Healthcare Redesign of Medication Management for Parkinson’s Inpatients
Abstract
1. Introduction
2. Materials and Methods
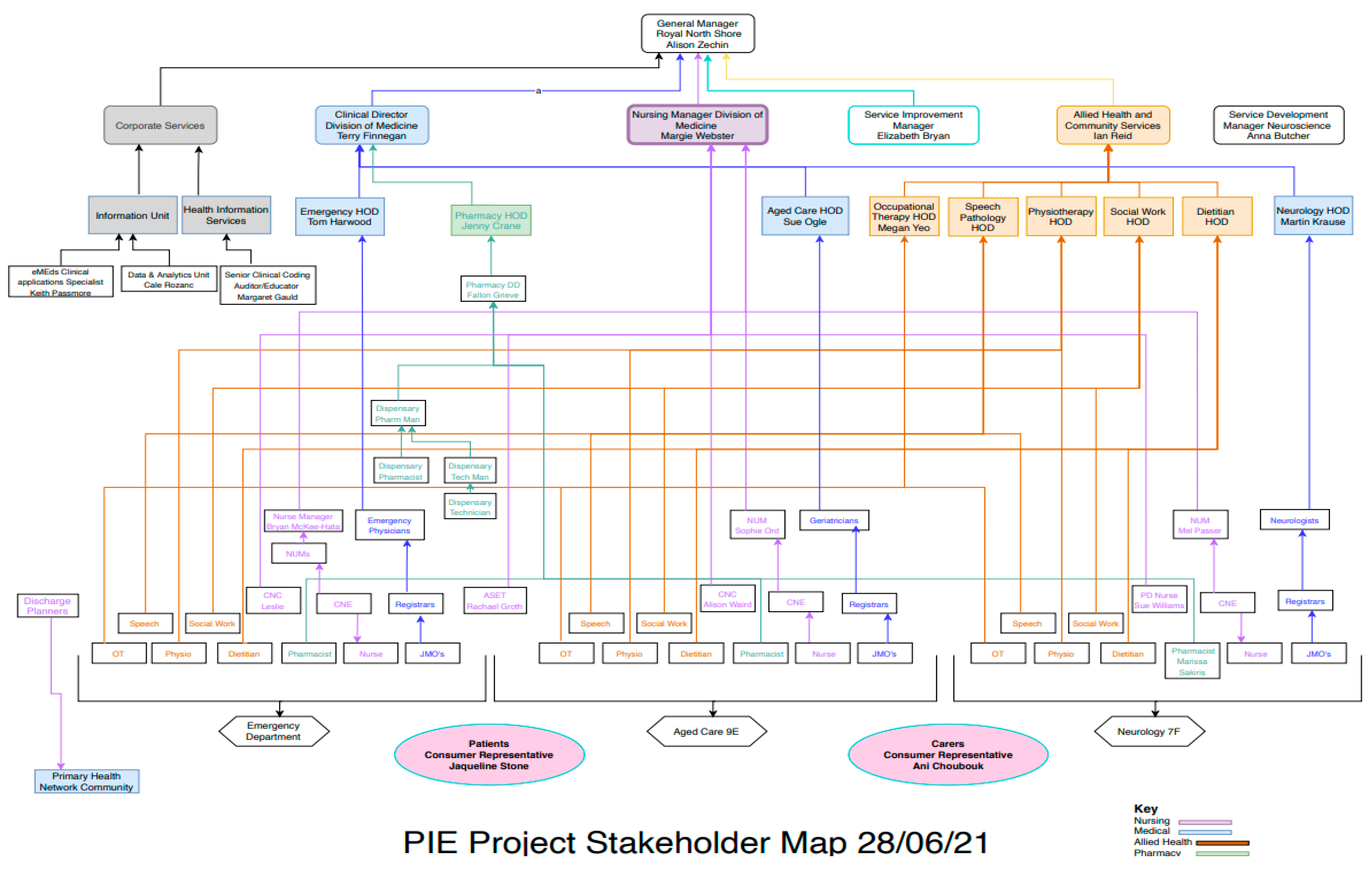
2.1. Setting
2.2. Solutions Design Phase
2.2.1. Data Collection and Analysis
Quantitative Data
Qualitative Data
2.3. Implementation Phase
2.4. Evaluation Phase
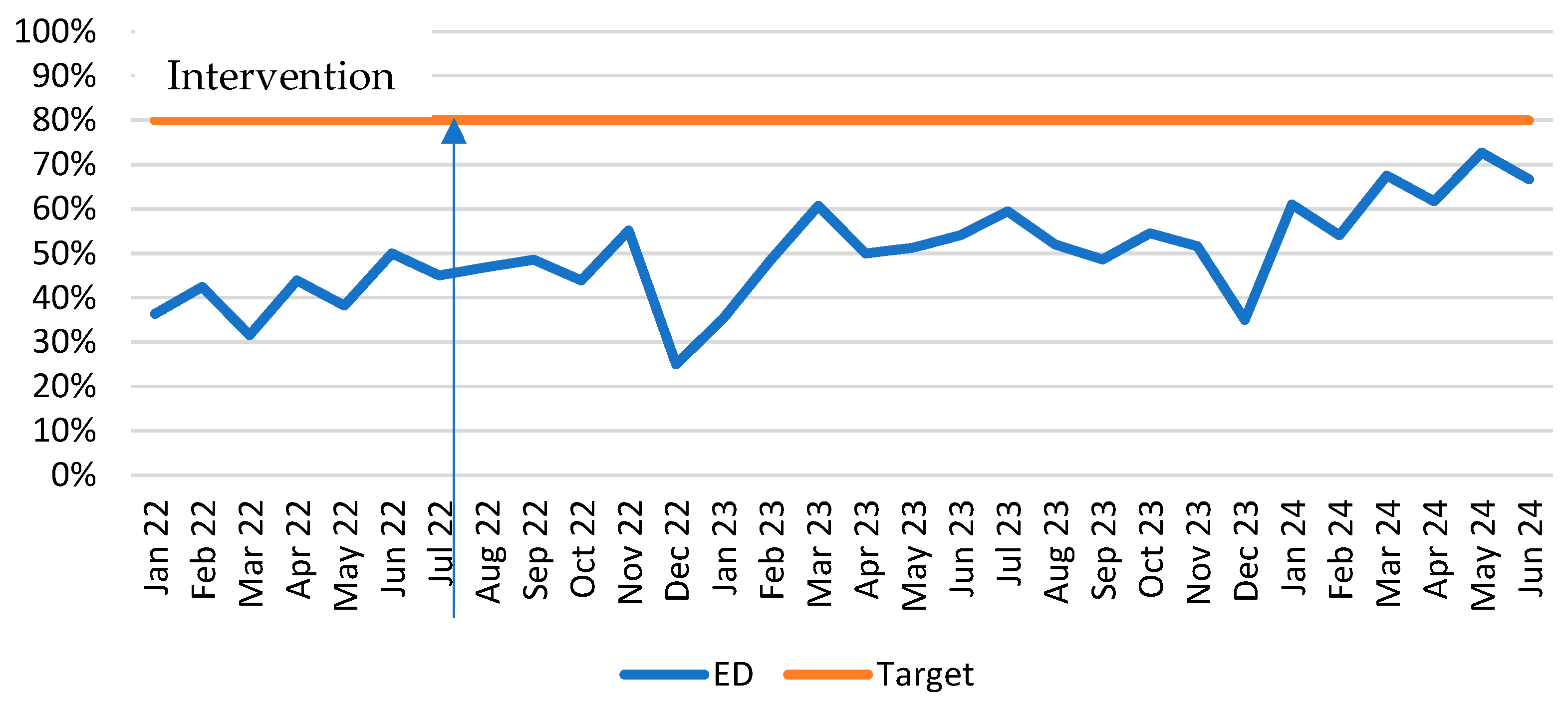
