Epigallocatechin Gallate as a Targeted Therapeutic Strategy Against the JAK2V617F Mutation: New Perspectives for the Treatment of Myeloproliferative Neoplasms and Acute Myeloid Leukemia
Abstract
1. Introduction
Epigallocatechin Gallate
2. Literature Review and Bioinformatics Analysis Approach
2.1. Selection of Clinical Case Reports
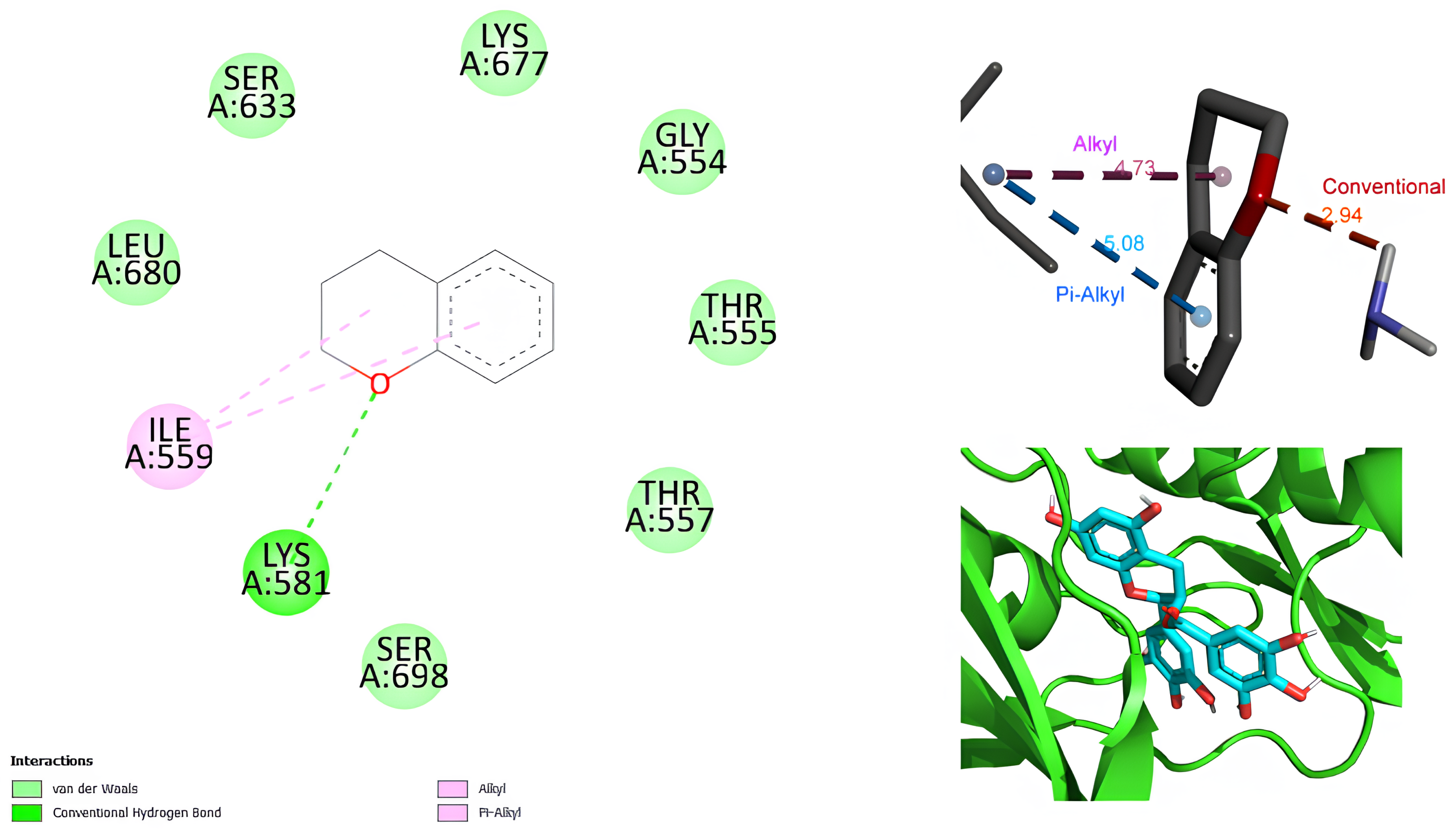
2.2. Pocket Analysis and Molecular Docking
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khoury, J.D.; Solary, E.; Abla, O.; Ebert, B.L.; Konopleva, M.; Ogawa, S.; Pullarkat, V.; Schnittger, S.; Vardiman, J.; Vyas, P. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, R.; Hoffman, R.; Mahmud, M.; Vasireddy, S.; Gowin, K.; Amaraneni, A. Myeloproliferative Neoplasms: Contemporary Review and Molecular Landscape. Int. J. Mol. Sci. 2023, 24, 17383. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef]
- Aaronson, D.S.; Horvath, C.M. A road map for those who don’t know JAK–STAT. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef]
- Xia, D.; Hasserjian, R.P. Molecular testing for JAK2, MPL, and CALR in myeloproliferative neoplasms. Am. J. Hematol. 2016, 91, 1277–1280. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Erdogan, F.; Radu, T.B.; Orlova, A.; Lobo, J.; Constantin, A.; Andrei, S.; Ichim, D.; Ciolac, O.A.; Slavescu, A.C.; Cojocneanu, R.; et al. JAK-STAT core cancer pathway: An integrative cancer interactome analysis. J. Cell. Mol. Med. 2022, 26, 2049–2062. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546, Erratum in Drugs 2017, 77, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ott, N.; Faletti, L.; Heeg, M.; Andreani, V.; Grimbacher, B. JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences. J. Clin. Immunol. 2023, 43, 1326–1359. [Google Scholar] [CrossRef]
- Dusa, A.; Mouton, C.; Pecquet, C.; Herman, M.; Constantinescu, S.N. JAK2 V617F Constitutive Activation Requires JH2 Residue F595: A Pseudokinase Domain Target for Specific Inhibitors. PLoS ONE 2010, 5, e11157. [Google Scholar] [CrossRef]
- Gnanasambandan, K.; Magis, A.; Sayeski, P.P. The constitutive activation of Jak2-V617F is mediated by a π stacking mechanism involving Phenylalanines 595 and 617. Biochemistry 2010, 49, 9972–9984. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Gotlib, J. JAK2 V617F and beyond: Role of genetics and aberrant signaling in the pathogenesis of myeloproliferative neoplasms. Expert Rev. Hematol. 2010, 3, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.P.; Silva, S.N.; Reichert, A.; Lima, F.; Júnior, E.; Rueff, J. Prevalence of the janus kinase 2 V617F mutation in philadelphia-negative myeloproliferative neoplasms in a Portuguese population. Biomed. Rep. 2017, 7, 370–376. [Google Scholar] [CrossRef][Green Version]
