Abstract
Introduction: The Mediterranean Diet is known for its protective effects against cardiovascular and metabolic diseases. Metabolic syndrome, characterized by multiple health risk factors, is increasingly concerning in older populations. Understanding dietary impacts on metabolic health is key for promoting healthy ageing. Objectives: This feasibility study aimed to explore the relationship between adherence to the Mediterranean Diet and metabolic risk factors in older adults participating in a community exercise program and to evaluate the feasibility of applying validated tools in this setting. Methods: A cross-sectional design was used. Adherence to the Mediterranean Diet was evaluated using the PREDIMED questionnaire, while Metabolic Syndrome was evaluated according to National Cholesterol Education Program criteria. Blood samples were taken following WHO guidelines. Results: Ten participants (mean age 73.1 years; 90% women) were included. 50% showed high adherence to the Mediterranean Diet, while 40% had moderate or low adherence. No participants met the full criteria for Metabolic Syndrome. Significant associations were found between Mediterranean Diet adherence and chronic disease (r = 0.869, p < 0.01), and an inverse correlation with the number of Metabolic Syndrome criteria (r = –0.707, p < 0.05). The Mediterranean Diet score was also inversely related to cholesterol (r = –0.740, p < 0.05). Conclusions: Higher adherence to the Mediterranean Diet was associated with better metabolic profiles, highlighting its potential protective role. The study demonstrates the feasibility of incorporating nutritional screening in community exercise programs for older adults. Future research should include larger and longitudinal samples and integrate inflammatory biomarkers.
1. Introduction
The global population is rapidly ageing, with 21.6% of the European Union (EU) population being aged 65 years and over [1]. Although this demographic shift is occurring across all EU countries, Portugal stands out as one of the oldest nations in Europe, with 24.1% of its population classified as elderly, only surpassed by Italy at 24.3% [1]. While increased longevity represents an achievement of modern society [2] reflecting improved access to healthcare, medical advances, and overall enhanced quality of life [3], demographic ageing also introduces substantial public health challenges [4,5]. In particular, older populations face a disproportionate burden of chronic non-communicable diseases, which are now the leading cause of disability and mortality worldwide according to the World Health Organisation (WHO) [6]. Within this broad spectrum of chronic diseases, Metabolic Syndrome (MetS), defined by the clustering of abdominal obesity, hypertension, dyslipidaemia, and impaired glucose regulation [7], is of specific concern, standing out as a major risk factor for cardiovascular disease, type 2 diabetes, and all-cause mortality in this age group [8,9]. MetS prevalence has been rising globally, largely driven by increasing rates of obesity and type 2 diabetes [10], with recent meta-analyses indicating a wider range, from 12.5% to 31.4%, depending on the specific diagnostic criteria used (National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) or International Diabetes Federation (IDF)) [11]. Epidemiological studies across Europe applying the ATP III criteria report that approximately 24.3% of the population is affected by MetS, with prevalence rising markedly with advancing age [12]. Regional disparities are evident, with the Eastern Mediterranean Countries showing particularly high rates compared with other parts of the world [11]. In Portugal, the most recent national survey (2015) estimated an overall prevalence of 33.4% among adults aged 25–74 years, with slightly higher rates observed in men (35.6%) than in women (31.3%) [13].
Diet is a vital determinant of healthy ageing, particularly given the specific nutritional needs of older adults [14]. Among various dietary patterns, the Mediterranean Diet (MedDiet) has received particular attention for its capacity to provide comprehensive nutritional support while also exerting protective effects on metabolic health [15,16]. Characterised by high intakes of vegetables, fruits, legumes, whole grains, nuts, and olive oil, as well as moderate consumption of fish, poultry, and dairy, the MedDiet features low intake of red and processed meats, an emphasis on fresh herbs, and encourages social eating [17]. Accumulating evidence from both observational and interventional research consistently indicates that greater adherence to the MedDiet lowers the risk of a wide range of chronic diseases [18]. Large prospective cohort studies, such as those from the European Prospective Investigation into Cancer and Nutrition (EPIC) project, have demonstrated that sustained adherence to the MedDiet is associated with significant reductions in cancer incidence and type 2 diabetes [19]. Building on these foundations, the DIABEPIC-2 programme, which combined a Mediterranean-based dietary pattern with calorie restriction and structured exercise, demonstrated that 38% of participants achieved remission of type 2 diabetes [20]. The landmark PREDIMED (Prevención con Dieta Mediterránea) randomised controlled trial reported that adherence to the MedDiet significantly reduces the risk of cardiovascular events [21,22]. Participants adhering closely to this diet had a 30% lower chance of developing type 2 diabetes, along with notable improvements in central obesity and glycaemic control [23]. Additionally, it lowers the risk of developing Metabolic Syndrome [24,25,26], resulting in a 30% to 50% reduction in the odds of MetS, mainly due to improvements in cardiovascular health [27,28], glucose levels [29,30] and inflammatory markers [31]. High adherence to MedDiet was also associated with a 13.9% decrease in the prevalence of MetS, with rates at 31.3% for those with high adherence compared to 45.2% for those with low adherence [26]. Further evidence from ageing cohorts reinforces these benefits. The Italian Longitudinal Study on Ageing (ILSA) found that higher adherence to the MedDiet was associated with a 34% reduction in all-cause mortality risk among older adults compared with those with the lowest adherence [32].
Despite this strong body of evidence, adherence to the MedDiet varies widely across populations and settings [33,34]. Paradoxically, countries traditionally associated with the Mediterranean lifestyle are experiencing rising rates of obesity, type 2 diabetes, and metabolic disorders [35], reflecting the progressive erosion of traditional dietary habits [33,36].