- Increase proportion of PD patients clearly identified in ED—flagged with a ‘PD problem code’ in the electronic medical record (EMR) from 31% to 80%;
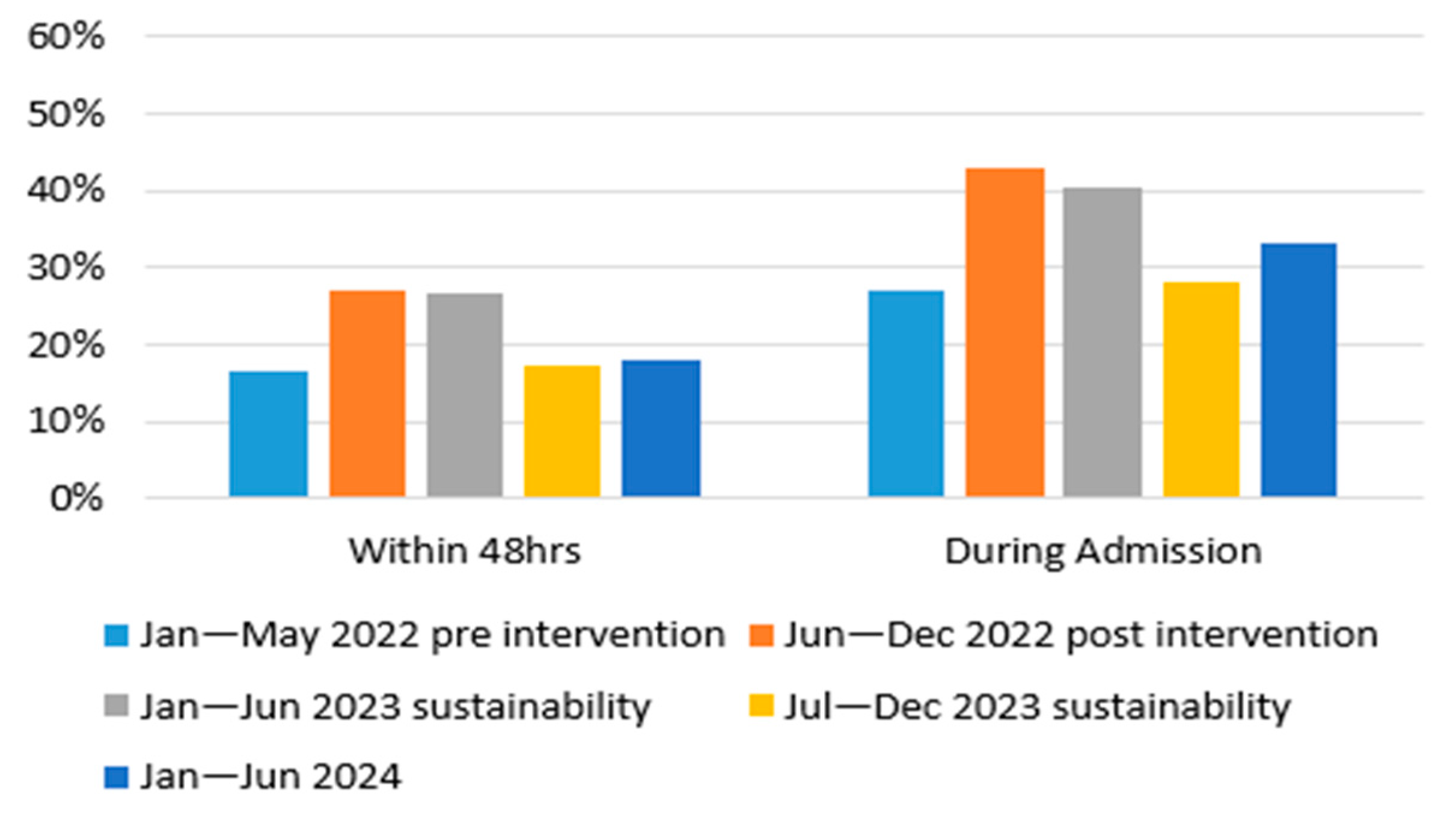
- Increase the proportion of PD patients who receive a pharmacist medication reconciliation within 48 h of admission from 16% to 60%;
- Increase the proportion of levodopa doses administered within 15 min of the prescribed time from 52% to 80%;
- Increase the proportion of pharmacist medication reconciliations free of PD medication prescribing errors from 38% to 80%;
- Improve service efficiency, experience of care, and health outcomes for PD patients by reducing their median length of stay by 10%.