- Da Silva, R.R.D.; Domingues Hatzlhofer, B.L.; De Faria Machado, C.G.; De Melo Lima, A.S.; De Albuquerque, D.M.; Dos Santos, M.N.N.; Fertrin, K.Y.; Costa, F.F.; Da Silva Araújo, A.; Bezerra, M.A.C. JAK2 V617F mutation prevalence in myeloproliferative neoplasms in Pernambuco, Brazil. Genet. Test. Mol. Biomark. 2012, 16, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Irfan, S.M.; Khan, S.R. Somatic JAK-2 V617F mutational analysis in Polycythemia rubra vera: A tertiary care center experience. Asian Pac. J. Cancer Prev. 2016, 17, 1053–1055. [Google Scholar] [CrossRef]
- Singdong, R.; Siriboonpiputtana, T.; Chareonsirisuthigul, T.; Kongruang, A.; Limsuwanachot, N.; Sirirat, T.; Chuncharunee, S.; Rerkamnuaychoke, B. Characterization and Prognosis Significance of JAK2 (V617F), MPL, and CALR Mutations in Philadelphia-Negative Myeloproliferative Neoplasms. Asian Pac. J. Cancer Prev. 2016, 17, 4647–4653. [Google Scholar] [CrossRef]
- Zulkeflee, R.H.; Zulkafli, Z.; Johan, M.F.; Husin, A.; Islam, M.A.; Hassan, R. Clinical and laboratory features of JAK2 V617F, CALR, and MPL mutations in Malaysian patients with classical myeloproliferative neoplasm (MPN). Int. J. Environ. Res. Public Health 2021, 18, 7582. [Google Scholar] [CrossRef]
- Hidalgo-López, J.E.; Kanagal-Shamanna, R.; Medeiros, L.J.; Luthra, R.; Khoury, J.D.; Wang, S.A. Morphologic and molecular characteristics of de novo acute myeloid leukemia with JAK2 V617F mutation. J. Natl. Compr. Canc. Netw. 2017, 15, 790–796. [Google Scholar] [CrossRef]
- Farasani, A. Screening of V617F Mutation in JAK2 Gene with Acute Myeloid Leukemia in the Saudi Population. Acta Biochim. Pol. 2022, 69, 211–214. [Google Scholar] [CrossRef]
- Constantinescu, S.N.; Vainchenker, W.; Levy, G.; Papadopoulos, N. Functional Consequences of Mutations in Myeloproliferative Neoplasms. Hemasphere 2021, 5, e578. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving Cognition of the JAK–STAT Signaling Pathway: Autoimmune Disorders and Cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Agashe, R.P.; Lippman, S.M.; Kurzrock, R. JAK: Not Just Another Kinase. Mol. Cancer Ther. 2022, 21, 1757–1764. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT Pathway: Extracellular Signals, Diseases, Immunity, and Therapeutic Regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Javadian, M.; Babaloo, Z.; Baradaran, B. Janus Kinase Inhibitors: A Therapeutic Strategy for Cancer and Autoimmune Diseases. J. Cell. Physiol. 2020, 235, 5903–5924. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Janus Kinase (JAK) Inhibitors in the Treatment of Inflammatory and Neoplastic Diseases. Pharmacol. Res. 2016, 111, 784–803. [Google Scholar] [CrossRef]