While international and national surveys provide valuable insights, Portuguese investigations often exclude individuals over 74 years of age, creating a significant gap in knowledge regarding the oldest and most vulnerable segments of the population. Given that Portuguese society is both ageing and facing a significant burden of metabolic disorders, there is an urgent need for updated evidence [37]. Moreover, since physical activity interacts with dietary habits to affect metabolic health, it is particularly important to study older adults who participate in structured community exercise programs, which are increasingly promoted in public health strategies for ageing populations. The relationship between the MedDiet and metabolic risk factors in older adults remains an important area for further investigation [38], particularly within the Portuguese context.
The present study aimed to assess adherence to the Mediterranean Diet among a group of older Portuguese adults participating in a multicomponent exercise program and to examine its association with the risk factors for MetS. Given the small sample size, this research was designed as a feasibility study with two main objectives: to generate preliminary evidence and to evaluate the practicality of incorporating validated tools, such as the PREDIMED questionnaire and ATP III criteria, into community-based health initiatives. The insights gained here are intended to inform the design of future large-scale and longitudinal studies focusing on the interplay between diet, ageing, and metabolic health.
2. Materials and Methods
2.1. Study Design and Participants
This cross-sectional feasibility study aimed to explore the relationship between adherence to the MedDiet and its potential association with MetS among older adults. This feasibility study aimed to assess the practicality and acceptance of integrating nutritional screening into a community-based exercise program for older adults. According to Whitehead et al. (2014) [39], such studies focus on whether an intervention can be implemented in real-world settings rather than on its effectiveness. This analysis serves as a foundational step for informing the design of future large-scale cohort studies.
Participants were community-dwelling adults aged 65 years or older, recruited from a multicomponent exercise training program specifically designed for this age group. Inclusion criteria were adults aged 65 years or older, enrolled in the exercise program for at least three months, and able to provide informed consent. Exclusion criteria comprised any cognitive impairment, acute or uncontrolled medical condition, or physical limitation that could compromise the safe execution of anthropometric measurements or blood collection procedures. Out of 50 individuals invited, 27 agreed to participate, 14 were excluded for not meeting the eligibility criteria, and only 10 participants were fully analysed. A detailed participant flow is illustrated in Figure 1.
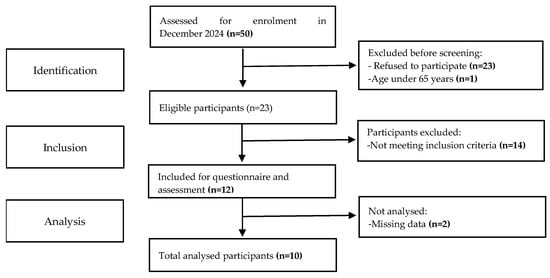
Figure 1.
STROBE flow chart of participants.
2.2. Data Collection Procedure and Instruments
The data collection process was a wide-ranging procedure, conducted exclusively during the morning period, that incorporated two key components: a self-administered two-part questionnaire and blood sample collection.
The first part questionnaire was focused on gathering essential sociodemographic variables included sex, age, and marital status, as well as health-related variables included the presence of chronic diseases, specifically diabetes, hypertension, and cardiovascular conditions. Anthropometric and body composition information was also using electrical bioimpedance analysis (InBody BWA 2.0), which provided detailed parameters including (among others) Body Mass Index (BMI, kg/m2) and Waist Circumference. The second part applied the PREDIMED questionnaire, to evaluate dietary adherence. This validated instrument is designed to measure how closely individuals adhere to the MedDiet. It contains 14 questions that focus on various dietary practices, along with a scoring system that classifies participants based on their adherence levels [40,41]. Participants receive 1 point for each item in the questionnaire that aligns with the established criteria. The total adherence score is determined by summing all the points earned. This score categorises adherence to the MedDiet into three levels: high adherence (11–14 points), moderate adherence (7–10 points), and low adherence (0–6 points) [41]. Participants were classified into specific categories according to established cut-off thresholds, allowing for a more straightforward interpretation of dietary patterns and their possible links to health outcomes.
MetS is diagnosed based on specific criteria outlined in The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [42]. According to ATP III, a diagnosis of MetS requires the presence of three or more components: abdominal obesity, characterized by a waist circumference greater than 102 cm for men or greater than 88 cm for women; high triglycerides, defined as levels of 150 mg/dL or higher; low HDL cholesterol, which is less than 40 mg/dL for men or less than 50 mg/dL for women; elevated blood pressure, measured at 130/85 mmHg or higher; and elevated fasting glucose, with levels of 110 mg/dL or higher [42].
A qualified healthcare professional collected all biological and anthropometric data in accordance with standardised procedures. Blood samples were obtained in the morning period (between 8:00 and 10:00 AM) after a minimum 8 h overnight fast, following the guidelines outlined by the WHO for specimen collection [43]. Samples were transported and processed within 3–4 h after venipuncture on the same collection day, ensuring their integrity [44].
2.3. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics (version 27.0). Descriptive statistics, including mean ± standard deviation (SD), were employed to summarize continuous variables, while categorical variables were presented as frequencies and percentages. The normality of data distribution was evaluated using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Due to the small sample size, the assumption of normality was not satisfied for several variables; thus, non-parametric tests were utilized to ensure the robustness of the analyses [45]. Specifically, differences in adherence to the MedDiet categories and the presence of MetS were examined using the Kruskal–Wallis test. Correlations between continuous variables were assessed using Spearman’s rank correlation coefficient, which is more suitable for non-normally distributed data. A confidence interval of 95% was applied, and statistical significance was defined as p < 0.05.