Data Collection and Analysis
3. Results
3.1. Solutions Design and Implementation Phase
3.2. Evaluation Phase
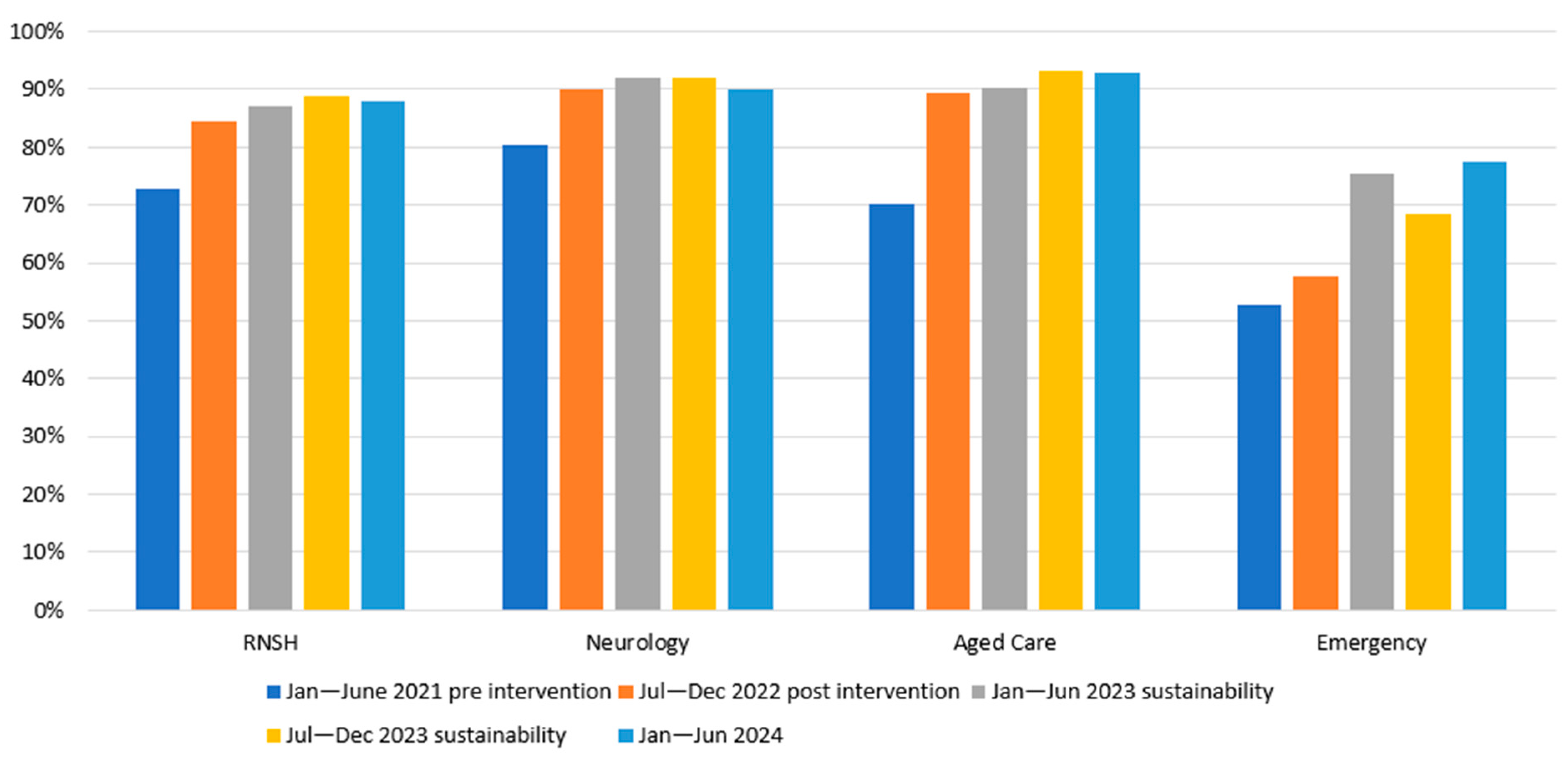
3.2.1. Solution 1: Patient Identification
3.2.2. Solution 2: Education
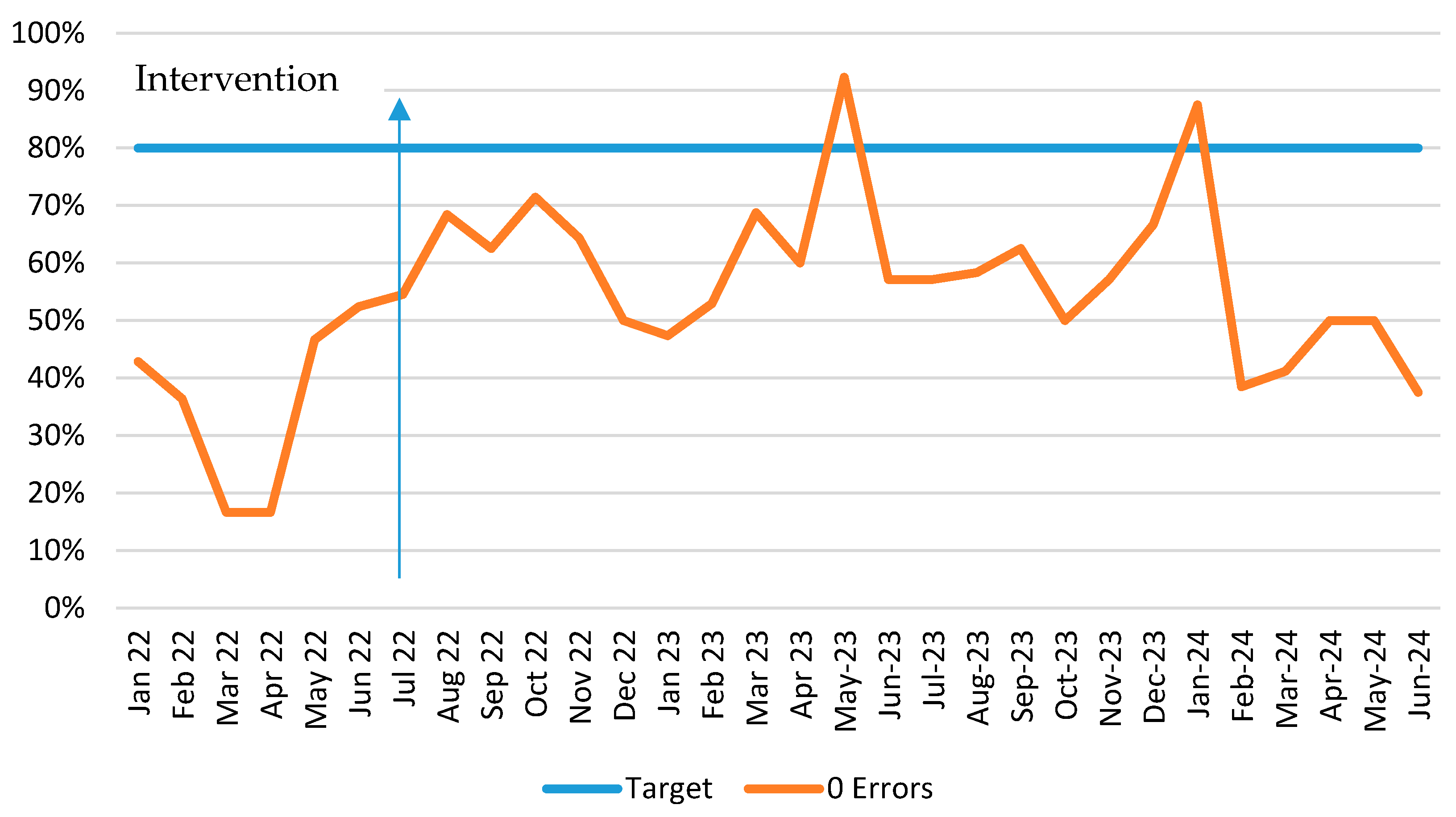
3.2.3. Solution 3: Medication Prescribing Errors
3.2.4. Solution 4: Ward Medication Stock
- Visual cues are beneficial—staff reported that displaying the posters next to where the medications are stored helped them to check the medications they administered easily and efficiently.
- Stock availability improved workflow—having the stock available was crucial for workflow and ensured that staff were able to access medications when needed, minimising delays in administration.
3.2.5. Solution 5: Systematic Prompting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| EMR | Electronic Medical Record |
| RNSH | Royal North Shore Hospital |
| NSLHD | Northern Sydney Local Health District |
| NSW | New South Wales |
| ED | Emergency Department |
| LOS | Length of Stay |
Appendix A
References
- Harris, M.; Fry, M.; Fitzpatrick, L. A clinical process redesign project to improve outcomes and reduce care variance for people with Parkinson’s disease. Australas. Emerg. Care 2019, 22, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Evans, A.H.; Fung, V.S.C.; Hayes, M.; Iansek, R.; Kimber, T.; O’SUllivan, J.D.; Sue, C.M. Practical approaches to commencing device-assisted therapies for Parkinson disease in Australia. Intern. Med. J. 2017, 47, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Azmi, H.; Cocoziello, L.; Nyirenda, T.; Douglas, C.; Jacob, B.; Thomas, J.; Cricco, D.; Finnerty, G.; Sommer, K.; Rocco, A.; et al. Adherence to a strict medication protocol can reduce length of stay in hospitalized patients with Parkinson’s Disease. Clin. Park. Relat. Disord. 2020, 3, 100076. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.D.; Neumiller, J.J.; Carlson, J.; Setter, S.M.; Corbett, C.F. Challenges of medication management in hospitalized patients with Parkinson’s disease. Am. J. Health Syst. Pharm. 2010, 67, 2059–2063. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Cowley, E.; Miller, M.R.; Yin, C.; Kelly, L. Correlation between Medication Administration–Related Errors in Patients with Parkinson Disease and Timing of Pharmacy-Led Best Possible Medication Histories. Can. J. Hosp. Pharm. 2021, 74, 15. [Google Scholar] [CrossRef]
- Lertxundi, U.; Isla, A.; Solinís, M.Á.; Echaburu, S.D.; Hernandez, R.; Peral-Aguirregoitia, J.; Medrano, J.; García-Moncó, J.C. Medication errors in Parkinson’s disease inpatients in the Basque Country. Park. Relat. Disord. 2017, 36, 57–62. [Google Scholar] [CrossRef]
- Buetow, S.; Henshaw, J.; Bryant, L.; O’Sullivan, D. Medication Timing Errors for Parkinson′ s Disease: Perspectives Held by Caregivers and People with Parkinson′s in New Zealand. Park. Dis. 2010, 2010, 432983. [Google Scholar]
- Yu, J.R.T.; Walter, B.L. Addressing critical care gaps in inpatient Parkinson’s care–Minimizing the impact of comorbidities and developing new care delivery models. Park. Relat. Disord. 2022, 104, 121–122. [Google Scholar] [CrossRef]
