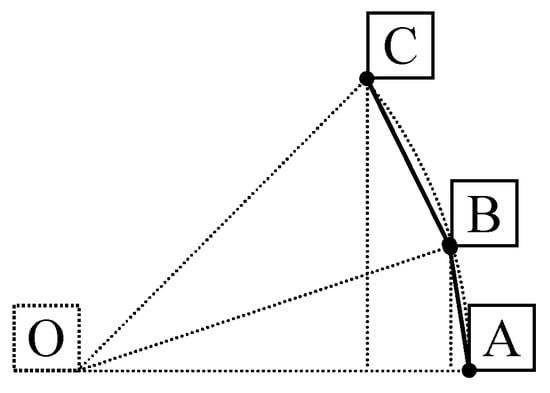
Figure 3.
Auxiliary representation for constructing a polyyne ring.
A simpler choice is using a program to obtaiin the radius (r) through successive approximations:
Starting with a rough approximation (this approximation gets closer to the exact solution with the increase of n): the iterations () converge on the exact solution leaving a residual below in 18 iterations for and in six iterations for .
4.2. Ring and Its {B,N}-Congeners
ring is very likely to stabilize the polyyne bonds (only about 5% of the energy increase is lost by the bending, as seen in Figure 2 and Equation (3); it is the biggest polyyne with unaltered symmetry, as seen in Table 2; bond lengths have very few differences (about 2%, see Equations (5)–(7)) to the limit infinite chain values). Previous reports [18,31] also show its potential from other perspectives.
Of interest is the substitution of Carbon atoms with Nitrogen and Boron in a regular, patterned manner [38]. Numerous congeners of carbon-based materials have been reported following such substitution [58], including inorganic benzene [59], boron nitride [60], nanotubes [61] and graphene-like materials [62].
Here, alternating between Boron and Nitrigen () and between Boron, Carbon and Nitrogen () congeners of have been designed, and their properties have been calculated and analyzed at BP/6-311G* level of theory (therefore with ).
Raman along with Infrared (IR) spectroscopy is commonly (in both organic and inorganic chemistry, in research and industry) used to provide a structural fingerprint by which molecules can be identified since is a simple and reliable technique [63,64,65]. Some molecular vibrational motions are associated with certain bonds or bonding environments or atoms groups, while others involve movement of almost all of the atoms in the molecule, being characteristic of the molecule as a whole. It is possible to calculate the vibrational frequencies associated with IR spectra, by using the data from quantum mechanics calculations. The nuclei are assumed to move much more slowly than the electrons, which are much lighter and thus more mobile. In this limit, the nuclei are assumed to move within an internuclear potential energy surface that is generated by the electrons (Born–Oppenheimer approximation [66]), and is accurate within fractions of a percent [67]. The calculation is based on optimal nucleus positions and electronic densities involving the evaluation of the electronic magnetic moment derivatives as detailed in [68].
Because vibrational frequencies are specific to a molecule’s chemical bonds and symmetry, the RAMAN spectra are also of interest (see Figure 4), where there is prominent one major peak shifting in the spectra.
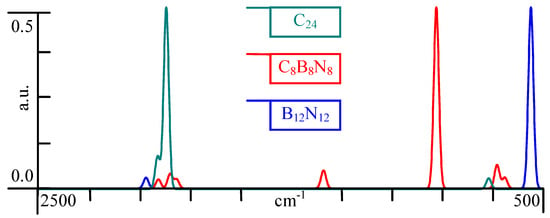
Figure 4.
Calculated RAMAN spectra of congeners.
Substituting the Carbon atoms with Boron and Nitrogen produces a shift in the RAMAN spectra, but since it is only one major peak, it keeps the symmetry at a comparable level everywhere.
Fundamental vibrations and associated rotational-vibrational structures are revealed by the IR spectra (see Figure 5).
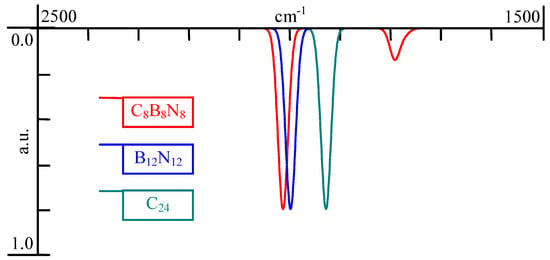
Figure 5.
Calculated IR spectra of congeners: zoom in into the region of interest ([ 2500 cm, 1500 cm]) for the mid-infrared spectra.
Substituting the Carbon atoms with Boron and Nitrogen produces a small shift in the IR spectra in the case of and also a small absorption peak appears in the case of . There is no symmetry reduction from ( point group symmetry; atom coordinates in Table A1) to ( point group symmetry; atom coordinates in Table A2) explainable by the tilting from polyyne (1.251 Å and 1.331 Å as bond lengths) to cumulene (1.321 Å bond length) bonds. It should be noted that, even if the Carbon atoms in are located on two circles (alternating), these have approximately the same radius (4.9441 Å and 4.9444 Å) but in the case of Boron atoms are accommodated on an interior circle (of 4.921 Å radius) significantly distinct from the exterior one (of 5.103 Å radius) accommodating Nitrogen atoms. Even more intriguing is the case of with point group symmetry (Figure 6 and Figure 7).
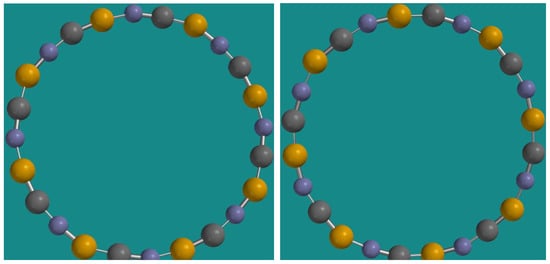
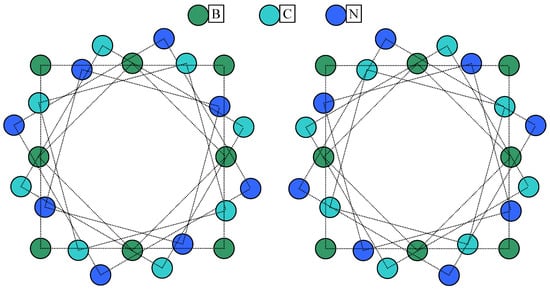
Figure 7.
The two conformers of (not to scale; point group symmetry).
In Figure 6, the radiuses (in Å) are as follows (in ascending order, from interior to exterior):
- Conformer 1: 4.998 (B), 5.006 (N), 5.012 (C), 5.018 (C), 5.064 (N), 5.145 (B);
- Conformer 2: 4.994 (B), 5.004 (C), 5.017 (N), 5.045 (C), 5.046 (N), 5.140 (B).
Since the differences between radiuses are very small, in order to illustrate more clearly the differences, Figure 7 represents the same conformers with the geometry not to scale. In Figure 7, it is visible that, while the inner ring is formed by the Boron atoms in both conformers (green balls in Figure 7), the next two interior rings are formed by Nitrogen and then Carbon for Conformer 1, while, in Conformer 2, the ring of Carbon appears next followed by the ring of Nitrogen.