2.4. Ethics
Informed consent was obtained from each participant before data collection, ensuring voluntary participation. Stringent adherence to the Declaration of Helsinki Oviedo Convention was ensured throughout the research process (approved protocol by Ethical Council of Unidade Local de Saúde do Nordeste, Nº41/2024). All personal data was stored separately from participant’s name with an alphanumeric code number.
3. Results
3.1. Population Characterisation
The sample consisted of 10 older adults with a mean age of 73.1 years (SD = 3.7; range: 69–81 years). The majority were female (90%), with only one male participant (10%). Regarding marital status, half of the participants were widowed (50%), 40% were married, and one participant (10%) did not report this information.
Self-reported health status varied: 40% classified their health as “Good,” 10% as “Very Good,” 40% as “Reasonable,” and 10% did not answer. Most participants (70%) reported having at least one chronic disease, while 20% denied any chronic condition, and 10% did not provide information. Specifically, 40% reported having diabetes, 60% reported high cholesterol levels, 20% had hypertension, and 20% had cardiovascular disease. Notably, 10% of participants did not report their disease status for some of these conditions.
Biochemical parameters revealed an average glucose level of 83.4 mg/dL (SD = 13.8; range: 64–109), which is within normal limits for most participants. Triglyceride levels ranged from 55 to 151 mg/dL, with a mean of 84.8 mg/dL (SD = 26.8), and only one participant had values above the threshold for hypertriglyceridemia. HDL cholesterol levels ranged from 51 to 84 mg/dL, with a mean of 68.7 mg/dL (SD = 11.9), indicating that all participants had values above the risk threshold (<40 mg/dL for men, <50 mg/dL for women). Total cholesterol ranged from 132 to 215 mg/dL, with a mean of 178.3 mg/dL (SD = 25.2).
Anthropometric measurements indicated a wide variation in body composition. The mean BMI was 27.5 kg/m2 (SD = 5.8), ranging from 19.0 (underweight) to 40.8 (obese), with several participants falling into the overweight and obesity categories. Abdominal circumference ranged from 65.1 cm to 113.1 cm, with a mean of 86.9 cm (SD = 13.0), and 40% of participants met the criteria for abdominal obesity based on sex-specific cutoffs.
3.2. Mediterranean Diet Adherence
Adherence to the MedDiet was classified into three levels based on a standardized scoring system. As demonstrated in Figure 2, half of the sample (50%) demonstrated high adherence, 20% had moderate adherence, and another 20% had low adherence. One participant (10%) did not provide sufficient data for classification. Table 1 presents the distribution of participants’ sociodemographic and clinical characteristics according to their level of adherence to the MedDiet. Overall, individuals with high adherence tended to have a better health profile, including lower rates of chronic diseases and cardiovascular risk factors. Notably, all participants with chronic diseases exhibited either high or moderate adherence, while participants without chronic conditions were predominantly in the low adherence group.
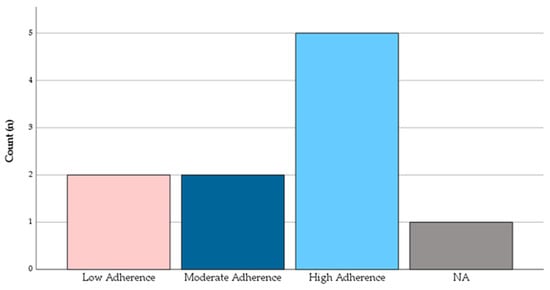
Figure 2.
Distribution of adherence to the Mediterranean Diet according to PREDIMED score categories (low: 0–6 points; moderate: 7–10 points; high: 11–14 points). Count is presented as n of total participants.

Table 1.
Sociodemographic and clinical characteristics of the participants.
3.3. Metabolic Syndrome
MetS was evaluated using the five standard criteria: abdominal obesity, elevated triglycerides, low HDL cholesterol, high blood pressure, and elevated fasting glucose. Although some individual risk factors were present, none of the 10 participants met the criteria for MetS, defined as the presence of three or more risk factors.
The most frequently observed component was abdominal obesity (40%), followed by high blood pressure (20%) and elevated triglycerides (10%). All participants had HDL levels above the risk threshold, and no participant exhibited impaired fasting glucose (>100 mg/dL).
3.4. The Association Between Adherence and the Development of Metabolic Syndrome
The analysis of MetS risk factors across Mediterranean Diet adherence categories revealed heterogeneous patterns (Figure 3). Participants in the high adherence group tended to show higher median values of both systolic and diastolic blood pressure when compared with those in the moderate and low adherence groups. In contrast, fasting glucose values were elevated among participants with moderate adherence, while triglycerides displayed considerable variability within this group. Interestingly, BMI and abdominal circumference were lowest in the low adherence group, suggesting that anthropometric indicators did not consistently increase with greater adherence to the MedDiet.
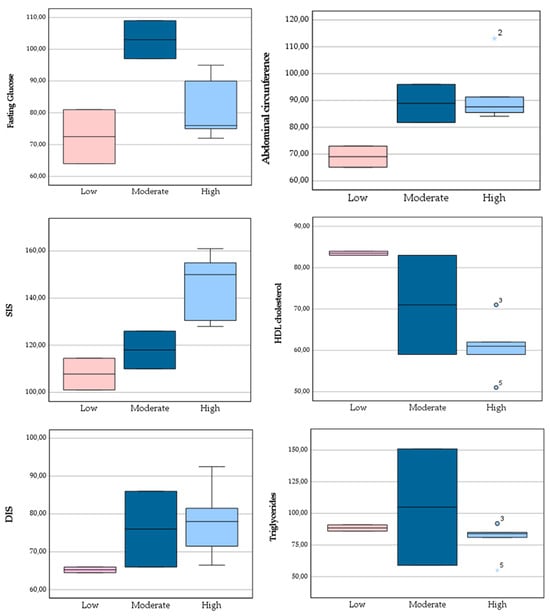
Figure 3.