- Ben-Tovim, D.I. Process Redesign for Health Care Using Lean Thinking: A Guide for Improving Patient Flow and the Quality and Safety of Care; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Titler, M.G. Translation Science and Context. Res. Theory Nurs. Pract. 2010, 24, 35–55. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Wieringa, S. Is it time to drop the ‘knowledge translation’ metaphor? A critical literature review. J. R. Soc. Med. 2011, 104, 501–509. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Clinical Studies. Taking Action Locally: Eight Steps to Putting Cancer Guidelines into Practice; National Institute of Clinical Studies: Melbourne, Australia, 2006.
- Williams, S.; Iannuzzi, M.A.; Prior, S.J. Using Healthcare Redesign to Identify Medication Management Issues in Parkinson’s Disease. Pharmacy 2025, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Liamputtong, P. Research Methods in Health: Foundations for Evidence Based Practice; Oxford University Press: Melbourne, UK, 2013. [Google Scholar]
- Creswell, J.W.; Creswell, J.D. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches; Sage Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Samaranayake, P.; Dadich, A.; Fitzgerald, A.; Zeitz, K. Developing an evaluation framework for clinical redesign programs: Lessons learnt. J. Health Organ. Manag. 2016, 30, 950–970. [Google Scholar] [CrossRef] [PubMed]
- Independent Hospital Pricing Authority (IHPA). ICD-10-AM/ACHI/ACS. 2022. Available online: www.ihpa.gov.au (accessed on 8 October 2024).
- Carroll, V.; Deutschmann, K.; Andrews, J. Purposeful collaboration: Enriching lives for people with Parkinson’s disease. Austral-Asian J. Neurosci. 2020, 30, 32–43. [Google Scholar] [CrossRef]
- Saleem, A.; Ungcharoen, N.; Bell, F.; Storton, J.; Bibi, H. Improving Parkinson’s Medicine Administration in Hospitals: The Impact of an Out-of-Hours Drug Box. Cureus 2023, 15, e48447. [Google Scholar] [CrossRef]
- Corrado, J.; Jackson, O.; Baxandall, D.; Robson, J.; Duggan-Carter, P.; Throssell, J.; Westgarth, T.; Chhokar, G.; Alty, J.; Cracknell, A. Get Parkinson’s medications on time: The Leeds QI project. Age Ageing 2020, 49, 865–872. [Google Scholar] [CrossRef]
- Lance, S.; Travers, J.; Bourke, D. Reducing medication errors for hospital inpatients with Parkinsonism. Intern. Med. J. 2021, 51, 385–389. [Google Scholar] [CrossRef]
- Russell, S. Improving the management of Parkinson’s disease in surgical patients. Age Ageing 2018, 47 (Suppl. 2), ii25–ii39. [Google Scholar]
- Chhokar, G.; Cracknell, A.; Alty, J.; Baxandall, D.; Corrado, J.; Diggles, A.; Duggan-Carter, P.; Gray, C.; Haigh, G.; Robson, J.; et al. A quality improvement project to improve the care of people with Parkinson’s in a large teaching hospitals trust. Age Ageing 2018, 47 (Suppl. 2), ii25–ii39. [Google Scholar]
- Skelly, R.; Brown, L.; Fakis, A.; Kimber, L.; Downes, C.; Lindop, F.; Johnson, C.; Bartliff, C.; Bajaj, N. Does a specialist unit improve outcomes for hospitalized patients with Parkinson’s disease? Park. Relat. Disord. 2014, 20, 1242–1247. [Google Scholar] [CrossRef]