- Kesarwani, M.; Huber, E.; Kincaid, Z.; Evelyn, C.R.; Biesiada, J.; Rance, M.; Thapa, M.B.; Shah, N.P.; Meller, J.; Zheng, Y.; et al. Targeting Substrate-Site in Jak2 Kinase Prevents Emergence of Genetic Resistance. Sci. Rep. 2015, 5, 14538. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent Advances on Tea Polyphenols. Front. Biosci. (Elite Ed.) 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; McClements, D.J.; Liu, X.; Liu, F. EGCG-based nanoparticles: Synthesis, properties, and applications. Crit. Rev. Food Sci. Nutr. 2024, 65, 2177–2198. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The Chemistry and Biotransformation of Tea Constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Chopra, S.; Kaur, D.; Wang, H.; Li, M.; Chen, P.; Zhang, W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets 2020, 21, 1099–1104. [Google Scholar] [CrossRef]
- Romano, A.; Martel, F. The Role of EGCG in Breast Cancer Prevention and Therapy. Mini-Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef]
- Han, L.; Koduru, P.; Cantu, M.; Fuda, F.; Chen, W. RUNX1::CBFA2T2 Rearranged Acute Myeloid Leukemia Transformed from JAK2 V617F Mutated Primary Myelofibrosis. EJHaem 2024, 5, 1330–1332. [Google Scholar] [CrossRef]
- Ohanian, M.; Bueso-Ramos, C.; Ok, C.Y.; Lin, P.; Patel, K.; Alattar, M.L.; Khoury, J.D.; Rozovski, U.; Estrov, Z.; Huh, Y.O.; et al. Acute Myeloid Leukemia with MYC Rearrangement and JAK2 V617F Mutation. Cancer Genet. 2015, 208, 571–574. [Google Scholar] [CrossRef][Green Version]
- Borsellino, B.; Savi, A.; Pascale, M.R.; Meddi, E.; Cristiano, A.; Ottone, T.; Rapanotti, M.C.; Divona, M.; Travaglini, S.; Attardi, E.; et al. Can Polycythemia Vera Evolve from Acute Myeloid Leukemia? A Case Report Showing a Simultaneous Minor JAK2 V617F Mutated Clone: De Novo Polycythemia Vera Following AML Remission. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022058. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Wei, Z.L.; Wang, N.N.; Huang, C.; Huang, J.; Yan, J.W.; Wang, R.; Yu, Z.Z.; Huang, D.P. Clinical Characteristics of a Patient with de Novo Acute Promyelocytic Leukemia with JAK2 V617F Mutation. Hematology 2022, 27, 1290–1293. [Google Scholar] [CrossRef]