Boxplots showing the distribution of BMI, abdominal circumference, fasting glucose, triglycerides, and systolic and diastolic blood pressure according to Mediterranean Diet adherence categories (low: 0–6; moderate: 7–10; high: 11–14).
None of the participants in this small sample met the diagnostic threshold for MetS. Nevertheless, individuals with higher adherence appeared less likely to accumulate multiple risk factors, supporting the potential protective role of the MedDiet. Correlation analysis (Figure 4) provided further insight into these associations. A significant negative correlation was observed between MedDiet adherence and the presence of chronic disease (r = –0.740, p = 0.023), suggesting that participants with a greater number of chronic conditions tended to report lower adherence. Abdominal obesity was also negatively correlated with the number of MetS diagnostic criteria (r = –0.707, p = 0.022), indicating that participants without abdominal obesity were less likely to fulfil multiple MetS components. Although correlations between MedDiet adherence and specific MetS risk factors such as triglycerides, blood pressure, or HDL cholesterol were not statistically significant, some exploratory patterns emerged. Higher PREDIMED scores appeared to associate with lower HDL cholesterol and higher BMI in this small sample, while triglycerides and total cholesterol showed near-zero to weak negative correlations. This may reflect the small sample size or the relatively low prevalence of some risk factors among participants.
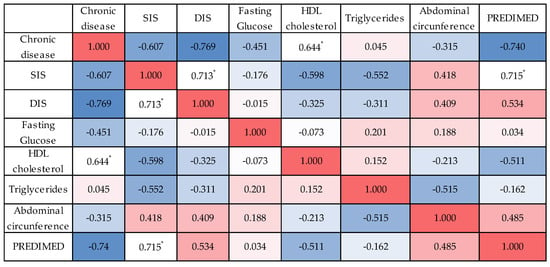
Figure 4.
Heatmap of Spearman’s correlations between Mediterranean Diet adherence (PREDIMED score), Metabolic Risk factors (systolic and diastolic blood pressure, fasting glucose, HDL cholesterol, triglycerides) and anthropometric measures (abdominal circumference). Strong positive correlations (r ≥ 0.60) were observed between PREDIMED score and systolic blood pressure, and between systolic and diastolic blood pressure. Moderate negative correlations were observed between adherence and HDL cholesterol, and between triglycerides and abdominal circumference. Asterisks indicate statistical significance of Spearman’s correlations. Red and blue colours represent positive and negative correlations, respectively, with colour intensity indicating the strength of the association. The full set of correlation results is presented in Supplementary Materials, Table S1: Correlation Results.
4. Discussion
This feasibility investigation aimed to evaluate older adults’ adherence to the MedDiet and its potential link to MetS. Several important descriptive trends were identified. Notably, half of the participants exhibited high adherence to MedDiet, and none met all the criteria for MetS. Although some participants displayed individual risk factors, we did not find significant links between diet adherence and specific MetS components, such as blood pressure, which could be explained by the limited statistical power of a study with ten participants, coupled with the generally healthy profile of the cohort. Participants in the high adherence group showed higher median systolic and diastolic blood pressure compared with those in the moderate and low adherence groups. This finding appears paradoxical, specifically considering that diet is a tractable modifier of vascular health and blood pressure [46]. MedDiet emphasises high potassium and low sodium intake, which helps balance sodium levels [47]. Foods rich in this mineral, such as fruits, vegetables, legumes, and nuts, support kidney function in excreting excess sodium, lowering blood pressure by reducing blood volume and vascular resistance [46,47]. In addition, studies indicate that MedDiet may also influence the composition of gut microbiota [48], which has been associated with the development and pathogenesis of blood pressure [49]. The consumption of polyphenols and monounsaturated fats, present in olive oil and nuts, may improve the production and release of nitric oxide (NO) from endothelial cells, reducing oxidative stress, improving microvascular endothelial function, promoting relaxation and vasodilation, which improves blood flow [50,51,52,53].
