Abstract
Neuropathic pain has emerged as a significant concern for patients dealing with persistent post-COVID-19 symptoms. Small fiber neuropathy (SFN) has been identified as a potential underlying mechanism contributing to long-term pain in these patients. Despite an increasing body of evidence associating post-COVID-19 SFN with immune dysregulation and neuroinflammation, the exact pathophysiology and optimal treatment remains unclear. This review aims to explore the pathophysiology, diagnosis, proposed mechanisms, and treatment of post-COVID-19 SFN. A comprehensive literature review was conducted, examining studies on SFN, as well as SFN in the context of COVID-19, including clinical manifestations, diagnostic criteria, and potential treatment modalities. Evidence was gathered from case studies, observational reports, and clinical trials addressing post-COVID-19 neuropathy and SFN. SFN in long COVID presents a heterogeneous range of sensory and autonomic symptoms. Diagnosis relies on clinical evaluation, quantitative sensory testing, and confirmatory skin biopsy. Proposed mechanisms include autoimmune dysregulation, molecular mimicry, direct viral invasion of neural structures, and inflammatory responses. Pharmacological treatments—such as gabapentin, antidepressants, and corticosteroids—have demonstrated symptom relief, while immunomodulatory therapies show promise in immune-mediated cases. Non-pharmacological strategies warrant further investigation. Post-COVID-19 SFN represents a complex and multifactorial condition requiring a multidisciplinary approach to diagnosis and management. While merging evidence supports immune-mediated pathogenesis, further research is needed to establish definitive mechanisms and optimize targeted therapeutic strategies. Continued investigation into post-COVID-19 SFN will be crucial in addressing the long-term neurological sequelae of SARS-CoV-2 infection.
1. Introduction
“Long COVID” is a term commonly used to describe the myriad of symptoms patients continue to experience following an acute COVID-19 infection. Approximately 65 million people worldwide have been identified as having long COVID symptoms [1]. Long COVID has also been defined as persistent or new symptoms that occur at least four weeks after the initial infection. Neuropathic pain is one of the 200 symptoms associated with long COVID [1,2]. It has been suggested that more than one in three patients with long COVID will develop neuropathic pain [2], which can last for weeks to months or even years. Recent studies regarding COVID-19 and long-term pain have identified correlation between acute SARS-COV-2 infection and resultant small fiber neuropathy (SFN) [1,3,4,5]. SFN is a peripheral neuropathy that commonly presents with chronic and diffuse pain, often described as constant burning pain [6]. Previous case studies have shown patients who have recovered from COVID-19 presenting with sensory and/or autonomic dysfunction indicative of SFN [4,5,7,8].
It has been established that infection is one of the main etiologies of SFN [6,9]. HIV [10] and Hepatitis C [11] are two common infectious agents that elicit immune-mediated, inflammatory SFN. It has been hypothesized that post-COVID-19 SFN may be the result of a similar mechanism of infectious autoimmune dysregulation [1,3]. However, due to the many etiologies associated with SFN, comprehensive patient history, physical examination, and diagnostic testing are necessary to establish a causal diagnosis of COVID-19-based SFN [7,8]. In this article, we will review SFN and, specifically, the proposed mechanisms and treatments modalities for COVID-19-based SFN.
2. Definition and Diagnosis of SFN
Small nerve fibers include small myelinated Aδ-fibers and unmyelinated C-fibers that receive sensory and autonomic input [12]. Small fiber neuropathy occurs when either of these small nerve fibers are damaged, resulting in sensory and/or autonomic dysfunction [9,12]. Small fibers with relatively diminished or absent myelination make them more vulnerable to stress-induced damage [4]. SFN can be present in two distinct manners. Length-dependent SFN is associated with distal axonal degeneration, and neuropathic pain typically presents in a “stocking-and-glove” pattern [9,12,13]. In contrast, non-length dependent SFN does not follow this traditional distribution. Neuropathic pain may occur in the trunk, face, and proximal extremities [9,13]. Skin biopsy markers have linked this non-traditional pattern with proximal neuronal degeneration [13].
