Abstract
Gamma knife radiosurgery (GKRS), a form of stereotactic radiosurgery (SRS), has gained importance in treating glioblastoma alongside conventional chemotherapy. This study aims to assess the efficacy of combining GKRS with surgery and chemotherapy to enhance treatment outcomes for glioblastoma patients. This prospective clinical study, adhering to STROBE guidelines, assessed 121 glioblastoma patients from June 2008 to December 2022. All patients who had not undergone prior radiotherapy underwent open surgical tumor resection, GKRS, and adjuvant chemotherapy. In the analyzed cohort, the median survival post-diagnosis was 21.2 months (95% CI: 11.4–26.7) and the median progression-free survival was 13.6 months (95% CI: 12.5–28.3). The median time to first recurrence post-treatment was 14.5 months (range: 4–33 months). The median prescribed dose for GKRS was 12 Gy (range: 10–17 Gy), with a median target volume of 6.0 cm3 (range: 1.6–68 cm3). Post GKRS, 92 patients experienced local recurrence, 21 experienced distant recurrence, and 87 received additional treatment, indicating diverse responses and treatment engagement. This study evaluates the use of GKRS for glioblastomas, emphasizing its efficacy and complications in a single-center trial. It suggests integrating GKRS into initial treatment and for recurrences, highlighting the comparable survival rates but underscoring the need for further research.
Keywords:
brain tumor; gamma knife; glioblastoma; oncology; radiotherapy; radiosurgery; cancer neuroscience 1. Introduction
Management of glioblastoma remains challenging, and decision-making is highly individualized [1]. Surgery, temozolomide, and fractionated radiotherapy are standards of care for newly diagnosed glioblastoma; however, approaches for improving the treatment outcome is still under on-going investigation [2,3]. The incidence of glioblastoma increases with age, with the highest rates observed in the elderly population ranging from an age of 75 to 84 years old [3]. Current new treatment modalities for high-grade gliomas include open surgical resection, adjuvant radiotherapy, and chemotherapy [3,4,5]. Despite the implementation of aggressive multi-modality therapeutic approaches in the treatment of high-grade gliomas such as glioblastoma, the prognosis remains grim with inevitable recurrence [1,5,6,7]. Many patients are treated with systemic chemotherapy as an initial and salvage management approach in the form of bevacizumab, temozolomide (TMZ), irinotecan, and nitrosoureas [1,7,8]. Despite recent advances in radiation and surgical care of patients with high-grade gliomas, treatment of these neoplasms remains challenging for neurooncologists and surgeons [2,9]. The current standard of care for the treatment of stage IV gliomas involves maximal possible surgical resection followed by radiotherapy and temozolomide chemotherapy [1,2,9].
Over the years, gamma knife stereotactic radiosurgery has become one of the highly effective options in the treatment of a range of both malignant and benign CNS lesions, providing a relatively safe and noninvasive treatment modality and offering promising results [10,11,12]. Stereotactic radiosurgery uses multiple focused beams of high-energy ionizing radiation at a precise target site, resulting in targeted tissue destruction and endothelial apoptosis induction while minimizing damage to the surrounding healthy brain parenchyma, making it a highly favorable option for the treatment of brain tumors [13,14,15]. Radiation necrosis and hemorrhage from the periphery of the irradiated focus have been implicated as a side effect of GKRS treatment with radiosurgery [13]. Even though glioblastomas are infiltrative with ill-defined margins, radiosurgery has been described to be an effective treatment modality [1,13,15]. Data advocate that in patients over 60 years old, treatment with temozolomide is associated with a longer survival than treatment with standard radiotherapy [16,17,18], and for those over 70 years old, temozolomide or hypofractionated radiotherapy is correlated with a longer survival than management with standard fractionated radiotherapy, especially for recurrent glioblastoma [17,19,20]. In this paper, the authors present a prospective clinical study analyzing a multifactorial approach involving radiosurgery for the treatment of glioblastoma.
2. Materials and Methods
This study was approved by our Committee for Human Research. We prospectively reviewed patients who underwent gamma knife radiosurgery treatment for glioblastoma at the Miami Neuroscience Center at Larkin Community Hospital between June 2008 and December 2022. The authors reviewed the outcomes of one hundred and twenty-one patients with confirmed histopathological diagnosis of glioblastoma who were treated following a modified version of the Stupp protocol. We included patients with pathologically confirmed glioblastoma who received comprehensive or radiosurgical care at our institution. The brain tumors were graded following the World Health Organization classification. Our center had no standardized GKRS patient selection criteria. The clinical research study was conducted following STROBE guidelines. GKRS was recommended by a multidisciplinary neurosurgical–oncology tumor group, composed of neurosurgical oncologists, neuroradiologists, physicists, physician neuroscientists, radiation oncologists, and medical physicists.