Regarding other properties of polyyne congeners calculated from the molecular modeling (DFT M3) and listed in Table 3, one should notice very little change in entropy from to and the very small HOMO-LUMO gap of (which, coupled with greatest heat capacity and smallest zero point energy, deems it a very interesting subject for further studies). The decrease of polarizability of relative to is conjugated with the increase of HOMO-LUMO gap to a value (4 eV) at limit when the near-degeneracy can be removed and the HOMO stabilized by distortion to a lower symmetry structure that brings about electron occupancy of the LUMO [69].

Table 3.
Calculated properties of congeners (with DFT M3).
The bond lengths in () are smaller than a typical Carbon-Carbon double bond () suggesting that has a very good potential to make compact, hard materials. In the case of , the bond length between Boron and Carbon ( and , alternating) suggests presence of the aromaticity ( is the typical length of the aromatic bond in benzene), while the bond length between Boron and Nitrogen ( and , alternating) suggests the presence of at least a double bond (typical Carbon - Carbon double bond length is ) while the bond length between Carbon and Nitrogen ( and , alternating) indicates an intermediate strength between a double ( is the typical length of the double bond between Carbon and Nitrogen) and a triple bond ( is the typical length of the triple bond between Carbon and Nitrogen).
5. Molecular Clusters
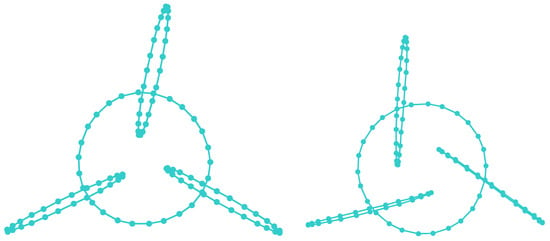
A molecular mechanics method [18] was involved in minimizing the energies of clusters, while polyyne cluster and , , and cumulene and cumulene-like cluster structures were further optimized at Hartree–Fock theory level using 6-311G* basis set [71]. Two different conformations were identified (see Figure 8).
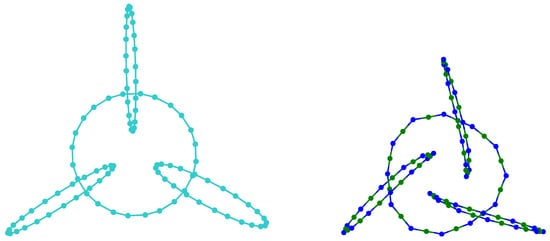
Cumulene conformation (build up from alternating Boron and Nitrogen) has an increased entropy (the geometry is more irregular and the outer rims are bended), suggesting that a longer circle length is stabilizing.
The result is consistent with previously reported data [18]. One different aspect: here, involving an upper theory level in the final geometry optimization, one can distinguish between the geometry of the polyyne Figure 8 left—preferring an axial alignment of the outer rims and the geometry of the cumulene Figure 8 right—preferring a paddle alignment of the outer rims.
The polyyne (Figure 8 left) has, in its geometrically optimized form (using HF 6-311G*), an entirely symmetrical form: the outer rims are perfectly perpendicular to the inner rim ( between the versors of their planes) and the angles between the planes of the outer rims are, again, in a perfect symmetry ( between them), the polyyne cluster having a molecular point group. The cumulene (Figure 8 right) has a small break in its symmetry—the angles of the outer rims with the inner rim are a little bigger (), the angles between the outer rims are a little smaller (), and the plane of their centers does not include the center of the inner rim (this is off by 0.023 Å). More importantly, the centers of the outer rims are significantly closer to the center of the inner rim in the case of the cumulene (4.969 Å) when compared with the polyyne (6.755 Å).
Figure 9 gives a picture of the polyyne cluster in its final, optimized conformation depicting the electronic density.
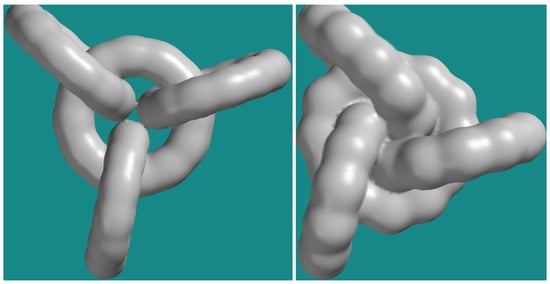
Figure 9.
Electronic density and electrostatic potential map—those two have essentially the same shape-of polyyne cluster (image to the left) and of cumulene (image to the right)-Spartan ’14 screen captures.
In the case of the polyyne cluster, the electronic density distribution reveals a natural accommodation of the crosses-no overlap and no gap between the 95% coverage of the electronic density, no bending due to the crossing. In the case of the cumulene, the electronic density distribution reveals a very good occupancy of the space inside of the inner rim by the 95% coverage of the electronic density suggesting a configuration stress.
Furthermore, the optimized geometry of cumulene (Figure 10) proves the fact that polyyne (Figure 8 left) is the optimal conformation, having a balanced use of the free space.
cumulene conformers reveal the existence of extra free space in their conformation. In the image to the left, the outer rims are not perfectly vertical (being positioned at , , and , respectively, relative to the inner circle). The angles between the outer rims are , , and , respectively. In the image to the right, the vertical alignment is improved (, , and respectively) but the departure to the perfect symmetry (angles of ) is pushed even further away (, , and , respectively).
6. Conclusions
Polyynes, possessing metallic and semiconducting properties, and susceptible for valuable superconductivity, are also very attractive for applications requiring dense materials with very good compressibility. Energy and conformation analyses employed in this study at different theory levels (including HF and DFT) revealed that cyclic conformation begins to stabilize itself for medium sized rings, having an optimum (characterized by only a 5% energy loss due to bending) at atoms. Polyyne conformations (alternations of single-like and triple-like bonds) are preferred by Carbon rings of sizes that are multiples of 4 (…, 16, 20, 24, 28, 32, …) while cumulene conformations are preferred for the rest of the cases (Carbon rings of sizes that are non-multiples of 4 (…, 18, 22, 26, 30, …) and alternations of Boron and Nitrogen atoms ( studied here). The bond length split present at (bond length between Boron and Carbon is and , alternating; bond length between Boron and Nitrogen is and , alternating; bond length between Carbon and Nitrogen is and , alternating) as well as the lengths of these bonds indicate an intermediate configuration (between polyyne and cumulene) which warrants further study. The existence of the polyyne and/or cumulene long chain is further stabilized when molecular clusters are formed. The analysis of , , , and clusters (all built on a cluster topology in which one central ring is crossed by other three rings) revealed that polyyne and cumulene are [4]catenane arrangements that are best balancing the free space between atoms.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Appendix A

Table A1.
Cartesian coordinates of cyclic cumulene having DFT BP 6-311G* optimal geometry.
Table A1.