Symptoms of SFN are representative of sensory and/or autonomic nerve damage. Sensory symptoms include burning, itching, numbness, tingling, and neuropathic pain [1,12]. Pain associated with SFN has been described as an electric shock, squeezing, burning, and cold [3,9,12]. A sensory symptom found to be strongly suggestive of SFN is allodynia, or pain triggered by stimuli that should not normally cause pain [6]. For example, allodynia is often reported in these patients with pain induced simply by bedsheets coming in contact with their feet [6,9]. Autonomic symptoms have been primarily presented as genitourinary and/or gastrointestinal involvement. Patients experience constipation, diarrhea, dysuria, and incontinence [3,6,9]. There have been multiple underlying etiologies identified for SFN [6,9,12]. The etiologies can be divided into metabolic, inflammatory, infectious, genetic, toxic agent, and idiopathic causes [6,12]. Diabetes is a metabolic condition that has frequently been associated with SFN [9]. Sjörgen’s syndrome and sarcoidosis are chronic inflammatory conditions commonly associated with SFN [6]. Hepatitis C, HIV and, more recently, COVID-19 are infectious agents that have been linked to SFN [3,12]. Toxic and medication-induced causes for SFN include alcohol-related neuropathy, chemotherapy-induced neuropathy, and even antibiotics.
Due to the considerable number of SFN causal agents, diagnosis should begin with a detailed medical, family, and social history [6,9,12]. Appropriate bloodwork and testing should be performed to determine the underlying etiology of the SFN. A proper neurological examination should be conducted; however, it has been noted that normal physical and neurological exams are often common with SFN patients [9]. Neurological exams can include testing sensation, vibration, strength, and reflexes [4,8,9]. Other diseases such as fibromyalgia, multiple sclerosis, complex regional pain syndrome, and chronic inflammatory demyelinating polyneuropathy (CIDP) may have similar presentations to SFN and can be ruled out with an effective history and physical exam.
3. Methods
A comprehensive literature review was conducted to investigate the pathophysiology, diagnosis, proposed mechanisms, and treatment strategies for SFN in the context of post-COVID-19 infection. The review focused on studies examining both COVID-19-associated SFN and general SFN pathologies to provide a broader understanding of potential mechanisms and treatment options.
3.1. Databases and Search Strategy
The literature review was performed using PubMed and Google Scholar. Keywords searched included combinations of the following: “small fiber neuropathy”, “SFN”, “COVID-19”, “SARS-COV-2”, “long COVID”, “neuropathic pain”, “post-COVID syndrome”, “autoimmune neuropathy”, “peripheral neuropathies”, “treatment”, “mechanism”, “chronic pain”, “inflammatory neuropathy”, “autonomic dysfunction”, and “post-viral neuropathy”. Boolean operators (AND/OR) were applied to refine the search results.
3.2. Time Frame
Articles published between January 2020 and January 2025 were prioritized, corresponding to the global emergence and ongoing evaluation of the COVID-19 pandemic. The earlier literature on SFN pathophysiology and treatment (pre-2020) was also reviewed when relevant to contextualize emergent findings.
3.3. Inclusion Criteria
- Peer-reviewed clinical trials, observational studies, case reports/series, and systematic reviews;
- Studies describing SFN and/or in relation to SFN post-COVID-19 infection or vaccination;
- English language publications.
3.4. Exclusion Criteria
- Animal studies, unless they provided novel mechanistic insights applicable to human pathology;
- Abstracts without full-text availability;
- Neuropathic pain studies not addressing SFN.
3.5. Data Extraction and Analysis
Articles were reviewed for information on pathophysiology, diagnostic criteria, clinical manifestations, proposed mechanisms, and treatment outcomes for post-COVID-19 SFN. Findings were synthesized to identify prevailing themes, areas of consensus, and current gaps in the understanding of post-COVID-19 SFN.
4. Proposed Mechanisms for COVID-19-Based SFN
There have been numerous proposed mechanisms (as depicted in Figure 1) for the development of small fiber neuropathy post-COVID-19 infection and vaccination; however, the exact methodology is still largely unknown. There is a substantial amount of literature suggesting an autoimmune response to COVID-19, including the release of antibodies against interferon-gamma and interleukin-2 [14]. There have also been case studies demonstrating positive anti-spike IgG levels. It is possible that this contributes to autoimmune neuropathy targeting small fibers, which may be particularly vulnerable due to their lack of a myelin sheath, limited capacity for axon repair, and increased metabolic requirements [15]. Similarly, the COVID-19 vaccine may also transiently increase autoantibodies and cause small fiber neuropathy through molecular mimicry, although the similarity between the viral protein and human nerve tissue protein structure is not strong [16]. Specifically, TS-HDS/FGFR3 antibodies have been identified in individuals with autonomic dysfunction due to SFN [17], and have also been associated with SFN following COVID-19 vaccination [18], although their exact role remains unclear and requires further investigation.