Our analysis showed no significant links between Mediterranean Diet (MedDiet) adherence and triglycerides or HDL cholesterol, with fasting glucose unexpectedly higher in those moderately adhering to the diet. The MedDiet supports lipid metabolism by replacing saturated fats with monounsaturated fatty acids (MUFA) from olive oil and nuts [54,55,56], alongside omega-3 polyunsaturated fatty acids (PUFA) from fish [57,58]. These components work to lower hepatic triglyceride synthesis and promotes fatty acid oxidation through activation of peroxisome proliferator-activated receptor-alpha (PPAR-α). These mechanisms lower very-low-density lipoprotein (VLDL) secretion, the main carrier of triglycerides [59,60]. Additionally, the MedDiet enhances clearance of triglyceride-rich particles by stimulating lipoprotein lipase (LPL) activity and reducing apolipoprotein C-III expression, which together accelerate triglyceride breakdown [61,62]. As a consequence, lower circulating triglycerides limit cholesteryl ester transfer protein (CETP)-mediated exchange between triglyceride-rich lipoproteins and HDL, preserving more stable particles that support reverse cholesterol transport [63,64]. Antioxidant and anti-inflammatory compounds, particularly olive oil polyphenols and plant flavonoids, further protect HDL functionality and vascular health [56,65,66]. Central obesity, on the other hand, is a key driver of metabolic dysfunction [67,68], as excess visceral fat promotes insulin resistance and dyslipidaemia by increasing hepatic lipid flux, which reduces HDL and elevates triglyceride levels, ultimately leading to atherogenic dyslipidaemia [68,69,70]. In our sample, BMI and waist circumference were lowest in the low adherence group, a pattern that contrasts with the established literature and with the significant negative correlation observed between abdominal obesity and the number of MetS criteria. Robust evidence indicates that greater adherence to the MedDiet is typically associated with reductions in central adiposity and improvements in lipid metabolism [71,72]. Central obesity is recognised as a major driver of metabolic dysfunction, as excess visceral fat contributes to dyslipidaemia and insulin resistance [73,74]. Mechanistically, abdominal obesity promotes lipid overflow to the liver, which reduces HDL cholesterol and elevates triglyceride levels, leading to an atherogenic dyslipidaemia profile [75,76]. For this reason, waist circumference, rather than BMI, is considered a more reliable marker for predicting MetS risk [75]. Dietary strategies like the MedDiet can effectively reduce abdominal circumference and visceral fat, thereby enhancing insulin sensitivity, decreasing inflammation, and addressing various components of MetS [71,72]. These benefits are largely mediated by a high intake of fibre-rich plant foods and monounsaturated fats that positively influence satiety, energy balance, and lipid metabolism [26]. In particular, the privileged intake of monounsaturated fats through olive oil improves glucose metabolism, enhances postprandial fat oxidation, increases diet-induced thermogenesis, and ultimately raises total daily energy expenditure, culminating in reductions in body weight [77]. Since excess body weight, especially abdominal visceral fat, is a key factor in the development of insulin resistance, and thereby type 2 diabetes, the MedDiet is capable of improving glycemic control both indirectly, by preventing obesity, and directly, through multiple mechanisms [77,78]. In particular, consumption of high content of fiber-rich foods can slow carbohydrate digestion and glucose absorption, by forming a gel-like substance in the gut, which delays gastric emptying and reduces the rate at which glucose enters the bloodstream, leading to reduced postprandial blood sugar spikes and more stable fasting glucose levels [79,80,81]. In addition, this diet emphasizes low-glycemic index foods that release glucose gradually into the bloodstream, decreasing insulin demand and improving glucose tolerance [82,83].
Regardless all paradoxical or contrary findings in our sample, there was a prominent negative correlation between adherence to the MedDiet and the prevalence of chronic diseases. These findings are strongly aligned with a significant body of research demonstrating that adherence to the MedDiet is associated with a reduced risk of chronic diseases and improved meabolic health among older adults [23,84,85]. The various mechanisms previously described support this relationship. The MedDiet contributes to reducing pro-inflammatory states by increasing levels of adiponectin, which in turn decreases inflammatory cytokines, enhances insulin sensitivity, and reduces vascular injury. These effects collectively help delay the progression of metabolic diseases. Together, these interconnected processes work synergistically to reduce abdominal obesity, improve metabolic regulation, and contribute to the prevention of chronic diseases among older adults.
Although the current study was constrained by a small sample size, which limits statistical power and generalizability, this study provides valuable preliminary insights into the health profile of older adults participating in community-based exercise programmes, and more diverse investigations to elucidate the relationship between adherence to the MedDiet and its health benefits, particularly in ageing populations, are needed. Additionally, the cross-sectional design precludes definitive conclusions regarding causality between MedDiet adherence and metabolic risk factors. Reliance on self-reported dietary data introduces potential biases, including recall inaccuracies and social desirability effects. Moreover, the absence of biomarkers related to inflammation and oxidative stress restricts insights into the physiological pathways involved. Despite these constraints, the study demonstrates the practicality of using straightforward nutritional screening tools, such as the PREDIMED questionnaire, within community-based programs targeting older adults. Conducting research in a community action setting adds both scientific and social value. It allows for the direct engagement of participants in real-life contexts, enhancing the ecological validity and applicability of findings. Such settings facilitate the implementation of lifestyle interventions, like promoting adherence to the MedDiet, in a supportive social environment that encourages motivation, shared experiences, and long-term adherence.
Future research should prioritise longitudinal monitoring of community participants to evaluate changes in metabolic risk factors and dietary adherence over time, as well as the inclusion of inflammatory and oxidative stress biomarkers to deepen the understanding of underlying mechanisms.
5. Conclusions
While no participants in our sample met the full criteria for metabolic syndrome, individual metabolic risk factors were present. Higher adherence to the Mediterranean Diet was associated with fewer risk factors and better overall health indicators. These findings suggest that adopting MedDiet, with attention to age-appropriate portion sizes and the inclusion of key foods such as vegetables, legumes, nuts, olive oil, whole grains, and fatty fish, may play a crucial role in maintaining metabolic health and promoting overall well-being among older adults. Further studies with larger, more heterogeneous populations are warranted to confirm these benefits and guide the development of targeted dietary interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jal6010005/s1. Table S1: Correlation Results.