- Mata, H.J.; Davis, S. Translational health research: Perspectives from health education specialists. Clin. Transl. Med. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Roberts, S.; Davies, G. The introduction of a Parkinson’s disease email alert system to allow for early specialist team review of inpatients. BMC Health Serv. Res. 2019, 19, 271. [Google Scholar] [CrossRef]
- Nance, M.A.; Boettcher, L.; Edinger, G.; Gardner, J.; Kitzmann, R.; Erickson, L.O.; Wichmann, R.; Wielinski, C.L. Quality Improvement in Parkinson’s Disease: A Successful Program to Enhance Timely Administration of Levodopa in the Hospital. J. Park. Dis. 2020, 10, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.A.; Richardson, S.J.; Yarnall, A.J.; Burn, D.J.; Allan, L.M. Identifying delirium in Parkinson disease: A pilot study. Int. J. Geriatr. Psychiatry 2020, 35, 547–552. [Google Scholar] [CrossRef]
- Oguh, O.; Videnovic, A. Inpatient management of Parkinson disease: Current challenges and future directions. Neurohospi-Talist 2012, 2, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bramble, M.; Wong, A.; Carroll, V.; Schwebel, D.; Rossiter, R. Using an economic evaluation approach to support specialist nursing services for people with Parkinson’s in a regional community. J. Adv. Nurs. 2021, 77, 4722–4732. [Google Scholar] [CrossRef]
- Moore, S.; Jackson, S.; Yousuf, A. Medication management for patients with Parkinson’s: The impact of a nurse specialist and non-medical prescribing in the hospital setting. J. Prescr. Pract. 2022, 4, 200–204. [Google Scholar] [CrossRef]



| Key Issue | Root Cause |
|---|---|
| PD patients are not readily identifiable to hospital staff | Staff are unaware that the PD alert icon in FirstNet (eMR system used in ED) exists and what it achieves |
| Staff are unaware of how to set up the PD alert icon in FirstNet | |
| No alert system in PowerChart for identification of PD patients on the ward | |
| No bedside (non-electronic) means of identification | |
| Pharmacist reviews of PD patients are not conducted in a timely fashion | Insufficient funding to increase pharmacist-to-patient ratios |
| Pharmacists have competing priorities | |
| PD patients are not readily identifiable to pharmacists in the ED due to poor use of the electronic PD alert icon | |
| No alert system exists in the eMR for the identification of PD patients on wards | |
| Staff lack knowledge about PD medications | Lack of education on PD medications |
| PD medications are often not administered on time | Competing nursing priorities prevent timely administration from being prioritised |
| Lack of education regarding the importance of administering medications on time | |
| eMR system does not advocate for the timely administration of PD medications | |
| High-risk or low-use medications may not be available on the ward | |
| Delays in obtaining medication, which is not available on the ward—there is no alert system for pharmacists and nurses to action ordering medications available only in the dispensary | |
| PD medications are often incorrectly prescribed | Medication regimens are complex, and the history is poorly taken due to heavy workload and lack of education |
| The eMR function to chart custom medication administration times is not user-friendly | |
| There is a lack of education on how to chart custom medication administration times |