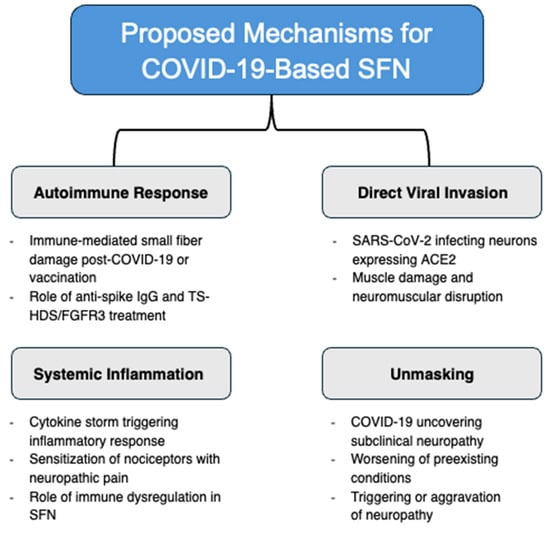
Figure 1.
Proposed mechanisms for COVID-19-based SFN: autoimmune response, direct viral invasion, systemic inflammation, and unmasking.
Multiple immunotherapy studies utilizing intravenous immunoglobulin (IVIG) to treat post-COVID-19 SFN have demonstrated significant improvements in neuropathic symptoms [19,20]. Therefore, there is a chance that the systemic autoimmune response leads to a generalized neuronal attack, affecting the small sensory cutaneous nerves to a greater initial degree due to their susceptible nature. Rather than an autoimmune reaction, there is also a suggestion of direct invasion of dorsal root ganglia neurons expressing the SARS-CoV-2 receptor, angiotensin-converting enzyme 2 (ACE2) [21,22]. The ACE-2 receptor facilitates virus entry into muscle, with myalgias and muscle fatigue being prevalent symptoms of COVID-19 infection [23,24]. Muscle damage causing disruption of neuromuscular junctions and connection impairment may lead to the neuropathic symptoms associated with SFN [25]. Possible small fiber damage resulting from cytokine storms in response to the COVID-19 virus may also contribute to the widespread neuropathy [26]. Dalakas suggests that this innate immunity—consisting of activated macrophages, dendritic cells, and cytokines—rather than adaptive immunity, plays a larger role in post-COVID-19 SFN by sensitizing skin nociceptors and triggering an inflammatory reaction, thereby causing neuropathic pain [27]. It has also been observed that COVID-19 has the potential to worsen neurological symptoms in patients with preexisting neurologic disease [28]. Therefore, it is possible that the infection does not directly cause neuropathy but rather unmasks it or exacerbates preexisting conditions already present in patients [29]. This likely varies on a case-by-case basis, and whether a particular mechanism is dominant in a certain patient population or with comorbidities remains to be determined.
Given the heterogeneity of post-COVID-19 SFN presentations, it is likely that not only one but rather multiple mechanisms are at play, potentially varying based on patient demographics, comorbidities, and immune responses. Further research on precision medicine is needed to determine which of these mechanisms predominate in specific patient populations, as well as to identify potential biomarkers for diagnosis and targeted treatment approaches.
5. Treatments for SFN
The primary treatment for SFN aims to target the underlying etiology [3,9]. Ideally, if the inciting agent can be identified and addressed, resolution of the primary problem may lead to an improvement or even the resolution of SFN symptoms. However, given the heterogeneity of SFN causes and the frequent difficulty in pinpointing an exact etiology, individualized treatment plans remain challenging [3]. Regardless of the underlying cause, treatment plans for SFN (Table 1) typically aim to relieve the symptoms, especially neuropathic pain [7,8,9,11].

Table 1.
Current treatments and associated mechanisms, side effects, and dosing for SFN.