For patients with multiple targets treated in the same GK session, we focused on parameters from the largest volumetric lesion. We further focused on the patients’ first GK session. The glioblastoma therapy was carried out sequentially by maximal surgical resection, radiosurgery, continuous daily TMZ (75 mg per square meter of body-surface area per day, 7 days per week from the first to the last day of radiotherapy), and six cycles of adjuvant TMZ (150 to 200 mg per square meter for 5 days during each 28-day cycle). The male-to-female ratio was 39:82, and the median age was 51.3 years (range, 18–73). Demographic and clinical center criteria are listed in Table 1.

Table 1.
Demographic and clinical center criteria.
2.1. Patient Selection and Radiosurgery Technique
Patients were deemed eligible for gamma knife stereotactic radiosurgery (GKRS) based on two key criteria: (1) a histological confirmation of glioblastoma and (2) completion of surgical resection without any history of other therapies, including conventional radiotherapy. The decision to proceed with GKRS following temozolomide chemotherapy was made by the treating medical team, consisting of the radiation oncologist, medical oncologist, and neurosurgeon. For each GKRS procedure, the patient first underwent a stereotactic head frame placement. This was followed by the acquisition of magnetic resonance imaging (MRI, 1.5 Tesla) with intravenous contrast to precisely identify the treatment area. Treatment plans were generated cooperatively by a neurosurgical oncologist, neuroradiologist, radiation oncologist, and medical physicist with target volumes based largely on the T1 postcontrast sequence. Fluid-attenuated inversion-recovery sequences were used to demarcate the full extent of tumor cellularity when T1 imaging was insufficient. The tumor recurrence was diagnosed with contrast-enhanced brain MRI including diffusion-weighted images, MR spectroscopy, and perfusion images. Targets were prescribed marginal doses of 10 to 20 Gy based on the in-house protocol, with decreasing doses for larger dose volumes. Dose planning was implemented with multiple isocenters to maximize the dose conformity and gradient index.
2.2. Statistical Analysis
Both the overall survival and progression-free survival were central to our endpoint analysis. Using the Kaplan–Meier method, overall survival was calculated from two different time points: initial time diagnosis and GKRS time. Variables affecting progression-free survival (PFS) and overall survival (OS) after GKRS were determined via univariate analysis. Progression-free survival was calculated from the GKRS time until either tumor recurrence, tumor progression, or death. We examined variables using a Cox proportional hazard analysis model to identify the independent predictors of survival. All statistical analyses were performed with a significance level of p < 0.05 using SPSS version 21.0 (SPSS, Chicago, IL, USA).
3. Results
Gamma Knife Radiosurgery Plan
In the studied cohort, the analysis of survival time post histopathological diagnosis reveals a median duration of 21.2 months, with the interquartile range delineated by a 95% Confidence Interval (CI) stretching from 11.4 to 26.7 months. Progression-free survival, which measures the duration patients live without any signs of disease progression, was observed at a median of 13.6 months, with the 95% CI ranging from 12.5 to 28.3 months. Regarding the recurrence of disease, the median time until the first recurrence post-treatment was 14.5 months, with observed cases varying widely from as short as 4 months to as long as 33 months.
In terms of treatment specifics, the median dose prescribed for GKRS was identified as 12 Gy, with treatment doses administered across the cohort fluctuating between 10 and 17 Gy. Furthermore, the median target volume for the GKRS treatment was established at 6.0 cm3, with a broad range in target volumes from 1.6 to 68 cm3, reflecting the variance in tumor sizes and treatment approaches within the patient population.
The median duration of follow-up after GKRS treatment was recorded as 16.5 months, with the range of follow-up periods extending from 8 to 33 months. This period represents the post-treatment observation time to monitor patient outcomes and disease progression or recurrence. Local recurrence was well defined as reappearance at the primary tumor site. CSF dissemination was defined as recurrence in the central nervous system in imaging studies. Local recurrence was noted in 92 patients.
Among the cohort, 87 patients underwent further treatment modalities following the initial intervention, categorized as follows: reoperation (n = 13), a second session of GKRS (n = 79), administration of bevacizumab and irinotecan (n = 3), and PCV chemotherapy (n = 12). Distant recurrence was observed in 21 patients. Of these, 10 received additional treatments, which are detailed as follows: radiotherapy in 2 patients, temozolomide therapy in 5 cases, PCV chemotherapy in 3 patients, and combination therapy of nimustine and cisplatin in 3 patients. Furthermore, cerebrospinal fluid (CSF) dissemination was identified in five individuals within the study population.
Following GKRS, a structured follow-up protocol was implemented. All patients underwent their first evaluation at 4 weeks post-treatment, with subsequent evaluations scheduled at intervals of 2 to 3 months. During each follow-up visit, patients were subjected to a contrast-enhanced magnetic resonance imaging (MRI) scan in addition to a comprehensive neurological assessment. As required, further diagnostic investigations, including MR spectroscopy, MRI perfusion, and/or positron emission tomography (PET) imaging, were conducted to accurately differentiate between radiation necrosis and tumor progression.