Cartesian coordinates of cyclic cumulene having DFT BP 6-311G* optimal geometry.
| 4.78618 | −1.24089 | 0 | 4.94409 | 0.00000 | 0 | 4.76540 | 1.31845 | 0 | 4.28171 | 2.47205 | 0 |
| 3.46773 | 3.52451 | 0 | 2.47205 | 4.28171 | 0 | 1.24089 | 4.78618 | 0 | 0.00000 | 4.94409 | 0 |
| −1.31845 | 4.76540 | 0 | −2.47205 | 4.28171 | 0 | −3.52451 | 3.46773 | 0 | −4.28171 | 2.47205 | 0 |
| −4.78618 | 1.24089 | 0 | −4.94409 | 0.00000 | 0 | −4.76540 | −1.31845 | 0 | −4.28171 | −2.47205 | 0 |
| −3.46773 | −3.52451 | 0 | −2.47205 | −4.28171 | 0 | −1.24089 | −4.78618 | 0 | 0.00000 | −4.94409 | 0 |
| 1.31845 | −4.76540 | 0 | 2.47205 | −4.28171 | 0 | 3.52451 | −3.46773 | 0 | 4.28171 | −2.47205 | 0 |

Table A2.
Cartesian coordinates of cyclic cumulene having DFT BP 6-311G* optimal geometry.
Table A2.
Cartesian coordinates of cyclic cumulene having DFT BP 6-311G* optimal geometry.
| 3.79257 | 3.41407 | 0 | 2.68051 | 4.12673 | 0 | 1.57740 | 4.85298 | 0 | 0.25800 | 4.91411 | 0 |
| −1.06039 | 4.99150 | 0 | −2.23359 | 4.38476 | 0 | −3.41411 | 3.79256 | 0 | −4.12675 | 2.68049 | 0 |
| −4.85296 | 1.57742 | 0 | −4.91411 | 0.25803 | 0 | −4.99151 | −1.06042 | 0 | −4.38475 | −2.23362 | 0 |
| −3.79257 | −3.41407 | 0 | −2.68051 | −4.12673 | 0 | −1.57740 | −4.85298 | 0 | −0.25800 | −4.91411 | 0 |
| 1.06039 | −4.99150 | 0 | 2.23359 | −4.38476 | 0 | 3.41411 | −3.79256 | 0 | 4.12675 | −2.68049 | 0 |
| 4.85296 | −1.57742 | 0 | 4.91411 | −0.25803 | 0 | 4.99151 | 1.06042 | 0 | 4.38475 | 2.23362 | 0 |

Table A3.
Cartesian coordinates of cyclic cumulene conformer 1 having DFT BP 6-311G* optimal geometry.
Table A3.
Cartesian coordinates of cyclic cumulene conformer 1 having DFT BP 6-311G* optimal geometry.
| 3.62802 | −3.64575 | 0 | 4.37263 | −2.44972 | 0 | 4.88467 | −1.33917 | 0 | 4.99818 | 0.00000 | 0 |
| 4.82863 | 1.36764 | 0 | 4.33202 | 2.50870 | 0 | 3.64575 | 3.62802 | 0 | 2.44972 | 4.37263 | 0 |
| 1.33917 | 4.88467 | 0 | 0.00000 | 4.99818 | 0 | −1.36764 | 4.82863 | 0 | −2.50870 | 4.33202 | 0 |
| −3.62802 | 3.64575 | 0 | −4.37263 | 2.44972 | 0 | −4.88467 | 1.33917 | 0 | −4.99818 | 0.00000 | 0 |
| −4.82863 | −1.36764 | 0 | −4.33202 | −2.50870 | 0 | −3.64575 | −3.62802 | 0 | −2.44972 | −4.37263 | 0 |
| −1.33917 | −4.88467 | 0 | 0.00000 | −4.99818 | 0 | 1.36764 | −4.82863 | 0 | 2.50870 | −4.33202 | 0 |

Table A4.
Cartesian coordinates of cyclic cumulene conformer 2 having DFT BP 6-311G* optimal geometry.
Table A4.
Cartesian coordinates of cyclic cumulene conformer 2 having DFT BP 6-311G* optimal geometry.
| 4.33233 | −2.50908 | 0 | 4.82779 | −1.36740 | 0 | 4.99764 | 0.00000 | 0 | 4.88379 | 1.33938 | 0 |
| 4.37257 | 2.45010 | 0 | 3.62884 | 3.64692 | 0 | 2.50908 | 4.33233 | 0 | 1.36740 | 4.82779 | 0 |
| 0.00000 | 4.99764 | 0 | −1.33938 | 4.88379 | 0 | −2.45010 | 4.37257 | 0 | −3.64692 | 3.62884 | 0 |
| −4.33233 | 2.50908 | 0 | −4.82779 | 1.36740 | 0 | −4.99764 | 0.00000 | 0 | −4.88379 | −1.33938 | 0 |
| −4.37257 | −2.45010 | 0 | −3.62884 | −3.64692 | 0 | −2.50908 | −4.33233 | 0 | −1.36740 | −4.82779 | 0 |
| 0.00000 | −4.99764 | 0 | 1.33938 | −4.88379 | 0 | 2.45010 | −4.37257 | 0 | 3.64692 | −3.62884 | 0 |

Table A5.
Cartesian coordinates of cyclic cumulene having HF 6-311G* optimal geometry.
Table A5.
Cartesian coordinates of cyclic cumulene having HF 6-311G* optimal geometry.