Pharmacological interventions play a major role in symptom control. The anticonvulsant gabapentin has been recommended as a first-line treatment for neuropathic pain [3,8,9]. In a case study [8], gabapentin 300 mg was administered orally, once daily, to symptomatically treat a patient with post-COVID-19 SFN who subsequently reported adequate pain relief. Pregabalin, a structural analog of gabapentin, is another common anticonvulsant used to treat SFN-associated neuropathic pain [3,9].
In addition, antidepressants have been recommended as symptomatic treatment of SFN-associated neuropathic pain [3,9,39]. Specifically, tricyclic antidepressants, such as nortriptyline, are used for their analgesic and anti-inflammatory effects [39]. Serotonin–norepinephrine reuptake inhibitors, such as duloxetine, are also used to reduce neuropathic pain [3,9,39]. These medications modulate pain perception by increasing serotonin and norepinephrine levels in the central nervous system, resulting in pain relief [3,9,39]. There has been some evidence that topical therapies, such as lidocaine patches, can provide localized pain relief [3,9,39]. These treatments can be particularly useful in patients who experience focal pain, or those who may not be able to tolerate systemic medications due to side effects. Additionally, opioids—while typically reserved for refractory cases due to dependency concerns—have shown some efficacy in managing severe SFN-related pain [3,9,39].
Corticosteroids have been shown to be effective in treating SFN-associated neuropathic pain through their anti-inflammatory effects [40]. One study [40] showed marked improvement in neuropathic pain in four patients with acute onset SFN after oral prednisone treatment. In terms of treatment for post-COVID-19 SFN, corticosteroids have been shown to be effective in treating neuropathic pain [4,5]. Oaklander et al. (2022) had 35.3% of their participants placed on corticosteroid treatment. One participant reported a 90% improvement in her symptoms [4]. The patient involved in a case study by Panagiotides et al. (2023) reported a modest reduction in pain when placed on high-dose cortisone [5]. However, low-dose cortisone was not effective [5]. Corticosteroid treatment is thought to diminish the inflammatory immune response incited by COVID-19 [4].
IV Immunoglobulin (IVIG) is an emerging SFN treatment, especially in post-COVID-19 cases [4,5]. IVIG therapy has been explored as a potential option for SFN patients, with varying outcomes. While some case series have reported significant symptom relief following IVIG administration, a randomized controlled trial did not find a statistically significant difference between the SFN patients and the controls receiving IVIG [41]. However, IVIG treatment may provide greater benefit when the etiology is known to be immune mediated [41]. COVID-19-related SFN has been posited to be induced by an autoimmune inflammatory reaction, which may be an indication for IVIG treatment [3]. Five patients with post-COVID-19 SFN received repeated IVIG treatment and reported improvements in their symptoms [4]. Another case study utilized IVIG treatment. Initially, the patient reported only modest improvement in pain and hand redness. However, after IVIG treatment was re-initiated a few months later, significant symptomatic improvement was reported [5]. While these findings are promising, further research is needed to establish the efficacy of IVIG treatment and to determine optimal patient selection criteria for its use in treating post-COVID-19 SFN [42].
In addition, non-pharmacologic interventions can play a role in managing SFN. Acupuncture and essential oil massage therapy on the feet may provide temporary pain relief [37]. Yoga and meditation have also been shown to improve quality of life in patients with neuropathic pain [37]. Other nonpharmacological options that have been used to symptomatically treat patients with SFN include cool socks, foot tents, and transcutaneous electrical nerve stimulation machines [9]. However, non-pharmacological options have been minimally explored in patients with post-COVID-19 SFN, and no clinical trials have been conducted to evaluate the effectiveness of these interventions [9].
Overall, SFN treatment remains highly individualized, with a multimodal approach often required to achieve optimal symptom control. Further research is needed to refine treatment strategies and explore emerging therapeutic options, particularly in cases with novel or poorly understood etiologies such as post-COVID-19 SFN.
6. Discussion
In the aftermath of the SARS-CoV-2 pandemic, global healthcare efforts have largely shifted toward managing acute infections and implementing prevention strategies. However, as time has passed and more data have become available, persistent post-infectious symptoms—often referred to as “long COVID”—have emerged as a significant public health concern. Among these symptoms, orthostatic lightheadedness, palpitations, gastrointestinal dysfunction, chronic pain, and dysesthesias suggest the possible involvement of small nerve fibers, raising the question of an association with small fiber neuropathy (SFN).