| Solution | Implementation Plan (Activities) | Additional Tasks | Outcome Measures |
|---|---|---|---|
| Solution 1. Formalise routine patient identification | Emergency Ward—PD patients entered can be identified within the ED by the Green PD icon on the FirstNet board in the ED. Neurology Ward—PD patients are included in the safety huddle, identifying issues and risks for patients on each shift. Neurology and Aged Care—Patients are highlighted on the nursing handover. | Training nurses in ED to check for and enter the code at triage and the bedside nursing assessment. Training pharmacists to scan FirstNet and the Patient Flow Portal to identify PD patients to Ongoing education sessions, quick reference instructions, and emails for nurses and pharmacists. Open feedback loop for staff. Periodic report of outcome measures to managers | Data collection: Report from EMR. Measure: proportion of PD patients who have the green icon activated in FirstNet. Measure: Proportion of PD patients who receive a pharmacist medication reconciliation within 48 h and during admission |
| Solution 2. Provide improved staff Education | Face-to-face/virtual educational sessions: PD medication overview Importance of routine identification of PD patients Email—Educational emails to reinforce the learnings from the educational sessions. Educational Packages Quick reference instructions—Provide staff easy access to reminders of how to enter the PD ‘problem’ into FirstNet and PowerChart. | Open a feedback loop for staff to provide feedback on the solution to the team. Monitoring via monthly audits to track the measurable benefits. PD education embedded into annual education programmes and orientation programs for JMO’s, nursing new graduates, departmental educational meetings, including Elderly Care, Neurology, Pharmacy, and Emergency. | Data collection: staff survey Measure: knowledge that PD medication on time means within 15 min of the prescribed time Data Collection: Report from EMR Measure: proportion of pharmacy medication reconciliations completed in 24 h. |
| Solution 3. Develop and install an Automated Prescriber alert | A pop-up prescriber alert in the medication administration record when any levodopa medication or entacapone is prescribed | Education sessions and quick reference instruction cards for JMO ID tags Periodic report of outcome measures | Data collection: Manual review of Pharmacy medication reconciliations. Measure: prescriber errors. |
| Solution 4. Review and amend ward PD medication stock | Review and amendment of ward stock medication available on 7F, 9E, and ED occurred in consultation with Nursing Unit Managers (NUMs) and Senior Pharmacists, improved access to PD medications on the wards. Poster listing what PD medications are available on the ward imprest and instructions for nurses to administer. Posters identifying PD medications included image of the medications and formulation details. | Ongoing education to nurses and pharmacists on what changes are made to the imprest supply and why timely administration benefits the patient. Posters and emails about the solution. Communication feedback loops. Annual review of imprest stock to confirm information on the posters remains current. | Data collection: Focus groups and feedback sought during education sessions embedded into business as usual to confirm resources and ward stock remain up to date and relevant Outcome: Resources and ward stock are reviewed annually. |
