A Feasibility Study of Two Cognitive Training Programs for Urban Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
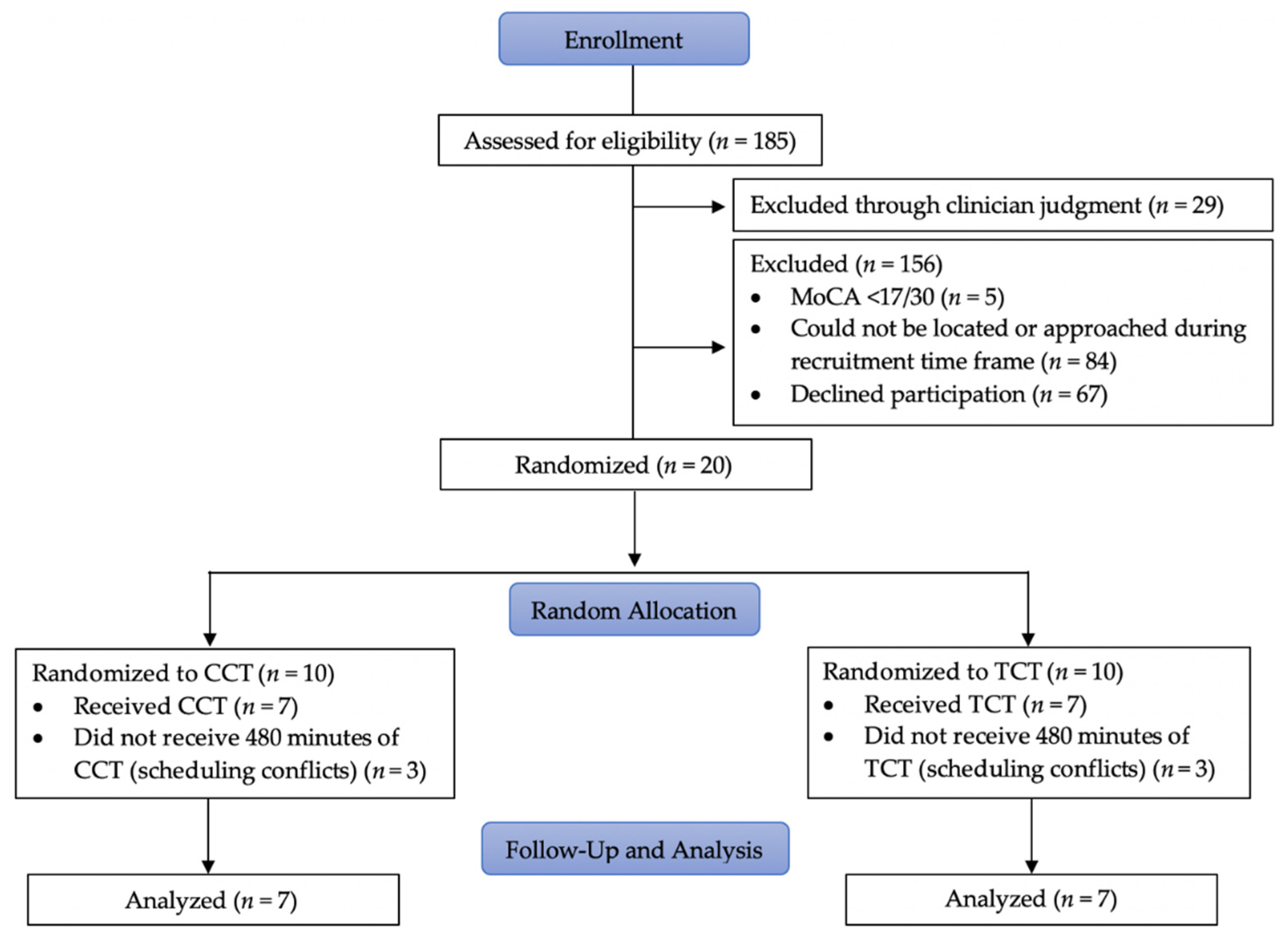
2.2. Participants
2.3. Instruments
2.4. Interventions
2.5. Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harada, C.N.; Natelson Love, M.C.N.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Judge, K.S.; Dawson, N.T. Cognitive Function. In Functional Performance in Older Adults, 4th ed.; Bonder, B., Dal Bello-Haas, V., Eds.; F.A. Davis Company: Philadelphia, PA, USA, 2018; pp. 93–108. [Google Scholar]
- Jeste, D.V.; Depp, C.A.; Vahia, I.V. Successful Cognitive and Emotional Aging. World Psychiatry 2010, 9, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pardasani, M.; Thompson, P. Senior Centers: Innovative and Emerging Models. J. Appl. Gerontol. 2010, 31, 52–77. [Google Scholar] [CrossRef]
- Lampit, A.; Hallock, H.; Suo, C.; Naismith, S.; Valenzuela, M. Cognitive Training-Induced Short-Term Functional and Long-Term Structural Plastic Change Is Related to Gains in Global Cognition in Healthy Older Adults: A Pilot Study. Front. Aging Neurosci. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Berch, D.B.; Helmers, K.F.; Jobe, J.B.; Leveck, M.D.; Marsiske, M.; Morris, J.N.; Rebok, G.W.; Smith, D.M.; Tennstedt, S.L.; et al. Effects of Cognitive Training Interventions with Older Adults. A Randomized Controlled Trial. JAMA 2002, 288, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Richards, M.; Marmot, M. Leisure Activities and Cognitive Function in Middle Age: Evidence from the Whitehall II Study. J. Epidemiol. Community Health 2003, 57, 907–913. [Google Scholar] [CrossRef]
- Tranter, L.J.; Koutstaal, W. Age and Flexible Thinking: An Experimental Demonstration of the Beneficial Effects of Increased Cognitively Stimulating Activity on Fluid Intelligence in Healthy Older Adults. Aging Neuropsychol. Cogn. 2008, 15, 184–207. [Google Scholar] [CrossRef]
- Reijnders, J.; van Heugten, C.; van Boxtel, M. Cognitive Interventions in Healthy Older Adults and People with Mild Cognitive Impairment: A Systematic Review. Ageing Res. Rev. 2013, 12, 263–275. [Google Scholar] [CrossRef]
- Willis, S.L.; Tennstedt, S.L.; Marsiske, M.; Ball, K.; Elias, J.; Koepke, K.M.; Morris, J.N.; Rebok, G.W.; Unverzagt, F.W.; Stoddard, A.M.; et al. Long-term Effects of Cognitive Training on Everyday Functional Outcomes in Older Adults. JAMA 2006, 296, 2805–2814. [Google Scholar] [CrossRef]