Although SFN may not be the most reported manifestation of long COVID, its complex and multifactorial pathophysiology makes it a particularly challenging condition to diagnose and manage. Case reports have documented patients with ongoing sensory and autonomic dysfunction months after the initial infection, with clinical presentations consistent with SFN. Infectious diseases have long been recognized as triggers for polyneuropathies [43], including Hepatitis C, HIV, and viral etiologies associated with Guillain–Barré syndrome, placing COVID-19 within a broader context of infection-related neuropathies.
SFN may be present in a length-dependent or non-length-dependent pattern. The former is typically characterized by a “stocking-and-glove” distribution of symptoms, while the latter may involve the trunk, face, or proximal extremities. Symptoms often include neuropathic pain, described as burning, itching, tingling, numbness, and hypersensitivity, such as allodynia. These result from dysfunctional small myelinated Aδ-fibers and unmyelinated C-fibers. Autonomic symptoms, including gastrointestinal and genitourinary disturbances, are also frequently observed.
Diagnosis of SFN begins with a thorough history and physical examination. The current gold standard for confirmation is a skin biopsy to evaluate intraepidermal nerve fiber density. Additional diagnostic tools, such as quantitative sensory testing and nerve conduction studies, may be employed to support the diagnosis and exclude other neuropathic processes.
While the precise pathophysiological mechanism linking COVID-19 to SFN remains uncertain, an autoimmune-mediated response following infection is a leading hypothesis. This is supported by elevated antibody levels against interferon-gamma, interleukins, and IgG observed in some patients. Another theory posits that COVID-19 may exacerbate or unmask preexisting neurological conditions in susceptible individuals rather than directly causing SFN.
One of the most significant challenges in studying COVID-19-related SFN is the limited sample size in current research. Small cohorts reduce the generalizability of findings and hinder the ability to fully assess the condition’s prevalence and impact within the broader population of individuals experiencing long COVID symptoms.
Treatment typically involves addressing any underlying etiology, with symptomatic management playing a key role. There is moderate to strong evidence supporting the use of anticonvulsants such as gabapentin for treating neuropathic pain. Moderate evidence also exists for other pharmacologic options, including tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors (SNRIs), and, to a lesser extent, topical lidocaine, opioids, and corticosteroids. Intravenous immunoglobulin (IVIG) has shown promise in some cases; however, the strength of evidence remains limited, and further studies are needed to evaluate its efficacy in this specific patient population.
7. Conclusions
SFN has gained increased attention due to its association with long COVID. Symptoms such as allodynia, dysesthesia, and autonomic dysfunction—previously underrecognized—are now more frequently linked to SFN through improved diagnostic evaluation. The immune-mediated and inflammatory responses to SARS-CoV-2 appear to contribute to the development or unmasking of this condition in certain individuals. SFN can substantially impact quality of life, affecting sleep, sensation, autonomic function, and daily activities. This highlights the importance of continued research—not only to manage COVID-19’s evolving clinical presentations but to better understand the mechanisms underlying its long-term neurological effects.
To improve outcomes, there is a critical need for more accurate epidemiological data regarding SFN in the context of long COVID. Better characterization of this patient subgroup may enable earlier diagnosis and more targeted treatment approaches. As the virus continues to evolve [44], addressing its long-term neurological sequelae, including SFN, will remain a crucial aspect of post-pandemic healthcare.