| Solution 5. Develop systematic prompts for nurses regarding the time-critical nature of PD medications | EMR Electronic component 1 ‘Order Comment’ for all levodopa and/or entacapone containing medications on the medication administration record. Time-critical must be administered within 15 min of prescribed time EMR Electronic component 2 Tile indicating administration is due, turns red after 15 min for all levodopa and/or entacapone—containing medications Pharmacy Electronic component 3 and Physical component Time-critical prompt automatically included on medication labels when dispensing levodopa-containing products. | Ongoing education provided to nurses and pharmacists on the need to prioritise the timely administration of PD medications. Posters and emails about solutions. Open feedback loop Periodic report of outcome measures to Nurse Unit Managers and Clinical Nurse Educators to provide feedback on performance. | Data Collection: Report from EMR. Measure: timeliness of PD medication administration. |
| Admissions | RNSH | ED | Neurology | Elderly Care |
|---|---|---|---|---|
| January–June 2022 (Pre-intervention) | 279 | 225 | 40 | 37 |
| July–December 2022 (Post-intervention) | 252 | 205 | 34 | 25 |
| January–June 2023 (Sustain phase) | 266 | 225 | 44 | 21 |
| July–December 2023 (Sustain phase) | 287 | 239 | 46 | 28 |
| January–June 2024 (Sustain phase) | 274 | 234 | 38 | 27 |
| RNSH Total Admissions | Male (%) | Average Age PD pt (Years) | Average Length of Stay (Days) | PD Primary Diagnosis, n (%) | G20, n (%) | U80.1, n (%) | |
|---|---|---|---|---|---|---|---|
| January–June 22 (Pre-intervention) | 279 | 61 | 79 | 11 | 26 (9) | 111 (40) | 168 (60) |
| July–December 22 (Post-intervention) | 252 | 65 | 78 | 12 | 11 (4) | 82 (33) | 170 (67) |
| January–June 23 (Sustain phase) | 266 | 58 | 78 | 17 | 31 (12) | 101 (38) | 165 (62) |
| July–December 23 (Sustain phase) | 287 | 62 | 78 | 13 | 23 (8) | 96 (33) | 191 (67) |
| January–June 24 (Sustain phase) | 274 | 62 | 78 | 12 | 24 (9) | 95 (35) | 179 (65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, S.; Iannuzzi, M.A.; Prior, S.J. Healthcare Redesign of Medication Management for Parkinson’s Inpatients. J. Ageing Longev. 2025, 5, 33. https://doi.org/10.3390/jal5030033
Williams S, Iannuzzi MA, Prior SJ. Healthcare Redesign of Medication Management for Parkinson’s Inpatients. Journal of Ageing and Longevity. 2025; 5(3):33. https://doi.org/10.3390/jal5030033
Chicago/Turabian StyleWilliams, Susan, Marissa Anne Iannuzzi, and Sarah J. Prior. 2025. "Healthcare Redesign of Medication Management for Parkinson’s Inpatients" Journal of Ageing and Longevity 5, no. 3: 33. https://doi.org/10.3390/jal5030033
APA StyleWilliams, S., Iannuzzi, M. A., & Prior, S. J. (2025). Healthcare Redesign of Medication Management for Parkinson’s Inpatients. Journal of Ageing and Longevity, 5(3), 33. https://doi.org/10.3390/jal5030033