| −1.91612 | 4.70183 | 0.00000 | −3.11384 | 4.01033 | 0.00000 | −3.90083 | 3.11278 | 0.00000 | −4.48926 | 1.86180 | 0.00000 |
| −4.72452 | 0.69121 | 0.00000 | −4.72452 | −0.69121 | 0.00000 | −4.48926 | −1.86180 | 0.00000 | −3.90083 | −3.11278 | 0.00000 |
| −3.11384 | −4.01033 | 0.00000 | −1.91612 | −4.70183 | 0.00000 | −0.74533 | −4.93461 | 0.00000 | 0.63227 | −4.81871 | 0.00000 |
| 1.76366 | −4.43716 | 0.00000 | 2.96087 | −3.74595 | 0.00000 | 3.85699 | −2.95692 | 0.00000 | 4.64617 | −1.82183 | 0.00000 |
| 5.02997 | −0.69150 | 0.00000 | 5.02997 | 0.69150 | 0.00000 | 4.64617 | 1.82183 | 0.00000 | 3.85699 | 2.95692 | 0.00000 |
| 2.96087 | 3.74595 | 0.00000 | 1.76366 | 4.43716 | 0.00000 | 0.63227 | 4.81871 | 0.00000 | −0.74533 | 4.93461 | 0.00000 |
| 1.90890 | 0.00000 | 0.68777 | 1.90890 | 0.00000 | −0.68777 | 2.20706 | 0.00000 | −1.84016 | 2.88387 | 0.00000 | −3.03723 |
| 3.72300 | 0.00000 | −3.88360 | 4.91360 | 0.00000 | −4.57357 | 6.06469 | 0.00000 | −4.88178 | 7.44086 | 0.00000 | −4.87949 |
| 8.59115 | 0.00000 | −4.56844 | 9.78112 | 0.00000 | −3.87716 | 10.62161 | 0.00000 | −3.03250 | 11.30748 | 0.00000 | −1.83940 |
| 11.61471 | 0.00000 | −0.68810 | 11.61471 | 0.00000 | 0.68810 | 11.30748 | 0.00000 | 1.83940 | 10.62161 | 0.00000 | 3.03250 |
| 9.78112 | 0.00000 | 3.87716 | 8.59115 | 0.00000 | 4.56844 | 7.44086 | 0.00000 | 4.87949 | 6.06469 | 0.00000 | 4.88178 |
| 4.91360 | 0.00000 | 4.57357 | 3.72300 | 0.00000 | 3.88360 | 2.88387 | 0.00000 | 3.03723 | 2.20706 | 0.00000 | 1.84016 |
| −3.03235 | −5.25218 | −4.88178 | −3.72043 | −6.44398 | −4.87949 | −4.29558 | −7.44015 | −4.56844 | −4.89056 | −8.47070 | −3.87716 |
| −5.31081 | −9.19859 | −3.03250 | −5.65374 | −9.79256 | −1.83940 | −5.80735 | −10.05863 | −0.68810 | −5.80735 | −10.05863 | 0.68810 |
| −5.65374 | −9.79256 | 1.83940 | −5.31081 | −9.19859 | 3.03250 | −4.89056 | −8.47070 | 3.87716 | −4.29558 | −7.44015 | 4.56844 |
| −3.72043 | −6.44398 | 4.87949 | −3.03235 | −5.25218 | 4.88178 | −2.45680 | −4.25530 | 4.57357 | −1.86150 | −3.22421 | 3.88360 |
| −1.44193 | −2.49750 | 3.03723 | −1.10353 | −1.91137 | 1.84016 | −0.95445 | −1.65315 | 0.68777 | −0.95445 | −1.65315 | −0.68777 |
| −1.10353 | −1.91137 | −1.84016 | −1.44193 | −2.49750 | −3.03723 | −1.86150 | −3.22421 | −3.88360 | −2.45680 | −4.25530 | −4.57357 |
| −5.65374 | 9.79256 | 1.83940 | −5.31081 | 9.19859 | 3.03250 | −4.89056 | 8.47070 | 3.87716 | −4.29558 | 7.44015 | 4.56844 |
| −3.72043 | 6.44398 | 4.87949 | −3.03235 | 5.25218 | 4.88178 | −2.45680 | 4.25530 | 4.57357 | −1.86150 | 3.22421 | 3.88360 |
| −1.44193 | 2.49750 | 3.03723 | −1.10353 | 1.91137 | 1.84016 | −0.95445 | 1.65315 | 0.68777 | −0.95445 | 1.65315 | −0.68777 |
| −1.10353 | 1.91137 | −1.84016 | −1.44193 | 2.49750 | −3.03723 | −1.86150 | 3.22421 | −3.88360 | −2.45680 | 4.25530 | −4.57357 |
| −3.03235 | 5.25218 | −4.88178 | −3.72043 | 6.44398 | −4.87949 | −4.29558 | 7.44015 | −4.56844 | −4.89056 | 8.47070 | −3.87716 |
| −5.31081 | 9.19859 | −3.03250 | −5.65374 | 9.79256 | −1.83940 | −5.80735 | 10.05863 | −0.68810 | −5.80735 | 10.05863 | 0.68810 |

Table A6.
Cartesian coordinates of cyclic cumulene having HF 6-311G* optimal geometry.
Table A6.
Cartesian coordinates of cyclic cumulene having HF 6-311G* optimal geometry.
| −1.76825 | 4.36002 | −0.01740 | −3.04477 | 4.06970 | −0.01277 | −3.92879 | 3.10051 | −0.01653 | −4.83161 | 2.15186 | −0.01709 |
| −4.93518 | 0.84670 | −0.01869 | −5.00212 | −0.45994 | −0.01947 | −4.41697 | −1.62735 | −0.01976 | −3.80821 | −2.78179 | −0.01951 |
| −2.89176 | −3.71136 | −0.01740 | −2.00208 | −4.67170 | −0.01277 | −0.72072 | −4.95269 | −0.01653 | 0.55224 | −5.26023 | −0.01709 |
| 1.73433 | −4.69734 | −0.01869 | 2.89938 | −4.10199 | −0.01947 | 3.61781 | −3.01153 | −0.01976 | 4.31321 | −1.90711 | −0.01951 |
| 4.66002 | −0.64866 | −0.01740 | 5.04685 | 0.60200 | −0.01277 | 4.64951 | 1.85218 | −0.01653 | 4.27937 | 3.10837 | −0.01709 |
| 3.20085 | 3.85064 | −0.01869 | 2.10274 | 4.56193 | −0.01947 | 0.79916 | 4.63888 | −0.01976 | −0.50499 | 4.68890 | −0.01951 |
| 0.45557 | −1.95282 | 1.12465 | 0.07075 | −1.97729 | −0.12663 | 0.52155 | −1.93908 | −1.35625 | 0.91464 | −1.93971 | −2.59202 |
| 1.82698 | −1.71765 | −3.50609 | 2.72566 | −1.51437 | −4.41968 | 3.91641 | −1.06917 | −4.72814 | 5.09941 | −0.63335 | −5.04421 |
| 6.25905 | −0.16813 | −4.66280 | 7.41781 | 0.29606 | −4.29618 | 8.23877 | 0.63337 | −3.33902 | 9.07144 | 0.97414 | −2.39785 |
| 9.34014 | 1.08722 | −1.12658 | 9.63091 | 1.20674 | 0.13815 | 9.27914 | 1.06213 | 1.38456 | 8.94865 | 0.92424 | 2.63883 |
| 8.07121 | 0.56300 | 3.53033 | 7.20429 | 0.20475 | 4.43813 | 6.02906 | −0.26606 | 4.73741 | 4.85237 | −0.73905 | 5.05123 |
| 3.68354 | −1.16113 | 4.67110 | 2.50644 | −1.59451 | 4.29879 | 1.65198 | −1.77217 | 3.33893 | 0.78031 | −1.97173 | 2.38048 |
| −2.88414 | −2.85712 | −4.72814 | −3.09820 | −4.09955 | −5.04421 | −3.27513 | −5.33643 | −4.66280 | −3.45251 | −6.57205 | −4.29618 |
| −3.57087 | −7.45167 | −3.33902 | −3.69209 | −8.34316 | −2.39785 | −3.72851 | −8.63241 | −1.12658 | −3.77038 | −8.94398 | 0.13815 |
| −3.71974 | −8.56704 | 1.38456 | −3.67391 | −8.21188 | 2.63883 | −3.54803 | −7.27137 | 3.53033 | −3.42482 | −6.34147 | 4.43813 |
| −3.24495 | −5.08829 | 4.73741 | −3.06622 | −3.83275 | 5.05123 | −2.84734 | −2.60948 | 4.67110 | −2.63411 | −1.37339 | 4.29879 |
| −2.36074 | −0.54457 | 3.33893 | −2.09772 | 0.31010 | 2.38048 | −1.91898 | 0.58187 | 1.12465 | −1.74776 | 0.92738 | −0.12663 |
| −1.94007 | 0.51787 | −1.35625 | −2.13716 | 0.17776 | −2.59202 | −2.40102 | −0.72339 | −3.50609 | −2.67431 | −1.60331 | −4.41968 |
| −5.55940 | 7.50491 | 1.38456 | −5.27474 | 7.28764 | 2.63883 | −4.52318 | 6.70837 | 3.53033 | −3.77947 | 6.13672 | 4.43813 |
| −2.78412 | 5.35435 | 4.73741 | −1.78615 | 4.57179 | 5.05123 | −0.83621 | 3.77061 | 4.67110 | 0.12767 | 2.96790 | 4.29879 |
| 0.70876 | 2.31674 | 3.33893 | 1.31741 | 1.66163 | 2.38048 | 1.46341 | 1.37095 | 1.12465 | 1.67701 | 1.04992 | −0.12663 |
| 1.41852 | 1.42121 | −1.35625 | 1.22252 | 1.76196 | −2.59202 | 0.57404 | 2.44104 | −3.50609 | −0.05135 | 3.11768 | −4.41968 |
| −1.03227 | 3.92630 | −4.72814 | −2.00121 | 4.73289 | −5.04421 | −2.98392 | 5.50457 | −4.66280 | −3.96530 | 6.27599 | −4.29618 |
| −4.66790 | 6.81830 | −3.33902 | −5.37935 | 7.36902 | −2.39785 | −5.61163 | 7.54519 | −1.12658 | −5.86052 | 7.73724 | 0.13815 |

Table A7.