- Asou, C.; Sakamoto, T.; Suzuki, K.; Okuda, I.; Osaki, A.; Abe, R.; Ito, Y.; Kakegawa, E.; Miyakawa, Y.; Terui, Y.; et al. Transformation into Acute Myeloid Leukemia with t(8;21)(q22;q22.1); RUNX1::RUNX1T1 from JAK2-Mutated Essential Thrombocythemia: A Case Report. J. Med. Case Rep. 2024, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, D.; Zhuang, C.; Wei, P.; Mou, W.; Zhang, L.; Liang, H.; Liu, Y. Essential thrombocythemia during treatment of acute myeloid leukemia with JAK2 V617F mutation: A case report of a CARE-compliant article. Medicine 2018, 97, e11331. [Google Scholar] [CrossRef]
- Langabeer, S.E.; Haslam, K.; Smyth, M.A.; Quinn, J.; Murphy, P.T. Protracted Clonal Trajectory of a JAK2 V617F-Positive Myeloproliferative Neoplasm Developing during Long-Term Remission from Acute Myeloid Leukemia. Case Rep. Hematol. 2018, 2018, 8713020. [Google Scholar] [CrossRef]
- Wang, S.; Yan, J.; Zhou, G.; Heintzelman, R.; Hou, J.S. Myeloproliferative Neoplasm or Reactive Process? A Rare Case of Acute Myeloid Leukemia and Transient Posttreatment Megakaryocytic Hyperplasia with JAK-2 Mutation. Case Rep. Hematol. 2016, 2016, 6054017. [Google Scholar] [CrossRef]
- Liu, K.G.; Verma, A.; Derman, O.; Kornblum, N.; Janakiram, M.; Braunschweig, I.; Battini, R. JAK2 V617F mutation, multiple hematologic and non-hematologic processes: An association? Biomark. Res. 2016, 4, 19. [Google Scholar] [CrossRef]
- Brattås, M.K.; Lilleeng, K.; Hovland, R.; Lægreid, I.J.; Vorland, M.; Leh, F.; Bruserud, Ø.; Gjertsen, B.T.; Reikvam, H. Philadelphia chromosome positive AML arising from JAK2-positive myelofibrosis. Biomark. Res. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.; Li, Q.; Li, K.; Dekker, C.; Vangrevelinghe, E.; Jones, M.; Chène, P.; MacHauer, R.; Radimerski, T.; Eck, M.J. Discovery and Structural Characterization of ATP-Site Ligands for the Wild-Type and V617F Mutant JAK2 Pseudokinase Domain. ACS Chem. Biol. 2019, 14, 587–593. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 3.0; Schrödinger, LLC: New York, NY, USA, 2025; Available online: https://pymol.org/#download (accessed on 1 June 2025).
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D. PrankWeb: A web server for ligand binding site prediction and visualization. Nucleic Acids Res. 2019, 47, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Epigallocatechin Gallate|C22H18O11|CID 65064—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/65064 (accessed on 1 June 2025).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dassault Systèmes. BIOVIA Discovery Studio, Versão 2025; Dassault Systèmes: Vélizy-Villacoublay, France, 2025; Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 1 June 2025).