- Clark, F.; Blanchard, J.; Sleight, A.; Cogan, A.; Florindez, L.; Gleason, S.; Heymann, R.; Hill, V.; Holden, A.; Murphy, M.; et al. Lifestyle Redesign: The Intervention Tested in the USC Well Elderly Studies, 2nd ed.; AOTA Press: Bethesda, MD, USA, 2015. [Google Scholar]
- Gaitán, A.; Garolera, M.; Cerulla, N.; Chico, G.; Rodriguez-Querol, M.; Canela-Soler, J. Efficacy of an Adjunctive Computer-Based Cognitive Training Program in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: A Single-Blind, Randomized Clinical Trial. Int. J. Geriatr. Psychiatry 2013, 28, 91–99. [Google Scholar] [CrossRef]
- Hill, N.T.M.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized Cognitive Training in Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, K.-C. Effects of a Computerized Cognitive Training on Cognitive Function, Depression, Self-esteem, and Activities of Daily Living among Older Adults with Mild Cognitive Impairment. Korean J. Adult Nurs. 2016, 28, 691–700. [Google Scholar] [CrossRef]
- Li, K.; Alonso, J.; Chadha, N.; Pulido, J. Does Generalization Occur Following Computer-Based Cognitive Retraining?—An Exploratory Study. Occup. Ther. Health Care 2015, 29, 283–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, K.J.; Dye, R.V.; Kim, J.; Jennings, J.L.; O’Toole, E.; Wong, J.; Siddarth, P. Effect of a Computerized Brain Exercise Program on Cognitive Performance in Older Adults. Am. J. Geriatr. Psychiatry 2013, 21, 655–663. [Google Scholar] [CrossRef]
- Kueider, A.M.; Parisi, J.M.; Gross, A.L.; Rebok, G.W. Computerized Cognitive Training with Older Adults: A Systematic Review. PLoS ONE 2012, 7, e40588. [Google Scholar] [CrossRef]
- Vieira, E.R.; Richard, L.; Da Silva, R.A. Perspectives on Research and Health Practice in Physical and Occupational Therapy in Geriatrics during and Post COVID-19. Phys. Occup. Ther. Geriatr. 2020, 38, 199–202. [Google Scholar] [CrossRef]
- Perez-Stable, E.; Anderson, N.; Cuervo, A.M.; Hendrie, H.; LaCroix, A.; Morimoto, R.; Wetle, F. Health Disparities Research and Minority Aging Researcher Training Review: National Institute on Aging. 2012. Available online: https://www.nia.nih.gov/about/health-disparities-research-and-minority-aging-researcher-training-review-national-institute (accessed on 8 March 2022).