Cartesian coordinates of cyclic cumulene conformer 1 having HF 6-311G* optimal geometry.
Table A7.
Cartesian coordinates of cyclic cumulene conformer 1 having HF 6-311G* optimal geometry.
| 5.17522 | −2.16282 | −0.01977 | 5.56532 | −1.03759 | −0.07769 | 5.63702 | 0.33740 | −0.13549 | 5.37488 | 1.50006 | −0.17104 |
| 4.75927 | 2.73256 | −0.19280 | 4.00232 | 3.65404 | −0.19612 | 2.92835 | 4.51716 | −0.18631 | 1.86519 | 5.05698 | −0.16723 |
| 0.52738 | 5.38546 | −0.13588 | −0.66453 | 5.37750 | −0.10275 | −1.98708 | 4.99293 | −0.05690 | −2.99345 | 4.35516 | −0.01090 |
| −3.92624 | 3.34283 | 0.05024 | −4.50001 | 2.29896 | 0.10662 | −4.89440 | 0.98032 | 0.17059 | −4.99399 | −0.20692 | 0.22177 |
| −4.81304 | −1.57200 | 0.26983 | −4.39339 | −2.68781 | 0.29825 | −3.59025 | −3.80752 | 0.31106 | −2.65626 | −4.54861 | 0.30416 |
| −1.38440 | −5.07842 | 0.28011 | −0.20326 | −5.23938 | 0.24879 | 1.16844 | −5.11679 | 0.20439 | 2.30607 | −4.76198 | 0.15996 |
| 3.50719 | −4.08933 | 0.10198 | 4.39863 | −3.29912 | 0.04727 | −0.87899 | 2.63500 | −3.09286 | −1.34480 | 3.24110 | −4.00690 |
| −2.03747 | 4.10794 | −4.81966 | −2.73770 | 4.95910 | −5.27247 | −3.61361 | 6.00017 | −5.47461 | −4.38687 | 6.89977 | −5.36240 |
| −5.24385 | 7.87533 | −4.90881 | −5.91246 | 8.61673 | −4.25841 | −6.55384 | 9.30393 | −3.25426 | −6.96435 | 9.71870 | −2.21536 |
| −7.24337 | 9.96135 | −0.89054 | −7.30220 | 9.95481 | 0.29953 | −7.15515 | 9.69771 | 1.64273 | −6.84875 | 9.27137 | 2.71237 |
| −6.30909 | 8.57284 | 3.76723 | −5.70725 | 7.82364 | 4.47170 | −4.89793 | 6.84306 | 4.99647 | −4.13837 | 5.94221 | 5.17361 |
| −3.24503 | 4.90402 | 5.04705 | −2.50271 | 4.05838 | 4.65514 | −1.73136 | 3.20136 | 3.90518 | −1.17868 | 2.60589 | 3.03351 |
| −0.70867 | 2.12983 | 1.83195 | −0.47517 | 1.92472 | 0.68207 | −0.40723 | 1.92980 | −0.69105 | −0.53058 | 2.14515 | −1.85596 |
| 8.07620 | 0.55943 | 5.02090 | 6.88637 | 0.49654 | 5.03583 | 5.55092 | 0.38891 | 4.72444 | 4.49530 | 0.27160 | 4.18424 |
| 3.46565 | 0.11470 | 3.28585 | 2.79211 | −0.02981 | 2.31359 | 2.31094 | −0.20001 | 1.03695 | 2.16715 | −0.33865 | −0.13691 |
| 2.31450 | −0.48521 | −1.49582 | 2.71998 | −0.58954 | −2.61128 | 3.48911 | −0.67819 | −3.74810 | 4.37412 | −0.72050 | −4.54487 |
| 5.58652 | −0.72836 | −5.19442 | 6.74039 | −0.69851 | −5.49025 | 8.11381 | −0.62467 | −5.50378 | 9.26990 | −0.53009 | −5.23118 |
| 10.48786 | −0.39250 | −4.60702 | 11.38062 | −0.25566 | −3.82977 | 12.16437 | −0.08652 | −2.71216 | 12.59041 | 0.06125 | −1.60925 |
| 12.76207 | 0.22321 | −0.25419 | 12.62500 | 0.34818 | 0.92282 | 12.14621 | 0.46621 | 2.20685 | 11.47729 | 0.54003 | 3.19017 |
| 10.45640 | 0.58731 | 4.11076 | 9.40718 | 0.59339 | 4.67549 | −0.99910 | −1.64745 | 1.62168 | −1.00974 | −1.47408 | 0.25800 |
| −1.16327 | −1.55568 | −0.91994 | −1.50734 | −1.91766 | −2.20095 | −1.93390 | −2.45412 | −3.17571 | −2.54684 | −3.29279 | −4.07722 |
| −3.14903 | −4.16651 | −4.61930 | −3.88622 | −5.28467 | −4.93266 | −4.52351 | −6.29140 | −4.91843 | −5.21500 | −7.42914 | −4.57295 |
| −5.74080 | −8.33643 | −4.00708 | −6.22811 | −9.23211 | −3.08394 | −6.52208 | −9.83236 | −2.09751 | −6.69407 | −10.28218 | −0.80911 |
| −6.68962 | −10.43905 | 0.37207 | −6.50774 | −10.34085 | 1.73192 | −6.20622 | −10.01863 | 2.83875 | −5.71190 | −9.39449 | 3.96035 |
| −5.18172 | −8.66630 | 4.74038 | −4.48720 | −7.65813 | 5.36747 | −3.84885 | −6.69000 | 5.64151 | −3.11292 | −5.52801 | 5.62888 |
| −2.51286 | −4.54216 | 5.33241 | −1.90474 | −3.49421 | 4.68135 | −1.48166 | −2.71856 | 3.88185 | −1.14355 | −2.02795 | 2.74145 |

Table A8.