- Tefferi, A.; Vardiman, J.W. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Yun, J. Reclassification of Acute Myeloid Leukemia According to the 2022 World Health Organization Classification and the International Consensus Classification Using Open-Source Data. Ann. Lab. Med. 2025, 45, 170–177. [Google Scholar] [CrossRef]
- Benton, C.B.; Boddu, P.C.; DiNardo, C.D.; Bose, P.; Wang, F.; Assi, R.; Pemmaraju, N.; Kc, D.; Pierce, S.; Patel, K.; et al. Janus kinase 2 variants associated with the transformation of myeloproliferative neoplasms into acute myeloid leukemia. Cancer 2019, 125, 1855–1866. [Google Scholar] [CrossRef]
- Schulze, S.; Stengel, R.; Jaekel, N.; Wang, S.Y.; Franke, G.N.; Roskos, M.; Schneider, M.; Niederwieser, D.; Al-Ali, H.K. Concomitant and noncanonical JAK2 and MPL mutations in JAK2V617F- and MPLW515L-positive myelofibrosis. Genes Chromosomes Cancer 2019, 58, 747–755. [Google Scholar] [CrossRef]
- Rodriguez Rojas, L.X.; Olave Rodriguez, J.A.; Navarrete, S.B.; Carvajal, L.V.; Albán Silva, J.J.; Martínez, L.D.; Nastasi Catanese, J.A. Germinal pathogenic CHEK2, novel APC and somatic JAK2V617F variants in a young patient with colorectal cancer, atypical leukemia, cerebral tumour and aggressive course. ecancermedicalscience 2025, 19, 1833. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Huang, D.; Chen, S. A rare case of concurrent JAK2V617F-positive essential thrombocythemia, multiple myeloma, and colorectal adenocarcinoma. Indian J. Pathol. Microbiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Highsmith, K.; Rexwinkle, A. Pharmacologic management of myelofibrosis. J. Oncol. Pharm. Pract. 2017, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 801–821. [Google Scholar] [CrossRef]
- Tefferi, A.; Vainchenker, W. Myeloproliferative neoplasms: Molecular pathophysiology, essential clinical understanding, and treatment strategies. J. Clin. Oncol. 2011, 29, 573–582. [Google Scholar] [CrossRef]
- Kalota, A.; Jeschke, G.R.; Carroll, M.; Hexner, E.O. Intrinsic resistance to JAK2 inhibition in myelofibrosis. Clin. Cancer Res. 2013, 19, 1729. [Google Scholar] [CrossRef]
- Lundberg, P.; Takizawa, H.; Kubovcakova, L.; Guo, G.; Hao-Shen, H.; Dirnhofer, S.; Orkin, S.H.; Manz, M.G.; Skoda, R.C. Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F. J. Exp. Med. 2014, 211, 2213. [Google Scholar] [CrossRef]
- Mascarenhas, M.I.; Bacon, W.A.; Kapeni, C.; Fitch, S.R.; Kimber, G.; Cheng, S.W.P.; Li, J.; Green, A.R.; Ottersbach, K. Analysis of Jak2 signaling reveals resistance of mouse embryonic hematopoietic stem cells to myeloproliferative disease mutation. Blood 2016, 127, 2298–2309. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (−)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK2/STAT3/AKT signalling pathways. Clin. Exp. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef]
- Fisher, D.A.C.; Laranjeira, A.B.A.; Kong, T.; Snyder, S.C.; Shim, K.; Fulbright, M.C.; Oh, S.T. Complementary and countervailing actions of Jak2 and Ikk2 in hematopoiesis in mice. Exp. Hematol. 2023, 128, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, J.T.S.; Feghali, R.; Ribeiro, R.P.; Ramos, M.J.; Fernandes, P.A. The importance of intramolecular hydrogen bonds on the translocation of the small drug piracetam through a lipid bilayer. RSC Adv. 2021, 11, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.G.; Haikarainen, T.; Vuorio, J.; Girych, M.; Virtanen, A.T.; Kurttila, A.; Karathanasis, C.; Heilemann, M.; Sharma, V.; Vattulainen, I.; et al. Molecular basis of JAK2 activation in erythropoietin receptor and pathogenic JAK2 signaling. Sci. Adv. 2024, 10, eadl2097. [Google Scholar] [CrossRef]
- Hu, M.; Yang, T.; Yang, L.; Niu, L.; Zhu, J.; Zhao, A.; Shi, M.; Yuan, X.; Tang, M.; Yang, J.; et al. Preclinical studies of Flonoltinib Maleate, a novel JAK2/FLT3 inhibitor, in treatment of JAK2 V617F-induced myeloproliferative neoplasms. Blood Cancer J. 2022, 12, 37. [Google Scholar] [CrossRef]