Author Contributions
Conceptualization, A.D. and S.R.; methodology, H.M.; validation, formal analysis, A.B.; investigation, A.D. and S.R.; data curation, H.M.; writing—original draft preparation by all authors.; writing—review and editing, A.D. and S.R.; visualization, A.B.; supervision, A.D. and S.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Zis, P. COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1672. [Google Scholar] [CrossRef]
- Gomez, F.; Mehra, A.; Ensrud, E.; Diedrich, D.; Laudanski, K. COVID-19: A modern trigger for Guillain-Barre syndrome, myasthenia gravis, and small fiber neuropathy. Front. Neurosci. 2023, 17, 1198327. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Mills, A.J.; Kelley, M.; Toran, L.S.; Smith, B.; Dalakas, M.C.; Nath, A. Peripheral Neuropathy Evaluations of Patients with Prolonged Long COVID. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1146. [Google Scholar] [CrossRef] [PubMed]
- Panagiotides, N.G.; Zimprich, F.; Machold, K.; Schlager, O.; Müller, M.; Ertl, S.; Löfller-Stastka, H.; Koppensteiner, R.; Wadowski, P.P. A Case of Autoimmune Small Fiber Neuropathy as Possible Post COVID Sequelae. Int. J. Environ. Res. Public Health 2023, 20, 4918. [Google Scholar] [CrossRef]
- Sène, D. Small fiber neuropathy: Diagnosis, causes, and treatment. Jt. Bone Spine 2017, 85, 553–559. [Google Scholar] [CrossRef]
- Burakgazi, A.Z. Small-Fiber Neuropathy Possibly Associated with COVID-19. Case Rep. Neurol. 2022, 14, 208–212. [Google Scholar] [CrossRef]
- Kumar, S.S.; Yong, M.; Koh, Y. Small fiber neuropathy in a patient with coronavirus disease 2019 (COVID-19). Neurol. Asia 2022, 27, 195–197. [Google Scholar] [CrossRef]
- Hovaguimian, A.; Gibbons, C.H. Diagnosis and Treatment of Pain in Small Fiber Neuropathy. Curr. Pain Headache Rep. 2011, 15, 193–200. [Google Scholar] [CrossRef]
- Amruth, G.; Praveen-kumar, S.; Nataraju, B.; Nagaraja, B.S. HIV Associated Sensory Neuropathy. J. Clin. Diagn. Res. 2014, 8, MC04–MC07. [Google Scholar] [CrossRef]
- Moretti, R.; Giuffrè, M.; Merli, N.; Caruso, P.; Di Bella, S.; Tiribelli, C.; Crocè, L.S. Hepatitis C Virus-Related Central and Peripheral Nervous System Disorders. Brain Sci. 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.A.; Mukhdomi, T. Small Fiber Neuropathy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Birnbaum, J.; Bingham, C.O. Non-length-dependent and length-dependent small-fiber neuropathies associated with tumor necrosis factor (TNF)-inhibitor therapy in patients with rheumatoid arthritis: Expanding the spectrum of neurological disease associated with TNF inhibitors. Semin. Arthritis Rheum. 2014, 43, 638–647. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022, 20, 129. [Google Scholar] [CrossRef]
- Schwartz, B.; Moudgil, S.; Pollina, F.; Bhargava, A. Small Fiber Neuropathy After SARS-CoV-2 Infection and Vaccination: A Case-Based Comparison. Cureus 2023, 15, e43600. [Google Scholar] [CrossRef]
- Khokhar, F.; Khan, A.; Hussain, Z.; Yu, J. Small Fiber Neuropathy Associated with the Moderna SARS-CoV-2 Vaccine. Cureus 2022, 14, e25969. [Google Scholar] [CrossRef]
- Trevino, J.A.; Novak, P. TS-HDS and FGFR3 antibodies in small fiber neuropathy and Dysautonomia. Muscle Nerve 2021, 64, 70–76. [Google Scholar] [CrossRef]
- Mastropaolo, M.; Hasbani, M.J. Small Fiber Neuropathy Triggered by COVID-19 Vaccination: Association with FGFR3 Autoantibodies and Improvement during Intravenous Immunoglobulin Treatment. Case Rep. Neurol. 2023, 15, 6–10. [Google Scholar] [CrossRef]
- McAlpine, L.; Zubair, A.S.; Joseph, P.; Spudich, S. Case-Control Study of Individuals with Small Fiber Neuropathy After COVID-19. Neurol. Neuroimmunol. Neuroinflammation 2024, 11, e200244. [Google Scholar] [CrossRef]
- Novak, P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: A case report. eNeurologicalSci 2020, 21, 100276. [Google Scholar] [CrossRef]