Furthermore, there was a statistically significant difference in survival between patients with tumor volumes smaller than 10 cm3 and those with tumor volumes larger than 10 cm3, with p-values of 0.019 and 0.006, respectively, as illustrated in Figure 1.
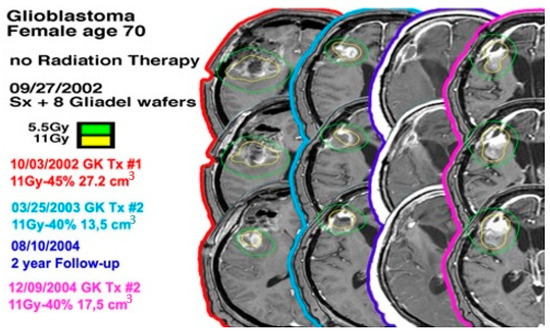
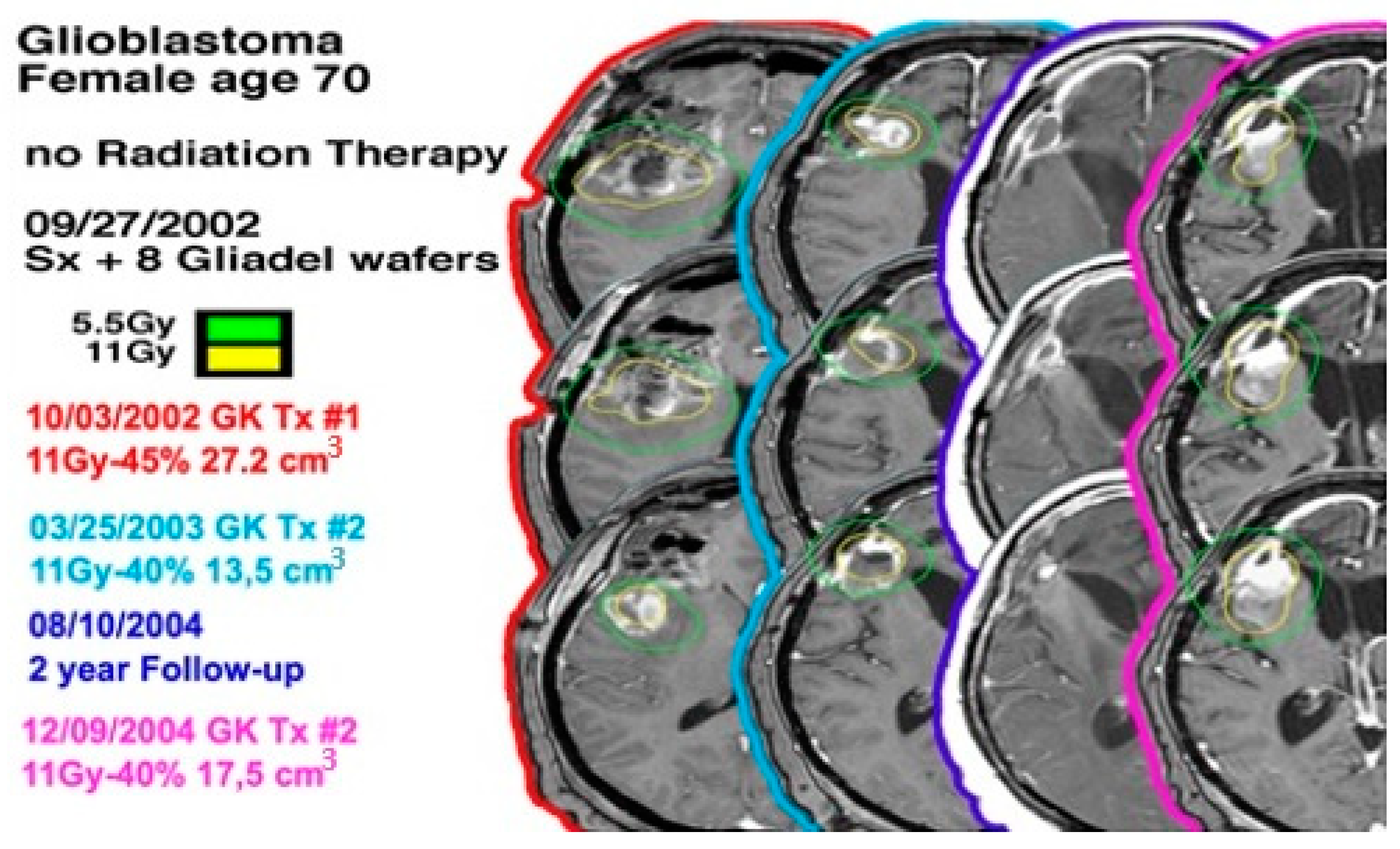
Figure 1.
A 70-year-old female with a left-sided frontal lobe glioblastoma underwent GKRS three times and did not undergo radiation therapy.