- Hammarén, H.M.; Ungureanu, D.; Grisouard, J.; Skoda, R.C.; Hubbard, S.R.; Silvennoinen, O. ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation. Proc. Natl. Acad. Sci. USA 2015, 112, 4642–4647. [Google Scholar] [CrossRef]

| Patient | Early Diagnosis | Disease Evolution | Initial Treatment | Recurrence and New Treatment | Clinical Outcome | Reference | |
|---|---|---|---|---|---|---|---|
| Woman, 62 years old | Diagnosed with breast cancer and treated with lumpectomy, anastrozole, radiation, and adjuvant chemotherapy. | Diagnosed with PMF with JAK2V617F, IDH2, and SRSF2 mutations. | Progression to AML with RUNX1::CBFA2T2 rearrangement. AML with adverse risk classification. | Use of azacitidine + ruxolitinib Remission for 4 months | Use of Cytarabine + Venetoclax + Enasidenib with modified chemotherapy regimens after relapse | Death 13 months after AML diagnosis. | [36] |
| Woman, 68 years old | Diagnosed with MDS with fibrosis MF-2 and JAK2V617F mutation. | Progression to AML with 16% initial blasts progressing to high grade. AML with intermediate risk classification. | Use of hypomethylating agent: 5-azacytidine | Death 2 months after MDS diagnosis. | [37] | ||
| Man, 60 years old | Diagnosed PMF with MF-3 with JAK2V617F mutation. | Diagnosed with BPDCN. | Progression to AML; with intermediate risk classification. | Use of hydroxyurea, splenic irradiation, and ruxolitinib | Death 1 month after diagnosis of persistent AML. | [37] | |
| Woman, 75 years old | Diagnosed with AML, treated with chemotherapy (7 + 3); 2 high-dose cytarabine consolidation cycles. AML with intermediate risk classification. | Progression to PV with JAK2V617F mutation (43.4%) and TET2 R550 mutation (46%). | After progression to PV: therapeutic phlebotomy + hydroxyurea. | Patient under follow-up after progression to PV, 19 months post-consolidation. | [38] | ||
| Woman, 44 years old | Diagnosed with APL with PML/RARα bcr3 mutation. APL with adverse risk classification. | Progression to PV and ET with JAK2V617F and TP53 P278R mutations. | Use of ATRA + ATO, with daunorubicin and cytarabine for cytoreduction. Use of dexamethasone for differentiation syndrome; induction followed by 3 consolidation cycles. | Treatment with interferon for MPN (PV/ET) 22 months after complete remission of APL. | Patient with stable clinical course under interferon treatment. | [39] | |
| Woman, 74 years old | Diagnosed with ET with JAK2V617F mutation. Using hydroxyurea for ET control. | Progression to secondary AML with t(8;21)(q22;q22.1); RUNX1::RUNX1T1; JAK2V617F mutation. AML with favorable risk classification. | Treatment with chemotherapy using venetoclax + azacitidine. | Hematologic and molecular remission of AML after chemotherapy, but JAK2V617F mutation still present in peripheral leukocytes. | [40] | ||
| Girl, 1 year old | Diagnosed with AML with 49% blasts, JAK2V617F mutation, and complex karyotype. AML with adverse risk classification. | Progression to possible underlying ET. | Induction 1: Daunorubicin + cytarabine + etoposide (MRD: 0.37%) Induction 2: Idarubicin + cytarabine + etoposide (MRD: 0.52%) | Induction 3: Mitoxantrone + HiDAC leading to hematologic failure and relapse. | Death 1 month after treatment discontinuation due to AML relapse. | [41] | |
| Woman, 64 years old | Diagnosed with AML with 49% blasts, JAK2V617F mutation, and normal karyotype. AML with intermediate risk classification. | Progression to unclassified MPN with JAKV617F mutation, diagnosed 7 years after AML remission, with initial fibrosis detected. | Treatment with daunorubicin + cytarabine (3 + 10), 3 consolidation cycles, and no active therapy after remission. | Patient in continuous remission, asymptomatic, and without active treatment. | [42] | ||