Cartesian coordinates of cyclic cumulene conformer 2 having HF 6-311G* optimal geometry.
Table A8.
Cartesian coordinates of cyclic cumulene conformer 2 having HF 6-311G* optimal geometry.
| 5.01853 | −1.97390 | −0.05084 | 5.35855 | −0.83697 | −0.05211 | 5.45303 | 0.55917 | −0.05390 | 5.26859 | 1.73149 | −0.05616 |
| 4.73281 | 3.02418 | −0.06039 | 4.02507 | 3.97698 | −0.06457 | 2.92685 | 4.84471 | −0.06744 | 1.83443 | 5.30885 | −0.06808 |
| 0.45020 | 5.51463 | −0.06866 | −0.73086 | 5.39831 | −0.06932 | −2.05693 | 4.95113 | −0.06972 | −3.06884 | 4.33110 | −0.06931 |
| −4.06772 | 3.35078 | −0.06992 | −4.70397 | 2.34891 | −0.07213 | −5.13979 | 1.01888 | −0.07525 | −5.20958 | −0.16595 | −0.07692 |
| −4.93893 | −1.53901 | −0.07488 | −4.43292 | −2.61257 | −0.07009 | −3.55687 | −3.70395 | −0.06362 | −2.62142 | −4.43423 | −0.05752 |
| −1.34977 | −5.01817 | −0.05099 | −0.18617 | −5.25153 | −0.04700 | 1.21122 | −5.17964 | −0.04494 | 2.34115 | −4.81639 | −0.04583 |
| 3.51832 | −4.05954 | −0.04734 | 4.32586 | −3.18984 | −0.04936 | 0.84609 | 2.30944 | −3.14470 | 0.21994 | 2.77315 | −4.03936 |
| −0.71104 | 3.44607 | −4.83601 | −1.61732 | 4.08859 | −5.25234 | −2.75412 | 4.88738 | −5.40469 | −3.71801 | 5.55941 | −5.24136 |
| −4.78137 | 6.29994 | −4.71734 | −5.56898 | 6.84945 | −4.02085 | −6.30538 | 7.37477 | −2.95527 | −6.73257 | 7.69342 | −1.89547 |
| −6.97399 | 7.90171 | −0.53478 | −6.94597 | 7.92702 | 0.65083 | −6.64198 | 7.77430 | 2.00630 | −6.16789 | 7.49485 | 3.05716 |
| −5.38592 | 7.00022 | 4.10469 | −4.57149 | 6.46261 | 4.77925 | −3.49188 | 5.72437 | 5.27224 | −2.52848 | 5.04534 | 5.40693 |
| −1.40845 | 4.23023 | 5.22060 | −0.52985 | 3.56681 | 4.77859 | 0.35880 | 2.86261 | 3.96104 | 0.94424 | 2.36783 | 3.05555 |
| 1.41271 | 1.95422 | 1.80494 | 1.60091 | 1.76741 | 0.64869 | 1.58300 | 1.75814 | −0.74965 | 1.36118 | 1.92686 | −1.90269 |
| 7.16651 | −0.42237 | 5.30666 | 6.02494 | −0.71211 | 5.44880 | 4.67836 | −1.04068 | 5.27022 | 3.60479 | −1.29209 | 4.83249 |
| 2.49714 | −1.53912 | 4.01673 | 1.74675 | −1.69264 | 3.11078 | 1.13210 | −1.80736 | 1.86042 | 0.86919 | −1.84364 | 0.70422 |
| 0.87800 | −1.82748 | −0.69419 | 1.15466 | −1.76327 | −1.84590 | 1.77907 | −1.61312 | −3.08751 | 2.53341 | −1.42860 | −3.98437 |
| 3.64143 | −1.14610 | −4.78814 | 4.71261 | −0.86720 | −5.21488 | 6.05509 | −0.51557 | −5.38003 | 7.19279 | −0.21617 | −5.22727 |
| 8.45028 | 0.11101 | −4.71259 | 9.38374 | 0.34906 | −4.02021 | 10.26203 | 0.56534 | −2.95471 | 10.77759 | 0.68356 | −1.89291 |
| 11.08548 | 0.73681 | −0.53069 | 11.07979 | 0.70720 | 0.65514 | 10.76193 | 0.58941 | 2.01098 | 10.24181 | 0.42470 | 3.06431 |
| 9.36130 | 0.16821 | 4.11897 | 8.42699 | −0.08961 | 4.80300 | −1.88024 | 0.60188 | 0.92464 | −1.81868 | 0.61393 | −0.47233 |
| −1.87196 | 0.36479 | −1.63080 | −2.03659 | −0.22329 | −2.88805 | −2.25401 | −0.94357 | −3.80519 | −2.57481 | −2.01218 | −4.64687 |
| −2.88611 | −3.05519 | −5.11849 | −3.28146 | −4.37623 | −5.34586 | −3.62241 | −5.50842 | −5.24988 | −4.01402 | −6.77184 | −4.79845 |
| −4.31902 | −7.71787 | −4.15077 | −4.63206 | −8.61334 | −3.12412 | −4.84307 | −9.14451 | −2.08453 | −5.01474 | −9.46707 | −0.73535 |
| −5.08627 | −9.46884 | 0.44880 | −5.07426 | −9.15811 | 1.81159 | −4.98093 | −8.64494 | 2.87708 | −4.77249 | −7.78232 | 3.95714 |
| −4.52060 | −6.87004 | 4.67246 | −4.15303 | −5.64028 | 5.22601 | −3.80012 | −4.52445 | 5.42024 | −3.36217 | −3.20198 | 5.30744 |
| −2.99619 | −2.14020 | 4.92523 | −2.59542 | −1.03816 | 4.16462 | −2.30052 | −0.28897 | 3.29332 | −2.03006 | 0.33051 | 2.06968 |
References
- Gorjizadeh, N.; Farajian, A.A.; Kawazoe, Y. Non-coherent transport in carbon chains. J. Phys. Condens. Matter. 2011, 23, 75301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, Y.; Wang, L.; Kong, E.S.W.; Chen, X.; Zhang, Y. A one-dimensional extremely covalent material: Monatomic carbon linear chain. Nanoscale Res. Lett. 2011, 6, 577. [Google Scholar] [CrossRef]
- Zirzlmeier, J.; Schrettl, S.; Brauer, J.C.; Contal, E.; Vannay, L.; Brémond, É.; Jahnke, E.; Guldi, D.M.; Corminboeuf, C.; Tykwinski, R.R.; et al. Optical gap and fundamental gap of oligoynes and carbyne. Nat. Commun. 2020, 11, 4797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-P.; Meng, S.-H.; Lu, H.-T.; Tohyama, T. First-principles study on the mechanics, optical, and phonon properties of carbon chains. Chin. Phys. B 2018, 27, 117101. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Shi, L. A review of linear carbon chains. Chin. Chem. Lett. 2020, 31, 1746–1756. [Google Scholar] [CrossRef]
- Da Silva, C.A.B.; Nisioka, K.R.; Moura-Moreira, M.; Macedo, R.F.; Del Nero, J. Tunneling rules for electronic transport in 1D systems. Mol. Phys. 2021, 119, e1976427. [Google Scholar] [CrossRef]
- Bamdad, M.; Mousavi, H. Harrison model of polyynic carbyne chains. ECS J. Solid State Sci. Technol. 2021, 10, 031001. [Google Scholar] [CrossRef]
- Zheng, P.; Zubatyuk, R.; Wu, W.; Isayev, O.; Dral, P.O. Artificial intelligence-enhanced quantum chemical method with broad applicability. Nat. Commun. 2021, 12, 7022. [Google Scholar] [CrossRef] [PubMed]
- Jäntschi, L. General Chemistry Course (Version 8); AcademicDirect: Cluj-Napoca, Romania, 2017; pp. 36–37. [Google Scholar]
- Dubrovinskaia, N.; Dubrovinsky, L. Aggregated diamond nanorods, the densest and least compressible form of carbon. Appl. Phys. Lett. 2005, 87, 083106. [Google Scholar] [CrossRef]