In the study cohort, adjuvant chemotherapy comprised 82% temozolomide (TMZ) and 18% procarbazine, lomustine (CCNU), and vincristine (PCV). An analysis of progression-free survival (PFS) concerning the radiation dose and the combination of chemotherapy did not reveal any statistically significant differences, as presented in case 1 (Figure 2). The median survival time from the point of initial diagnosis was determined to be 31.6 months, with a 95% Confidence Interval (CI) ranging from 17.4 to 44.7 months. Kaplan–Meier survival curves were utilized to delineate progression-free survival among patients treated with GKRS, as depicted in Figure 2. The overall survival rates at 1 year, 2 years, and 3 years post-treatment were 78%, 48%, and 18%, respectively.
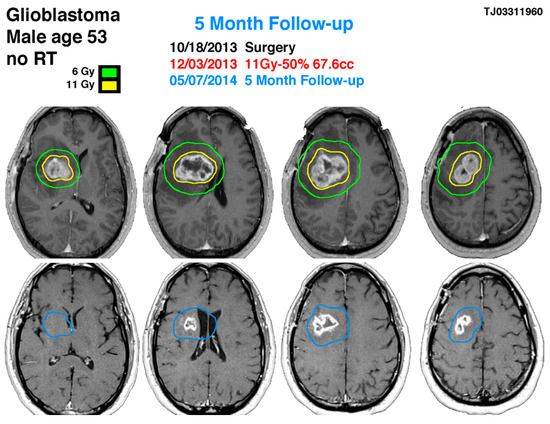
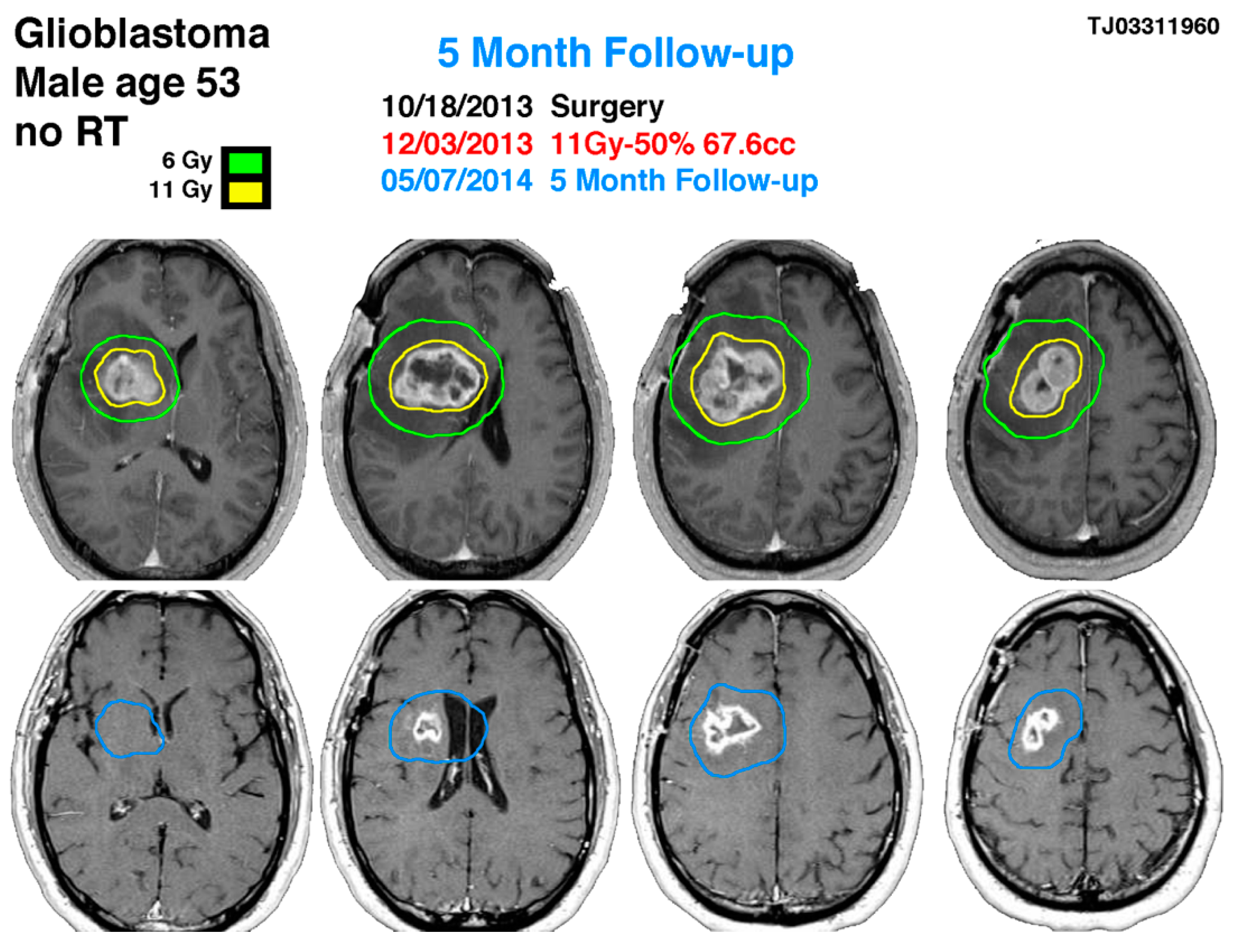
Figure 2.