- Weuve, J.; Barnes, L.L.; De Leon, C.F.M.; Rajan, K.B.; Beck, T.; Aggarwal, N.T.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Evans, D.A. Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Shadish, W.R.; Cook, T.D.; Campbell, D.T. Experimental and Quasi-Experimental Designs for Generalized Causal Inference; Houghton Mifflin: Boston, MA, USA, 2002. [Google Scholar]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for Assessment of Mild Cognitive Impairment in Older Adults. BMC Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Tu, Q.-Y.; Jin, H.; Ding, B.-R.; Yang, X.; Lei, Z.-H.; Bai, S.; Zhang, Y.-D.; Tang, X.-Q. Reliability, Validity, and Optimal Cutoff Score of the Montreal Cognitive Assessment (Changsha Version) in Ischemic Cerebrovascular Disease Patients of Hunan Province, China. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 25–36. [Google Scholar] [CrossRef]
- Baum, C.; Wolf, T. Executive Function Performance Test (EFPT). 2013. Available online: http://www.ot.wustl.edu/about/resources/executive-function-performance-test-efpt-308 (accessed on 8 March 2022).
- Baum, C.M.; Connor, L.T.; Morrison, T.; Hahn, M.; Dromerick, A.W.; Edwards, D.F. Reliability, Validity, and Clinical Utility of the Executive Function Performance Test: A Measure of Executive Function in a Sample of People with Stroke. Am. J. Occup. Ther. 2008, 62, 446–455. [Google Scholar] [CrossRef]
- Hahn, B.; Baum, C.; Moore, J.; Ehrlich-Jones, L.; Spoeri, S.; Doherty, M.; Wolf, T.J. Development of Additional Tasks for the Executive Function Performance Test. Am. J. Occup. Ther. 2014, 68, e241–e246. [Google Scholar] [CrossRef]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. Trail Making Test. In A Compendium of Neuropsychological Tests; Oxford University Press: New York, NY, SUA, 2006; pp. 655–659. [Google Scholar]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail Making Test: Normative Values from 287 Normal Adult Controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Fernández, E.; Bringas, M.L.; Salazar, S.; Rodríguez, D.; García, M.E.; Torres, M. Clinical impact of RehaCom Software for cognitive rehabilitation of patients with acquired brain injury. MEDICC Rev. 2012, 14, 32–35. [Google Scholar] [CrossRef]
- García-Fernández, L.; Cabot-Ivorra, N.; Rodríguez-García, V.; Pérez-Martín, J.; Dompablo, M.; Pérez-Gálvez, B.; Rodriguez-Jimenez, R. Computerized Cognitive Remediation Therapy, REHACOM, in First Episode of Schizophrenia: A Randomized Controlled Trial. Psychiatry Res. 2019, 281, 112563. [Google Scholar] [CrossRef]
- Messinis, L.; Nasios, G.; Kosmidis, M.; Zampakis, P.; Malefaki, S.; Ntoskou, K.; Nousia, A.; Bakirtzis, C.; Grigoriadis, N.; Gourzis, P.; et al. Efficacy of a Computer-Assisted Cognitive Rehabilitation Intervention in Relapsing-Remitting Multiple Sclerosis Patients: A Multicenter Randomized Controlled Trial. Behav. Neurol. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Schreier, M. Qualitative Content Analysis in Practice; SAGE: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Eldadah, B.A. Fatigue and Fatigability in Older Adults. PM&R 2010, 2, 406–413. [Google Scholar] [CrossRef]
- Williams, K.N.; Kemper, S. Interventions to Reduce Cognitive Decline in Aging. J. Psychosoc. Nurs. Ment. Heal. Serv. 2010, 48, 42–51. [Google Scholar] [CrossRef]
- Li, C.T.; Hung, G.K.; Fong, K.N.; Gonzalez, P.C.; Wah, S.-H.; Tsang, H.W. Effects of a Home-Based Occupational Therapy Telerehabilitation via Smartphone for Outpatients after Hip Fracture Surgery: A Feasibility Randomised Controlled Study. J. Telemed. Telecare 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Velayati, F.; Ayatollahi, H.; Hemmat, M. A Systematic Review of the Effectiveness of Telerehabilitation Interventions for Therapeutic Purposes in the Elderly. Methods Inf. Med. 2020, 59, 104–109. [Google Scholar] [CrossRef]
- National Council on Aging. COVID-19 Resources for Senior Centers. Available online: https://www.ncoa.org/news/ncoa-news/national-institute-of-senior-centers-news/covid-19-resources-for-senior-centers/ (accessed on 10 March 2020).