- Itzhaki, L.; Altus, E.; Basch, H.; Hoz, S. Harder than diamond: Determining the cross-sectional area and Young’s modulus of molecular rods. Angew. Chem. Int. Ed. 2005, 44, 7432–7435. [Google Scholar] [CrossRef]
- Smith, P.P.K.; Buseck, P.R. Carbyne forms of carbon: Do they exist? Science 1982, 216, 984–986. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Chen, W.; Travers, M.J.; Thaddeus, P. Microwave spectra of 11 polyyne carbon chains. Astrophys. J. 2000, 129, 611–623. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Chen, W.; Travers, M.J.; Thaddeus, P. The strongest and toughest predicted materials: Linear atomic chains without a Peierls instability. Matter 2022, 5, 1192–1203. [Google Scholar]
- Karpfen, A. Ab initio studies on polymers. I. The linear infinite polyyne. J. Phys. C: Solid State Phys. 1979, 12, 3227. [Google Scholar] [CrossRef]
- Rice, M.J.; Bishop, A.R.; Campbell, D.K. Unusual soliton properties of the infinite polyyne chain. Phys. Rev. Lett. 1983, 51, 2136–2139. [Google Scholar] [CrossRef]
- Rozental, E.; Altus, E.; Major, D.T.; Hoz, S. Shaping polyyne rods by using an electric field. ChemistryOpen 2017, 6, 733–738. [Google Scholar] [CrossRef]
- Jäntschi, L.; Bolboacă, S.D. Conformational study of C24 cyclic polyyne clusters. Int. J. Quantum Chem. 2018, 118, e25614. [Google Scholar] [CrossRef]
- Sladkov, A.M.; Kasatochkin, V.I.; Kudryavtsev, Y.P.; Korshak, V.V. Synthesis and properties of valuable polymers of carbon. Bull. Acad. Sci. USSR Div. Chem. Sci. 1968, 17, 2560–2565. [Google Scholar] [CrossRef]
- Brédas, J.L.; Chance, R.R.; Baughman, R.H.; Silbey, R. Ab initio effective Hamiltonian study of the electronic properties of conjugated polymers. J. Chem. Phys. 1982, 76, 3673–3678. [Google Scholar] [CrossRef]
- Akagi, K.; Nishiguchi, M.; Shirakawa, H.; Furukawa, Y.; Harada, I. One-dimensional conjugated carbyne-synthesis and properties. Synth. Met. 1987, 17, 557–562. [Google Scholar] [CrossRef]
- Johnson, B.F.G.; Kakkar, A.K.; Khan, M.S.; Lewis, J. Synthesis of novel rigid rod iron metal containing polyyne polymers. J. Organomet. Chem. 1991, 409, C12–C14. [Google Scholar] [CrossRef]
- Heimann, R.B. Linear finite carbon chains (carbynes): Their role during dynamic transformation of graphite to diamond, and their geometric and electronic structure. Diam. Relat. Mater. 1994, 3, 1151–1157. [Google Scholar] [CrossRef]
- Page, A.J.; Ding, F.; Irle, S.; Morokuma, K. Insights into carbon nanotube and graphene formation mechanisms from molecular simulations: A review. Rep. Prog. Phys. 2015, 78, 036501. [Google Scholar] [CrossRef]
- Anderson, H.L.; Patrick, C.W.; Scriven, L.M.; Woltering, S.L. A short history of cyclocarbons. Bull. Chem. Soc. Jpn. 2021, 94, 798–811. [Google Scholar] [CrossRef]
- Lagow, R.J.; Kampa, J.J.; Han-Chao, W.; Battle, S.L.; Genge, J.W.; Laude, D.A.; Harper, C.J.; Bau, R.; Stevens, R.C.; Haw, J.F.; et al. Synthesis of linear acetylenic Carbon: The “sp” Carbon allotrope. Science 1995, 267, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, R.; Johnson, T.R.; Walton, D.R.M. Silylation as a protective method for terminal alkynes in oxidative couplings: A general synthesis of the parent polyynes H(C≡C)nH (n = 4–10, 12). Tetrahedron 1972, 28, 4601–4616. [Google Scholar] [CrossRef]
- Zheng, Q.; Gladysz, J.A. A synthetic breakthrough into an unanticipated stability regime: Readily isolable complexes in which C16−C28 polyynediyl chains span two Platinum atoms. J. Am. Chem. Soc. 2005, 127, 10508–10509. [Google Scholar] [CrossRef]
- Gao, E.; Li, R.; Baughman, R.H. Predicted confinement-enhanced stability and extraordinary mechanical properties for Carbon nanotube wrapped chains of linear Carbon. ACS Nano 2020, 14, 17071–17079. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D. Cyclic polyynes as examples of the quantum mechanical particle on a ring. J. Chem. Educ. 2012, 89, 724–727. [Google Scholar] [CrossRef]
- Jäntschi, L.; Bolboacă, S.D.; Janežic, D. Cyclic Carbon polyynes. Carbon Mater. Chem. Phys. 2016, 9, 423–436. [Google Scholar]
- Hoffmann, R. Extended Hückel theory–v: Cumulenes, polyenes, polyacetylenes and Cn. Tetrahedron 1966, 22, 521–538. [Google Scholar] [CrossRef]
- Hutter, J.; Lüthi, H.P.; Diederich, F. Structures and vibrational frequencies of the carbon molecules C2−C18 calculated by density functional theory. J. Am. Chem. Soc. 1994, 116, 750–756. [Google Scholar] [CrossRef]
- Hunter, J.; Fye, J.; Jarrold, M.F. Annealing : Synthesis of fullerenes and large Carbon rings. Science 1993, 260, 784–786. [Google Scholar] [CrossRef]
- Springborg, M.; Kavan, L. On the stability of polyyne. Chem. Phys. 1992, 168, 249–258. [Google Scholar] [CrossRef]
- Springborg, M.; Kavan, L. Can linear carbon chains be synthesized? Synth. Met. 1993, 57, 4405–4410. [Google Scholar] [CrossRef]
- Li, P. DFT studies on configurations, stabilities, and IR spectra of neutral carbon clusters. J. At. Mol. Sci. 2012, 3, 308–322. [Google Scholar] [CrossRef]
- Jäntschi, L.; Bálint, D.; Pruteanu, L.L.; Bolboacă, S.D. Elemental factorial study on one-cage pentagonal face nanostructure congeners. Mater. Discov. 2016, 5, 14–21. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Foing, B.H. Fullerenes and cosmic Carbon. Science 2010, 329, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Black, S.P.; Stefankiewicz, A.R.; Smulders, M.M.J.; Sattler, D.; Schalley, C.A.; Nitschke, J.R.; Sanders, J.K.M. Generation of a dynamic system of three-dimensional tetrahedral polycatenanes. Angew. Chem. Int. Ed. 2013, 52, 5749–5752. [Google Scholar] [CrossRef]