Author Contributions
Conceptualization, S.B.A. and A.P.; methodology, S.B.A.; software, S.B.A.; validation, J.V., A.F. and A.P.; formal analysis, S.B.A., L.M.d.S. and C.A. investigation, S.B.A. and E.M.; resources, H.F. and A.M.M.; data curation, S.B.A.; writing—original draft preparation, S.B.A.; writing—review and editing, S.B.A. and A.P.; visualization, A.F.; supervision, A.F.; project administration, H.F.; funding acquisition, H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Regional Development Fund (FEDER) through the programme INTERREG VI-A Spain—Portugal (POCTEP) 2021–2027: Novas Sociedades Longevas (0137_NSL_6_E).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Unidade Local de Saúde do Nordeste (protocol code nº41/2024 and date of approval 12 June 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to ethical restrictions and the need to protect participants’ confidentiality, individual-level health data cannot be publicly shared. However, all aggregated and anonymized data supporting the findings of this study are included within the article. Additional information may be made available from the corresponding author upon reasonable request and with the approval of the ethics committee.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EU | European Union |
| WHO | World Health Organization |
| MetS | Metabolic Syndrome |
| NCEP | National Cholesterol Education Program |
| ATP III | Adult Treatment Panel III |
| IDF | International Diabetes Federation |
| MedDiet | Mediterranean Diet |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| DIABEPIC-2 | Diabetes Prevention with a Mediterranean-based Intervention Program |
| PREDIMED | Prevención con Dieta Mediterránea |
| ILSA | Italian Longitudinal Study on Ageing |
| SD | Standard Deviation |
References
- Population Structure and Ageing—Statistics Explained—Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 17 September 2025).
- Higo, M.; Khan, H.T.A. Global Population Aging: Unequal Distribution of Risks in Later Life between Developed and Developing Countries. Glob. Soc. Policy 2015, 15, 146–166. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Good Health Adds Life to Years: Global Brief for World Health Day 2012; World Health Organization: Geneva, Switzerland, 2012.
- Khan, H.T.A.; Addo, K.M.; Findlay, H. Public Health Challenges and Responses to the Growing Ageing Populations. Public Health Chall. 2024, 3, e213. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.T.A. Population Ageing in a Globalized World: Risks and Dilemmas? J. Eval. Clin. Pract. 2019, 25, 754–760. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. New Data: Noncommunicable Diseases Cause 1.8 Million Avoidable Deaths and Cost US$ 514 Billion Every Year, Reveals New WHO/Europe Report. Available online: https://www.who.int/europe/news/item/27-06-2025-new-data--noncommunicable-diseases-cause-1-8-million-avoidable-deaths-and-cost-us-514-billion-USD-every-year--reveals-new-who-europe-report (accessed on 22 September 2025).
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.P. Metabolic Syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic Diseases and Healthy Aging: Identifying Environmental and Behavioral Risk Factors and Promoting Public Health. Front. Public Health 2023, 11, 1253506. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Raso, F.U.M.; Muiesan, M.L.; Ryliškyte, L.; Rietzschel, E.; et al. Metabolic Syndrome across Europe: Different Clusters of Risk Factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- Alves, R.; Santos, A.J.; Kislaya, I.; Nunes, B.; Freire, A.C. Metabolic Syndrome in Portugal: Prevalence and Associated Factors. Acta Med. Port. 2022, 35, 633–643. [Google Scholar] [CrossRef]
- Duarte Junior, M.A.; Cabanas-Sánchez, V.; Pintos-Carrillo, S.; Ortolá, R.; Rodríguez-Artalejo, F.; Sotos-Prieto, M.; Martinez-Gomez, D. Association of Adherence to Mediterranean Diet and Changes over Time with All-Cause Mortality in Older Adults: The Seniors-ENRICA Cohorts. Am. J. Clin. Nutr. 2025, 122, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Petkoska, A.T.; Ognenoska, V.; Trajkovska-Broach, A. Mediterranean Diet: From Ancient Traditions to Modern Science—A Sustainable Way Towards Better Health, Wellness, Longevity, and Personalized Nutrition. Sustainability 2025, 17, 4187. [Google Scholar] [CrossRef]
- Giugliano, D.; Esposito, K. Mediterranean Diet and Metabolic Diseases. Curr. Opin. Lipidol. 2008, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, 673–675. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study Populations and Data Collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Dione, V.; Iglesies-Grau, J.; Latour, E.; Besnier, F.; Gagnon, C.; Gayda, M.; Pelletier, V.; Selcer, S.; Vrinceanu, T.; Debray, A.; et al. Optimizing Cardiovascular Health with a Type 2 Diabetes Remission Program: Ultraprocessed Food-Intake Reduction, Mediterranean Diet, Chrononutrition and Physical Training—The DIABEPIC-2 Pilot Study. Diabetes Obes. Metab. 2025, 27, 7374–7384. [Google Scholar] [CrossRef]
- López-Laguna, N.; Toledo, E.; Hershey, M.S.; Babio, N.; Sorlí, J.V.; Ros, E.; Muñoz, M.Á.; Estruch, R.; Lapetra, J.; Muñoz-Bravo, C.; et al. Life’s Simple 7 and Risk of Peripheral Artery Disease: Results from the PREDIMED Study and an Updated Meta-Analysis. Nutrients 2025, 17, 2058. [Google Scholar] [CrossRef]