| Demographic Characteristic | CCT (n = 7) M (SD) or n (%) | TCT (n = 7) M (SD) or n (%) | Fisher’s Exact Test or Mann–Whitney U p |
|---|---|---|---|
| Sex (Female) (Male) | 4 (57.14%) 3 (42.86%) | 6 (85.71%) 1 (14.29%) | 0.56 |
| Age, Years | 68.00 (5.39) | 75.71 (10.00) | 0.21 |
| Race (Unreported) (Black or African American) | 1 (14.29%) 6 (85.71%) | 0 (0%) 7 (100%) | 1.00 |
| MoCA, baseline (x/30) (Suspected MCI 17–25) | 22.29 (2.36) 7 (100%) | 21.57 (3.26) 6 (85.71%) | 0.51 |
| Education (High school) (≤12 years of education) | 5 (71.43%) 2 (28.57%) | 5 (71.43%) 2 (28.57%) | 1.00 |
| Home assist (Lives alone) (Lives with family) | 4 (57.14%) 3 (42.86%) | 3 (42.86%) 4 (57.14%) | 1.00 |
| Categorized Responses | CCT (n = 7) n (%) | TCT (n = 6) * n (%) | McNemar’s Test p |
|---|---|---|---|
| 1. Helpful | 7 (100%) | 6 (100%) | 1.00 |
| 2. Enjoyment | 7 (100%) | 6 (100%) | 1.00 |
| 3. Opinion | 6 (85.7%) | 6 (100%) | 0.46 |
| 4. Independence to do on own | 3 (42.9%) | 3 (50%) | 1.00 |
| 5. Assistance support would be required to continue | 5 (71.4%) | 3 (50%) | 1.00 |
| 6. Continuation of training provided | 6 (85.7%) | 4 (66.7%) | 0.56 |
| Cognitive Performance Outcome Measurement | CCT (n = 7) M (SD) | TCT (n = 7) M (SD) |
|---|---|---|
| EFPT Pre | 11.43 (4.83) | 25.29 (11.00) |
| Post | 11.14 (5.49) | 21.71 (9.69) |
| Pre-Post Change | −0.29 (4.57) | −3.57 (4.93) |
| p | 0.86 | 0.11 |
| TMT-A Pre | 66.57 (54.71) | 63.29 (18.39) |
| Post | 54.71 (27.26) | 66.29 (22.35) |
| Pre-Post Change | −11.86 (36.02) | 3 (13.87) |
| p | 0.61 | 0.61 |
| TMT-B Pre | 169.14 (187.00) | 176.71 (60.46) |
| Post | 118.14 (77.09) | 186.71 (57.67) |
| Pre-Post Change | −51 (133.62) | 10 (52.91) |
| p | 0.17 | 0.61 |
| Cognitive Performance Outcome Measurement | CCT M (SD) | TCT M (SD) | p | |||
|---|---|---|---|---|---|---|
| EFPT | −0.29 (4.57) | −3.57 (4.93) | 0.21 | |||
| TMT-A (in seconds) | −11.86 (36.02) | 3 (13.87) | 0.46 | |||
| TMT-B (in seconds) | −51 (133.62) | 10 (52.91) | 0.26 | |||
| Cognitive Performance Outcome Measurement | CCTM (SD) | TCT M (SD) | p | t | df | 95% CI |
| Duration of time of sessions (in minutes) | 43.64 (15.60) (total of 76 sessions among 7 subjects) | 44.27 (16.10) (total of 74 sessions among 7 subjects) | 0.81 | 0.24 | 12 | −4.45–5.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benham, S.; Otchet, K.; Senft, D.; Potter, A.M. A Feasibility Study of Two Cognitive Training Programs for Urban Community-Dwelling Older Adults. J. Ageing Longev. 2022, 2, 74-84. https://doi.org/10.3390/jal2020007
Benham S, Otchet K, Senft D, Potter AM. A Feasibility Study of Two Cognitive Training Programs for Urban Community-Dwelling Older Adults. Journal of Ageing and Longevity. 2022; 2(2):74-84. https://doi.org/10.3390/jal2020007
Chicago/Turabian StyleBenham, Sara, Kelly Otchet, Diana Senft, and Ann Marie Potter. 2022. "A Feasibility Study of Two Cognitive Training Programs for Urban Community-Dwelling Older Adults" Journal of Ageing and Longevity 2, no. 2: 74-84. https://doi.org/10.3390/jal2020007
APA StyleBenham, S., Otchet, K., Senft, D., & Potter, A. M. (2022). A Feasibility Study of Two Cognitive Training Programs for Urban Community-Dwelling Older Adults. Journal of Ageing and Longevity, 2(2), 74-84. https://doi.org/10.3390/jal2020007