| Man, 34 years old | Diagnosed with gout; chronic pain following a traffic accident; and bipolar disorder. | Diagnosed with AML NPM1 and JAK2V617F mutations. AML with intermediate risk classification. | Progression to transient underlying MPN. | Treatment with FLAG-IDA (fludarabine + cytarabine + idarubicin + G-CSF). | Allogeneic peripheral blood stem cell transplant after megakaryocytic hyperplasia and JAK2 positivity. | Patient in complete remission with no relapse of AML or MPN until the last evaluation. | [43] |
| Man, 68 years old | Diagnosed with multiple cardiovascular comorbidities (hypertension, coronary artery disease, deep vein thrombosis, pulmonary embolism) and pulmonary conditions (COPD, stage IA lung adenocarcinoma). | Diagnosed with ET with JAK2V617F mutation. | Progression to Chronic Myelomonocytic Leukemia with adverse risk classification Development of DLBC. Progression to AML-M5 with adverse risk classification. | Use of hydroxyurea for MPN Use of R-CHOP for DLBCL Use of chemotherapy (7 + 3) with cytarabine and idarubicin for AML-M5 | Use of decitabine, platelet apheresis for thrombocytosis, and allogeneic transplantation. | Death following transplant failure, AML relapse, and palliative care. | [44] |
| Man, 80 years old | Diagnosis of type 2 diabetes; atrial fibrillation; cerebrovascular disease; polymyalgia rheumatica; and osteoporosis. | Diagnosis of primary myelofibrosis with JAK2V617F mutation. | Progression to AML with BCR-ABL1 e6a2 mutation (Ph+) with adverse classification. Development of pancreatic adenocarcinoma (advanced stage). | Use of hydroxyurea + anagrelide + dasatinib + valproic acid. | Doses and schedules adjusted due to toxicity; anagrelide and dasatinib discontinued due to side effects; transition to imatinib with partial response; partial control of AML with TKI. | Death weeks after progression to pancreatic cancer | [45] |
| Score | Probability | SAS Points | Suface Atoms | Center (x, y, z) | |
|---|---|---|---|---|---|
| Pocket1 | 8.43 | 0.496 | 81 | 44 | (−20.25, 8.93, 145.79) |
| Pocket2 | 7.87 | 0.461 | 68 | 38 | (−15.91, 8.93, 157.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, L.S.D.; Farias, I.M.; Nogueira, B.M.D.; Machado, C.B.; Pessoa, F.M.C.D.P.; Oliveira, D.D.S.; de Morais, G.P.; Thé, A.P.; Thé, P.M.P.; Moraes Filho, M.O.D.; et al. Epigallocatechin Gallate as a Targeted Therapeutic Strategy Against the JAK2V617F Mutation: New Perspectives for the Treatment of Myeloproliferative Neoplasms and Acute Myeloid Leukemia. Int. J. Transl. Med. 2025, 5, 43. https://doi.org/10.3390/ijtm5030043
Cunha LSD, Farias IM, Nogueira BMD, Machado CB, Pessoa FMCDP, Oliveira DDS, de Morais GP, Thé AP, Thé PMP, Moraes Filho MOD, et al. Epigallocatechin Gallate as a Targeted Therapeutic Strategy Against the JAK2V617F Mutation: New Perspectives for the Treatment of Myeloproliferative Neoplasms and Acute Myeloid Leukemia. International Journal of Translational Medicine. 2025; 5(3):43. https://doi.org/10.3390/ijtm5030043
Chicago/Turabian StyleCunha, Leidivan Sousa Da, Isabelle Magalhães Farias, Beatriz Maria Dias Nogueira, Caio Bezerra Machado, Flávia Melo Cunha De Pinho Pessoa, Deivide De Sousa Oliveira, Guilherme Passos de Morais, André Pontes Thé, Patrícia Maria Pontes Thé, Manoel Odorico De Moraes Filho, and et al. 2025. "Epigallocatechin Gallate as a Targeted Therapeutic Strategy Against the JAK2V617F Mutation: New Perspectives for the Treatment of Myeloproliferative Neoplasms and Acute Myeloid Leukemia" International Journal of Translational Medicine 5, no. 3: 43. https://doi.org/10.3390/ijtm5030043
APA StyleCunha, L. S. D., Farias, I. M., Nogueira, B. M. D., Machado, C. B., Pessoa, F. M. C. D. P., Oliveira, D. D. S., de Morais, G. P., Thé, A. P., Thé, P. M. P., Moraes Filho, M. O. D., Moraes, M. E. A. D., & Moreira-Nunes, C. A. (2025). Epigallocatechin Gallate as a Targeted Therapeutic Strategy Against the JAK2V617F Mutation: New Perspectives for the Treatment of Myeloproliferative Neoplasms and Acute Myeloid Leukemia. International Journal of Translational Medicine, 5(3), 43. https://doi.org/10.3390/ijtm5030043