53-year-old male with right-sided insular lobe basal ganglia GBM. The blue line indicates the previous glioblastoma contouring treated with gamma knife on 12 March 2013 showing the significance response 5 months later with a reduction of the tumor size in more than 60% of volume.
A 53-year-old male with post-operative glioblastoma underwent initial gamma knife radiosurgery (GKRS) at 11 Gy and NovoTTF-100A therapy after partial resection on 18 October 2013. Treatment included six cycles of temozolomide. Follow-up MRI and methionine PET showed a uniformly enhancing lesion. A second GKRS procedure targeted a 67.6 cm3 lesion with 11 Gy, followed by twelve temozolomide cycles, achieving tumor reduction. After 12 months, MRI indicated a decreased lesion size, with no neurological symptoms. On 26 November 2015, imaging showed right frontal lobe progression, necessitating a third GKRS procedure.
4. Discussion
The literature supporting GKRS for glioblastoma is multifaceted and developing [3,15,19]. Retrospective series report diverging results regarding the efficacy of adding GKRS to conventional treatment, but randomized trials have shown no clear benefit to dose escalation or boosts [21,22,23,24,25,26,27,28]. Despite the implementation of aggressive multi-modality therapeutic approaches to treat glioblastoma, the prognosis remains grim with inevitable recurrence [1,8,10]. Furthermore, glioblastoma tumors recur in the majority of patients and the disease is often terminal. Progress in new treatment procedures and scientific innovations to improve the overall survival of these patients are urgently imperative [18,20,29,30,31,32,33]. Observational studies demonstrated that GKRS improves patient survival as a chemotherapy adjuvant therapy [2,16,19]. Without treatment intervention, the median survival time for patients diagnosed with grade IV glioblastoma is 6 months [3].
With advanced therapeutic interventions, patient survival can be extended beyond 16 months. The standard treatment for grade IV gliomas involves maximal surgical removal of the tumor, followed by adjuvant conventional radiotherapy and temozolomide (TMZ) chemotherapy. However, while surgical removal and radiotherapy are cornerstone treatments for high-grade gliomas, they come with significant potential side effects, such as radiation-induced necrosis, loss of pituitary function, visual problems, cognitive decline, leukoencephalopathy, and the risk of developing new tumors from radiation exposure. Additionally, the literature indicates that glioblastoma may recur anytime within 6 to 18 months after the initial surgical removal, highlighting the urgent need for innovative treatment options.
Our current treatment regimen includes comprehensive surgical removal of the tumor, followed by radiosurgery, administration of temozolomide or targeted chemotherapy, and NovoTTF (optune) therapy in selected cases. Here, gamma knife stereotactic radiosurgery (GKRS) offers a minimally invasive, safe alternative for post-surgical treatment and to reduce the recurrence of glioblastoma, particularly when patients cannot undergo further surgery due to severe comorbidities, with markedly reduced neurotoxicity.
4.1. Role of SRS in Glioblastoma
The management of glioblastoma remains a complex challenge, necessitating personalized decision-making processes. The application of highly conformal techniques such as GKRS is highly advantageous, especially given the propensity for patients to experience tumor recurrence close to the site of the original brain tumor. Recent glioblastoma treatment guidelines acknowledge that Level III evidence supports the use of all reirradiation techniques, including radiosurgery, although the specific patient population that would derive the most benefit from these interventions has yet to be precisely identified.
Our analysis, drawing on over a decade of experience from an institutional community neuroscience center, represents the largest single-institution experience examining the application of GKRS in the treatment of glioblastoma to date. The literature, including a study by Brown et al., suggests that the cognitive side effects associated with GKRS are less pronounced among patients with one to three brain metastases. Notably, our findings reveal no reported instances of cognitive decline either from patients or clinicians involved in the study, suggesting that GKRS might be preferable in preserving cognitive function, potentially offering a superior strategy compared, in a greater or lesser extent, to whole-brain radiation therapy (WBRT), and intensity modulated radiation therapy (IMRT) when evaluated in the context of the current research and understanding of the neuroplasticity of brain treatment outcomes [34].
Furthermore, while all GKRS, WBRT and IMRT are associated with certain side effects, such as acute postoperative edema, this condition can be effectively managed with corticosteroids. This management strategy underscores the importance of tailored treatment approaches in mitigating the adverse effects associated with glioblastoma therapies, thereby enhancing patient care and outcomes in this challenging clinical landscape.