- Akita, M.; Tanaka, Y. Carbon-rich organometallics: Application to molecular electronics. Coord. Chem. Rev. 2022, 461, 214501. [Google Scholar] [CrossRef]
- Safarowsky, O.; Windisch, B.; Mohry, A.; Vögtle, F. Nomenclature for catenanes, rotaxanes, molecular knots, and assemblies derived from these structural elements. J. Prakt. Chem. 2000, 342, 437–444. [Google Scholar] [CrossRef]
- Span, R.; Lemmon, E.W.; Jacobsen, R.T.; Wagner, W.; Yokozeki, A. A reference equation of state for the thermodynamic properties of Nitrogen for temperatures from 63.151 to 1000 K and pressures to 2200 MPa. J. Phys. Chem. Ref. Data 2000, 29, 1361–1433. [Google Scholar] [CrossRef]
- Johnson, R.D., III. Experimental data for N2 (Nitrogen diatomic). NIST Stand. Ref. Database 2020, 101, 1361–1433. Available online: http://cccbdb.nist.gov/exp2x.asp?casno=7727379 (accessed on 12 September 2022).
- Liu, Z.; Lu, T.; Chen, Q. Comment on “Theoretical investigation on bond and spectrum of cyclo[18]carbon (C18) with sp-hybridized”. J. Mol. Model. 2021, 27, 42. [Google Scholar] [CrossRef] [PubMed]
- Hartree, D.R. The wave mechanics of an atom with a non-Coulomb central field. Part I. Theory and methods. Math. Proc. Cambridge Phil. Soc. 1928, 24, 89–110. [Google Scholar] [CrossRef]
- Hartree, D.R. The wave mechanics of an atom with a non-Coulomb central field. Part II. Some results and discussion. Math. Proc. Cambridge Phil. Soc. 1928, 24, 111–132. [Google Scholar] [CrossRef]
- Fock, V.A. Approximation method for solving the quantum mechanical multibody problem. Z. Phys. 1930, 61, 126–148. (In German) [Google Scholar] [CrossRef]
- Fock, V.A. Self-consistent field with substitution for sodium. Z. Phys. 1930, 62, 795–805. (In German) [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Schrödinger, E. An undulatory theory of the mechanics of atoms and molecules. Phys. Rev. 1926, 28, 1049–1070. [Google Scholar] [CrossRef]
- Sitkiewicz, S.P.; Zalesny, R.; Ramos-Cordoba, E.; Luis, J.P.; Matito, E. How reliable are modern density functional approximations to simulate vibrational spectroscopies? J. Phys. Chem. Lett. 2022, 13, 5963–5968. [Google Scholar] [CrossRef] [PubMed]
- Cotton, A.F.; Wilkinson, G. The heat of formation of ferrocene. J. Am. Chem. Soc. 1952, 74, 5764–5766. [Google Scholar] [CrossRef]
- Jaffé, H.H. The electronic structure of ferrocene. J. Chem. Phys. 1953, 21, 156–157. [Google Scholar] [CrossRef]
- Sánchez, M.; Sabio, L.; Gálvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Bystritskaya, E.V.; Pomerantsev, A.L.; Rodionova, O.Y. Nonlinear regression analysis: New approach to traditional implementations. J. Chemom. 2000, 14, 667–692. [Google Scholar] [CrossRef]
- Lin, Y.; Connell, J.W. Advances in 2D Boron nitride nanostructures: Nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale 2012, 4, 6908–6939. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.; Pohland, E. Boron hydrogens, VIII. For knowledge of the B2H6 and the B5H11. Berichte 1926, 59, 2210–2215. (In German) [Google Scholar]
- Mulliken, R.S. The vibrational isotope effect in the brand spectrum of Boron nitride. Science 1923, 58, 164–166. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kuroda, S.; Muramatsu, Y. Electronic structure and intercalation chemistry of graphite-like layered material with a composition of BC6N. J. Phys. Chem. Solids 2008, 69, 1171–1178. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, Q.; Wang, Y.; Nishimura, K.; Yang, G.; Zhang, B.; Jiang, N.; Li, H. Optical properties of bulk single-crystal diamonds at 80–1200 K by vibrational spectroscopic methods. Materials 2021, 14, 7435. [Google Scholar] [CrossRef] [PubMed]
- Susniak, K.; Krysa, M.; Kidaj, D.; Szymanska-Chargot, M.; Komaniecka, I.; Zamlynska, K.; Choma, A.; Wielbo, J.; Ilag, L.L.; Sroka-Bartnicka, A. Multimodal spectroscopic imaging of pea root nodules to assess the Nitrogen fixation in the presence of biofertilizer based on nod-factors. Int. J. Mol. Sci. 2021, 22, 12991. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Datta, A.; Jágerská, J. Integrated nanophotonic waveguide-based devices for IR and Raman gas spectroscopy. Sensors 2021, 21, 7224. [Google Scholar] [CrossRef]
- Born, M.; Oppenheimer, J.R. Zur Quantentheorie der Molekeln. Ann. Phys. 1927, 389, 457–484. [Google Scholar] [CrossRef]
- Johnson, B.G.; Gill, P.M.W.; Pople, J.A. The performance of a family of density functional methods. J. Chem. Phys. 1993, 98, 5612–5626. [Google Scholar] [CrossRef]
- Hunt, K.L.C.; Harris, R.A. Vibrational circular dichroism and electric-field shielding tensors: A new physical interpretation based on non local susceptibility densities. J. Chem. Phys. 1991, 94, 6995–7002. [Google Scholar] [CrossRef]
- Pearson, R.G. Symmetry rule for predicting molecular structure and reactivity. J. Am. Chem. Soc. 1969, 91, 1252–1254. [Google Scholar] [CrossRef]
- Ghanty, T.K.; Ghosh, S.K. A density functional approach to hardness, polarizability, and valency of molecules in chemical reactions. J. Phys. Chem. 1996, 100, 12295–12298. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).