- Furbatto, M.; Lelli, D.; Antonelli Incalzi, R.; Pedone, C. Mediterranean Diet in Older Adults: Cardiovascular Outcomes and Mortality from Observational and Interventional Studies—A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3947. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2016, 146, 920S–927S. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean Diet Supplemented with Nuts on Metabolic Syndrome Status: One-Year Results of the PREDIMED Randomized Trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean Diet Is Inversely Associated with Metabolic Syndrome Occurrence: A Meta-Analysis of Observational Studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Basterrechea, G.; Elío, I.; Alonso, G.; Otero, L.; Gutiérrez-Bardeci, L.; Puente, J.; Muñoz-Cacho, P. Adherence to the Mediterranean Diet Is Inversely Associated with the Prevalence of Metabolic Syndrome in Older People from the North of Spain. Nutrients 2022, 14, 4536. [Google Scholar] [CrossRef] [PubMed]
- Angelis, A.; Chrysohoou, C.; Tzorovili, E.; Laina, A.; Xydis, P.; Terzis, I.; Ioakeimidis, N.; Aznaouridis, K.; Vlachopoulos, C.; Tsioufis, K. The Mediterranean Diet Benefit on Cardiovascular Hemodynamics and Erectile Function in Chronic Heart Failure Male Patients by Decoding Central and Peripheral Vessel Rheology. Nutrients 2021, 13, 108. [Google Scholar] [CrossRef]
- Akgüllü, Ç.; Siriken, F.; Eryilmaz, U.; Akdeniz, M.; Ömürlü, I.K.; Pekcan, G.; Güngör, H.; Kurtoʇlu, T. The Relation between Compliance to the Mediterranean Diet and the Extensiveness of Coronary Artery Disease. Turk. Kardiyol. Dern. Ars. 2015, 43, 340–349. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Richardson, L.A.; Izuora, K.; Basu, A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review. Int. J. Environ. Res. Public. Health 2022, 19, 12762. [Google Scholar] [CrossRef]
- Pignanelli, M.; Just, C.; Bogiatzi, C.; Dinculescu, V.; Gloor, G.B.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; et al. Mediterranean Diet Score: Associations with Metabolic Products of the Intestinal Microbiome, Carotid Plaque Burden, and Renal Function. Nutrients 2018, 10, 779. [Google Scholar] [CrossRef]
- Limongi, F.; Noale, M.; Gesmundo, A.; Crepaldi, G.; Maggi, S. Adherence to the Mediterranean Diet and All-Cause Mortality Risk in an Elderly Italian Population: Data from the ILSA Study. J. Nutr. Health Aging 2017, 21, 505–513. [Google Scholar] [CrossRef]
- Obeid, C.A.; Gubbels, J.S.; Jaalouk, D.; Kremers, S.P.J.; Oenema, A. Adherence to the Mediterranean Diet among Adults in Mediterranean Countries: A Systematic Literature Review. Eur. J. Nutr. 2022, 61, 3327. [Google Scholar] [CrossRef]
- Boujelbane, M.A.; Ammar, A.; Salem, A.; Kerkeni, M.; Trabelsi, K.; Bouaziz, B.; Masmoudi, L.; Heydenreich, J.; Schallhorn, C.; Müller, G.; et al. Regional Variations in Mediterranean Diet Adherence: A Sociodemographic and Lifestyle Analysis across Mediterranean and Non-Mediterranean Regions within the MEDIET4ALL Project. Front. Public Health 2025, 13, 1596681. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.; McColl, K.; Al-Jawaldeh, A. Proposed Policy Priorities for Preventing Obesity and Diabetes in the Eastern Mediterranean Region; World Health Organization, Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2017. [Google Scholar]
- Da Silva, R.; Bach-Faig, A.; Raidó Quintana, B.; Buckland, G.; Vaz De Almeida, M.D.; Serra-Majem, L. Worldwide Variation of Adherence to the Mediterranean Diet, in 1961–1965 and 2000–2003. Public Health Nutr. 2009, 12, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.E.; Bragada, J.; Bragada, J.; Coelho, J.; Pinto, I.; Reis, L.; Magalhães, P. The Prevalence of Metabolic Syndrome and Its Components in Bragança District, North-Eastern Portugal: A Retrospective Observational Cross-Sectional Study. Rev. Port. Endocrinol. Diabetes Metab. 2022, 17, 51–57. [Google Scholar] [CrossRef]
- Gómez-Sánchez, L.; Gómez-Sánchez, M.; Tamayo-Morales, O.; Lugones-Sánchez, C.; González-Sánchez, S.; Martí-Lluch, R.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A. Relationship between the Mediterranean Diet and Metabolic Syndrome and Each of the Components That Form It in Caucasian Subjects: A Cross-Sectional Trial. Nutrients 2024, 16, 1948. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Sully, B.G.O.; Campbell, M.J. Pilot and Feasibility Studies: Is There a Difference from Each Other and from a Randomised Controlled Trial? Contemp. Clin. Trials 2014, 38, 130–133. [Google Scholar] [CrossRef]
- Afonso, L.; Moreira, T.; Oliveira, A. Índices de Adesão Ao Padrão Alimentar Mediterrânico—A Base Metodológica Para Estudar a Sua Relação Com a Saúde. Rev. Factores Risco 2014, 31, 48–55. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. New Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- WHO. Capillary Sampling. In WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. The Blood Cold Chain; World Health Organization, Department of Blood Safety and Clinical Technology: Geneva, Switzerland, 2002; p. 74. [Google Scholar]
- Da Cunha, D.; Ramires, N.; Tibana, A.; Ferreira De Melo, G.; Prestes, J. Testes de Normalidade em Análises Estatísticas: Uma Orientação Para Praticantes em Ciências Da Saúde e Atividade Física. Rev. Mackenzie Educ. Física Esporte 2015, 14, 73–77. [Google Scholar]
- Jennings, A.; Berendsen, A.M.; De Groot, L.C.P.G.M.; Feskens, E.J.M.; Brzozowska, A.; Sicinska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef]
- Perez, V.; Chang, E.T. Sodium-to-Potassium Ratio and Blood Pressure, Hypertension, and Related Factors. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Avery, E.G.; Bartolomaeus, H.; Maifeld, A.; Marko, L.; Wiig, H.; Wilck, N.; Rosshart, S.P.; Forslund, S.K.; Müller, D.N. The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ. Res. 2021, 128, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña, J.D.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Mediterranean Diet and Endothelial Function: A Review of Its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of Nitric Oxide Production in Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L. Dietary Fibre Modifies Gut Microbiota: What’s the Role of (Poly)Phenols? Int. J. Food Sci. Nutr. 2020, 71, 783–784. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef]