Analyzing current studies and outcomes in brain neuroplasticity, GKRS could result in less cognitive deterioration compared to WBRT and IMRT [15,23,34,35,36]. Other side effects that have been observed in both therapies may include acute post-operative edema. However, this edema is very well managed by treatment with corticosteroids. The use of GKRS as a salvage treatment followed by administration of chemotherapeutic treatment in patients with glioblastoma has been shown to increase progression-free survival by a median of 15–16 months [24,37]. Although there are limited data currently available, the use of GKRS alone or as a co-adjuvant treatment in a multimodality treatment plan can significantly improve survival rates in patients with glioblastoma. The association of results from the current study shows that GKRS could be considered as an alternative to conventional radiotherapy after tumor resection. Stereotactic radiosurgery is increasingly used in patients with recurrent GBM, and the efficacy of this technique has shown benefits in the outcomes of the patients, highlighting the value of this therapy for glioblastomas [35].
4.2. Identifying Radiosurgery Candidates
Identifying optimal candidates for GKRS involves considering the tumor size, with smaller tumors (<10 cm3) showing significantly better survival outcomes. This suggests that patients with smaller glioblastoma lesions are ideal candidates for GKRS, as they tend to have both an improved procedural tolerance and prognosis. Selecting appropriate GKRS patients remains challenging [29,35,38,39,40]. Early GKRS intervention also appears to extend the life of patients significantly without compromising the patient’s overall quality of life. However, since treatment includes different grades of resection and the use of Optune, Gliadel wafers, and different chemotherapy schemes, these variables could be a confounding factor. This prospective clinical study demonstrates that there is still a window of opportunity for gamma knife radiosurgery as an initial early treatment after surgical resection.
4.3. Differential Diagnosis of Glioma
After 2021 the WHO made a new classification for gliomas, and now basically every glioma which is IDH wild type is considered glioblastoma, and IDH mutation in a glioma is considered a better prognosis compared to wild type IDH. Future stratification would be needed in order to objectively make the assessment of the effect of any medical treatment including radiosurgery in conjunction with chemotherapy or IMRT and chemotherapy to define the control of the disease. Likewise, 1p/19q deletion identifies a good prognosis since these gliomas are related to oligodendrogliomas, which tend to be more radiosensitive and chemosensitive [41,42,43,44].
4.4. Limitations
This prospective study’s limitations include a selection bias, because GKRS is offered to patients with a greater performance status and a characteristic tendency for longer survival. Additionally, many patients had nodular enhancement on gamma knife pre-plan MRI, and the natural history of further re-recurrences may be worse. In cases where patients underwent a complete resection and showed no enhancement, the patient would still receive GKRS as per the author’s protocol.
Apart from a subset of subjects from our main patient registry who were enrolled in a prospective brain registry, no stringent patient selection criteria were applied. As described, this prospective report included a heterogeneous population, and some patients underwent several therapies, including systemic chemotherapy, before receiving radiosurgery. Many patients also received additional salvage therapies after their GKRS. It would be incorrect to attribute the full survival benefit to GKRS alone. The author believes that this study presents a new opportunity, suggesting that radiosurgery could be an effective alternative to conventional treatments for glioblastoma, potentially leading to a significantly improved quality of life as it is current applied.
5. Conclusions
This study presents a comprehensive evaluation of gamma knife stereotactic radiosurgery (GKRS) for glioblastomas, conducted as a large, prospective analysis of single-center clinical data. The findings contribute valuable insights into the use of GKRS in a community hospital setting, highlighting its efficacy and potential complications. The results advocate for incorporating single-fraction radiosurgery, as an alternative to conventional radiation therapy, into the initial treatment regimen for glioblastoma following complete surgical resection, as well as for its use as a primary treatment for recurrences when surgical intervention is not feasible. The observed post-GKRS survival rates of approximately 12–16 months are comparable to or exceed those associated with other current treatment modalities. Despite these promising outcomes, there is a clear need for further research through prospective, randomized, clinical trials to thoroughly assess the effectiveness of GKRS in specific clinical scenarios and to solidify its role in the treatment landscape for glioblastoma.
Author Contributions
J.E.V., A.W., X.W., N.S.R., M.F.G., M.B. and A.M.A.-P. made substantial contributions to the conception, design, execution, and analysis of this research, as well as to the preparation and critical revision of the manuscript. J.E.V. and A.M.A.-P. served dual roles as both senior and first authors of the study and the accompanying case series. The contributions of all authors satisfy the following criteria: 1. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. 2. Drafting the work or revising it critically for important intellectual content. 3. Final approval of the version to be published. 4. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Larkin Community Hospital Miami Neuroscience Center (LCH 1- 022015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available per request. Per privacy guidelines, data is not available in a public database.