- Gemignani, F. Small Fiber Neuropathy and SARS-COV-2 Infection. Another piece in the long COVID puzzle? Muscle Nerve 2022, 65, 369–370. [Google Scholar] [CrossRef]
- Shiers, S.; Ray, P.R.; Wangzhou, A.; Sankaranarayanan, I.; Tatsui, C.E.; Rhines, L.D.; Li, Y.; Uhelski, M.L.; Dougherty, P.M.; Price, T.J. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: Implications for SARS-CoV-2 virus neurological effects. Pain 2020, 161, 2494–2501. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Martínez, A.; Caballero-García, A.; Pérez-Valdecantos, D.; Roche, E.; Noriega-González, D.C. Peripheral Neuropathies Derived from COVID-19: New Perspectives for Treatment. Biomedicines 2022, 10, 1051. [Google Scholar] [CrossRef]
- Üçeyler, N.; Kafke, W.; Riediger, N.; He, L.; Necula, G.; Toyka, K.V.; Sommer, C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010, 74, 1806–1813. [Google Scholar] [CrossRef]
- Dalakas, M.C. Post-COVID Small Fiber Neuropathy, Implications of Innate Immunity, and Challenges on IVIG Therapy. Neurol. Neuroimmunol. Neuroinflammation 2024, 11, e200248. [Google Scholar] [CrossRef]
- Kubota, T.; Kuroda, N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: A systematic review. Clin. Neurol. Neurosurg. 2021, 200, 106349. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Maizels, M.; McCarberg, B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am. Fam. Physician 2005, 71, 483–490. [Google Scholar]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, L.; Haanpää, M.; Hansson, P.; Jensen, T.S. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Dhesi, M.; Maldonado, K.A.; Patel, P.; Maani, C.V. Tramadol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Duehmke, R.M.; Derry, S.; Wiffen, P.J.; Bell, R.F.; Moore, R.A. Tramadol for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 6, CD003726. [Google Scholar] [CrossRef] [PubMed]
- Couch, B.; Hayward, D.; Baum, G.; Sakthiyendran, N.A.; Harder, J.; Hernandez, E.J.; MacKay, B. A systematic review of steroid use in peripheral nerve pathologies and treatment. Front. Neurol. 2024, 15, 1434429. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, V.B.; Rayi, A. Intravenous Immunoglobulin (IVIG). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tavee, J.O. Office approach to small fiber neuropathy. Clevel. Clin. J. Med. 2018, 85, 801–912. [Google Scholar] [CrossRef]
- Hwang, M.S.; Lee, H.Y.; Choi, T.Y.; Lee, J.H.; Ko, Y.S.L.; Jo, D.C.; Do, K.; Lee, J.H.; Park, T.Y. A systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine 2020, 99, e19837. [Google Scholar] [CrossRef]
- Brouwer, B.A.; de Greef, B.T.A.; Hoeijmakers, J.G.J.; Geerts, M.; van Kleef, M.; Merkies, I.S.J.; Faber, C.G. Neuropathic Pain due to Small Fiber Neuropathy in Aging: Current Management and Future Prospects. Drugs Aging 2015, 32, 611–621. [Google Scholar] [CrossRef]
- Dabby, R.; Gilad, R.; Sadeh, M.; Lampl, Y.; Watemberg, N. Acute steroid responsive small-fiber sensory neuropathy: A new entity? J. Peripher. Nerv. Syst. 2006, 11, 47–52. [Google Scholar] [CrossRef]
- Geerts, M.; de Greef, B.T.A.; Sopacua, M.; van Kuijk, S.M.J.; Hoeijmakers, J.G.J.; Faber, C.G.; Merkies, I.S.J. Intravenous Immunoglobulin Therapy in Patients with Painful Idiopathic Small Fiber Neuropathy. Neurology 2021, 96, e2534–e2545. [Google Scholar] [CrossRef]
- Abrams, R.M.C.; Simpson, D.M.; Navis, A.; Jette, N.; Zhou, L.; Shin, S.C. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve 2022, 65, 440–443. [Google Scholar] [CrossRef]
- De León, A.M.; Garcia-Santibanez, R.; Harrison, T.B. Article Topic: Neuropathies Due to Infections and Antimicrobial Treatments. Curr. Treat. Options Neurol. 2023, 10, 213–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, S.; Cavany, S.; Perkins, T.A.; España, G.F.C. Projecting the future impact of emerging SARS-CoV-2 variants under uncertainty: Modeling the initial Omicron outbreak. Epidemics 2024, 47, 100759. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).