- Estruch, R.; Camafort, M. The Mediterranean Diet and Plasma Lipid Profile. Rev. Española Cardiol. 2015, 68, 279–281. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 318. [Google Scholar] [CrossRef]
- Del Bo’, C.; Perna, S.; Allehdan, S.; Rafique, A.; Saad, S.; AlGhareeb, F.; Rondanelli, M.; Tayyem, R.F.; Marino, M.; Martini, D.; et al. Does the Mediterranean Diet Have Any Effect on Lipid Profile, Central Obesity and Liver Enzymes in Non-Alcoholic Fatty Liver Disease (NAFLD) Subjects? A Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2023, 15, 2250. [Google Scholar] [CrossRef]
- Galli, C.; Marangoni, F. N-3 Fatty Acids in the Mediterranean Diet. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. Fatty Acid Regulation of Hepatic Lipid Metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Modulation of Hepatic Steatosis by Dietary Fatty Acids. World J. Gastroenterol. 2014, 20, 1746. [Google Scholar] [CrossRef]
- Pérez-Vega, K.A.; Castañer, O.; Sanllorente, A.; Lassale, C.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Alonso-Gómez, Á.M.; et al. Mediterranean Diet, Energy Restriction, Physical Activity, and Atherogenicity of Very-Low Density Lipoproteins: Findings from Two Randomized Controlled Trials. Mol. Nutr. Food Res. 2023, 67, 2200338. [Google Scholar] [CrossRef]
- Campanella, A.; Iacovazzi, P.A.; Misciagna, G.; Bonfiglio, C.; Mirizzi, A.; Franco, I.; Bianco, A.; Sorino, P.; Caruso, M.G.; Cisternino, A.M.; et al. The Effect of Three Mediterranean Diets on Remnant Cholesterol and Non-Alcoholic Fatty Liver Disease: A Secondary Analysis. Nutrients 2020, 12, 1674. [Google Scholar] [CrossRef]
- Koivuniemi, A.; Vuorela, T.; Kovanen, P.T.; Vattulainen, I.; Hyvönen, M.T. Lipid Exchange Mechanism of the Cholesteryl Ester Transfer Protein Clarified by Atomistic and Coarse-Grained Simulations. PLoS Comput. Biol. 2012, 8, e1002299. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl Ester Transfer Protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef]
- Abumweis, S.S.; Barake, R.; Jones, P.J.H. Plant Sterols/Stanols as Cholesterol Lowering Agents: A Meta-Analysis of Randomized Controlled Trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef]
- Zambrano, A.K.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Guevara-Ramírez, P.; Frias-Toral, E.; Simancas-Racines, D. Impact of Fundamental Components of the Mediterranean Diet on the Microbiota Composition in Blood Pressure Regulation. J. Transl. Med. 2024, 22, 417. [Google Scholar] [CrossRef]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of Obesity-Induced Metabolic and Vascular Dysfunctions. Front. Biosci. 2019, 24, 890. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of Obesity and Its Associated Diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Kim, S.P.; Catalano, K.J.; Hsu, I.R.; Chiu, J.D.; Kabir, M.; Hucking, K.; Ader, M. Why Visceral Fat Is Bad: Mechanisms of the Metabolic Syndrome. Obesity 2006, 14, 16S–19S. [Google Scholar] [CrossRef] [PubMed]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and Insulin Resistance in Humans: The Role of the Different Tissue and Cellular Lipid Depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean Diet in the Management and Prevention of Obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef]
- Paley, C.A.; Johnson, M.I. Abdominal Obesity and Metabolic Syndrome: Exercise as Medicine? BMC Sports Sci. Med. Rehabil. 2018, 10, 7. [Google Scholar] [CrossRef]
- Björntorp, P. Metabolic Implications of Body Fat Distribution. Diabetes Care 1991, 14, 1132–1143. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Després, J.P. Abdominal Obesity: The Most Prevalent Cause of the Metabolic Syndrome and Related Cardiometabolic Risk. Eur. Heart J. Suppl. 2006, 8, B4–B12. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Milenkovic, T.; Bozhinovska, N.; Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Asimi, Z.V.; Burekovic, A. Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review. Nutrients 2021, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, L.A. Dietary Fiber Influence on Overall Health, with an Emphasis on CVD, Diabetes, Obesity, Colon Cancer, and Inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of Soluble Dietary Fibre on Postprandial Blood Glucose Response and Its Potential as a Functional Food Ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber Intake and Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef]
- Vitale, M.; Costabile, G.; Bergia, R.E.; Hjorth, T.; Campbell, W.W.; Landberg, R.; Riccardi, G.; Giacco, R. The Effects of Mediterranean Diets with Low or High Glycemic Index on Plasma Glucose and Insulin Profiles Are Different in Adult Men and Women: Data from MEDGI-Carb Randomized Clinical Trial. Clin. Nutr. 2023, 42, 2022–2028. [Google Scholar] [CrossRef]
- Bergia, R.E.; Giacco, R.; Hjorth, T.; Biskup, I.; Zhu, W.; Costabile, G.; Vitale, M.; Campbell, W.W.; Landberg, R.; Riccardi, G. Differential Glycemic Effects of Low-versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial. Nutrients 2022, 14, 706. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The Effect of Mediterranean Diet on Metabolic Syndrome and Its Components: A Meta-Analysis of 50 Studies and 534,906 Individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Roman, B.; Carta, L.; Martínez-González, Á.M.; Serra-Majem, L. Effectiveness of the Mediterranean Diet in the Elderly. Clin. Interv. Aging 2008, 3, 97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.