Acknowledgments
The authors express appreciation to the Latino America Valerio Foundation and Silvana Valerio for their substantial support, which has advanced neurosurgical research across Latin America and the Caribbean.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| EGFR | Epidermal Growth Factor Receptor |
| GBM | Glioblastoma Multiforme |
| GK | Gamma Knife |
| GKRS | Gamma Knife Radiosurgery |
| IDH | Isocitrate Dehydrogenase |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| OS | Overall Survival |
| PCV | Procarbazine, Lomustine (CCNU), Vincristine |
| PET | Positron Emission Tomography |
| PFS | Progression-Free Survival |
| SRS | Stereotactic Radiosurgery |
| TERT | Telomerase Reverse Transcriptase |
| TMZ | Temozolomide |
| WBRT | Whole-Brain Radiation Therapy |
| WHO | World Health Organization |
References
- Norden, A.D.; Lesser, G.J.; Drappatz, J.; Ligon, K.L.; Hammond, S.N.; Lee, E.Q.; Reardon, D.R.; Fadul, C.E.; Plotkin, S.R.; Batchelor, T.T.; et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013, 15, 930–935. [Google Scholar] [CrossRef]
- Omuro, A.; Chan, T.A.; Abrey, L.E.; Khasraw, M.; Reiner, A.S.; Kaley, T.J.; DeAngelis, L.M.; Lassman, A.B.; Nolan, C.P.; Gavrilovic, I.T.; et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013, 15, 242–250. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef]
- Cheon, Y.J.; Jung, T.Y.; Jung, S.; Kim, I.Y.; Moon, K.S.; Lim, S.H. Efficacy of Gamma Knife Radiosurgery for Recurrent High-Grade Gliomas with Limited Tumor Volume. J. Korean Neurosurg. Soc. 2018, 61, 516–524. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes. Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Lau, D.; Magill, S.T.; Aghi, M.K. Molecularly targeted therapies for recurrent glioblastoma: Current and future targets. Neurosurg. Focus 2014, 37, E15. [Google Scholar] [CrossRef]
- Taylor, A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012, 14 (Suppl. S5), v1–v49. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, S.H.; Lee, J.I.; Seol, H.J.; Nam, D.-H.; Kim, S.T.; Park, K.; Kim, J.H.; Kong, D.-S. Outcome of radiosurgery for recurrent malignant gliomas: Assessment of treatment response using relative cerebral blood volume. J. Neurooncology 2015, 121, 311–318. [Google Scholar] [CrossRef]
- Alomari, A.; Rauch, P.J.; Orsaria, M.; Minja, F.J.; Chiang, V.L.; Vortmeyer, A.O. Radiologic and histologic consequences of radiosurgery for brain tumors. J. Neurooncology 2014, 117, 33–42. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Sheean, T.; Bonney, P.A.; Maurer, A.J.; Teo, C. Aggressive repeat surgery for focally recurrent primary glioblastoma: Outcomes and theoretical framework. Neurosurg. Focus 2015, 38, E11. [Google Scholar] [CrossRef] [PubMed]
- Imber, B.S.; Kanungo, I.; Braunstein, S.; Barani, I.J.; Fogh, S.E.; Nakamura, J.L.; Berger, M.S.; Chang, E.F.; Molinaro, A.M.; Cabrera, J.R.; et al. Indications and Efficacy of Gamma Knife Stereotactic Radiosurgery for Recurrent Glioblastoma: 2 Decades of Institutional Experience. Neurosurgery 2017, 80, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Yen, C.P.; Starke, R.M.; Lee, C.-C.; Sheehan, J.P. Unyielding progress: Recent advances in the treatment of central nervous system neoplasms with radiosurgery and radiation therapy. J. Neurooncology 2014, 119, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Krex, D.; Klink, B.; Hartmann, C.; von Deimling, A.; Pietsch, T.; Simon, M.; Sabel, M.; Steinbach, J.P.; Heese, O.; Reifenberger, G.; et al. Long-term survival with glioblastoma multiforme. Brain 2007, 130 Pt 10, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.W.; Peterson, H.E.; Fairbanks, R.K.; Lamoreaux, W.T.; Mackay, A.R.; Call, J.A.; Demakas, J.J.; Cooke, B.S.; Lee, C.M. Long-Term Survival and Improved Quality of Life following Multiple Repeat Gamma Knife Radiosurgeries for Recurrent Glioblastoma Multiforme: A Case Report and Review of the Literature. Case Rep. Oncol. Med. 2013, 2013, 431857. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Bélanger, K.; Mason, W.P.; Fulton, D.; Kavan, P.; Easaw, J.; Shields, C.; Kirby, S.; Macdonald, D.R.; Eisenstat, D.D.; et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010, 28, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Abacioglu, U.; Caglar, H.B.; Yumuk, P.F.; Akgun, Z.; Atasoy, B.M.; Sengoz, M. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J. Neurooncology 2011, 103, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Pontoriero, A.; Arpa, D.; Siragusa, C.; Tomasello, C.; Romanelli, P.; Cardali, S.; Granata, F.; De Renzis, C.; Tomasello, F. Efficacy and toxicity of CyberKnife reirradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. 2012, 154, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nwokedi, E.C.; DiBiase, S.J.; Jabbour, S.; Herman, J.; Amin, P.; Chin, L.S. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery 2002, 50, 41–47. [Google Scholar] [CrossRef]
- Skeie, B.S.; Enger, P.Ø.; Brøgger, J.; Ganz, J.C.; Thorsen, F.; Heggdal, J.I.; Pedersen, P.-H. Gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012, 78, 658–669. [Google Scholar] [CrossRef]
- Valerio, J.E.; Ramirez-Velandia, F.; Fernandez-Gomez, M.P.; Rea, N.S.; Alvarez-Pinzon, A.M. Bridging the Global Technology Gap in Neurosurgery: Disparities in Access to Advanced Tools for Brain Tumor Resection. Neurosurg. Pract. 2024, 5, e00090. [Google Scholar]
- Elaimy, A.L.; Mackay, A.R.; Lamoreaux, W.T.; Demakas, J.J.; Fairbanks, R.K.; Cooke, B.S.; Lamm, A.F.; Lee, C.M. Clinical outcomes of Gamma Knife radiosurgery in the salvage treatment of patients with recurrent high-grade glioma. World Neurosurg. 2013, 80, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Souhami, L.; Seiferheld, W.; Brachman, D.; Podgorsak, E.B.; Werner-Wasik, M.; Lustig, R.; Schultz, C.J.; Sause, W.; Okunieff, P.; Buckner, J.; et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.A.; Prados, M.; Lamborn, K.R.; Smith, V.; Sneed, P.K.; Chang, S.; Nicholas, K.M.; Wara, W.M.; Devriendt, D.; Kunwar, S.; et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1397–1404. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Tosoni, A.; Cavallo, G.; Bertorelle, R.; Gioia, V.; Franceschi, E.; Biscuola, M.; Blatt, V.; Crinò, L.; Ermani, M. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: Phase II study from Gruppo Italiano Cooperativo Di Neuro-Oncologia (GICNO). Br. J. Cancer 2006, 95, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Valerio, J.E.; Ochoa, S.; Alvarez, S.; Borro, M.; Alvarez-Pinzon, A.M. 5-Aminolevulinic Acid-A Biomarker for Worse Prognosis in IDH-Wildtype II Tumors? Evolution of a Fluorescence-Positive Diffuse Astrocytoma: A Case Report. J. Neurol. Surg. Rep. 2022, 83, e95–e99. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef]
- Sneed, P.K.; McDermott, M.W.; Gutin, P.H. Interstitial brachytherapy procedures for brain tumors. Semin. Surg. Oncol. 1997, 13, 157–166. [Google Scholar] [CrossRef]
- Tselis, N.; Kolotas, C.; Birn, G.; Röddiger, S.; Filipowicz, I.; Kontova, M.; Fountzilas, G.; Selviaridis, P.; Baltas, D.; Heyd, R.; et al. CT-guided interstitial HDR brachytherapy for recurrent glioblastoma multiforme. Long-term results. Strahlenther. Onkol. 2007, 183, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Archavlis, E.; Tselis, N.; Birn, G.; Ulrich, P.; Baltas, D.; Zamboglou, N. Survival analysis of HDR brachytherapy versus reoperation versus temozolomide alone: A retrospective cohort analysis of recurrent glioblastoma multiforme. BMJ Open 2013, 3, e002262. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Haldbo-Classen, L.; Amidi, A.; Wu, L.M.; Lukacova, S.; Oettingen, G.V.; Gottrup, H.; Zachariae, R.; Høyer, M. Long-term cognitive dysfunction after radiation therapy for primary brain tumors. Acta Oncol. 2019, 58, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; I Laack, N.N.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.; Conti, A.; Pontoriero, A.; Ricciardi, G.K.; Tomasello, F.; De Renzis, C.; Innocenzi, G.; Esposito, V.; Cantore, G. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg. Focus. 2009, 27, E8. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Masciopinto, J.E.; Levin, A.B.; Mehta, M.P.; Rhode, B.S. Stereotactic radiosurgery for glioblastoma: A final report of 31 patients. J. Neurosurg. 1995, 82, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Oldfield, E.H.; Markesbery, W.R.; Haack, D.; Tibbs, P.A.; McCombs, P.; Chin, H.W.; Maruyama, Y.; Meacham, W.F. Reoperation for glioblastoma. J. Neurosurg. 1981, 55, 917–921. [Google Scholar] [CrossRef]
- Kondziolka, D.; Flickinger, J.C.; Bissonette, D.J.; Bozik, M.; Lunsford, L.D. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery 1997, 41, 776–785. [Google Scholar] [CrossRef]
- Fernandes, R.T.; Teixeira, G.R.; Mamere, E.C.; Bandeira, G.A.; Mamere, A.E. The 2021 World Health Organization classification of gliomas: An imaging approach. Radiol. Bras. 2023, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Vollmuth, P.; Foltyn-Dumitru, M.; Sahm, F.; Ahn, S.S.; Chang, J.H.; Kim, S.H. The 2021 WHO Classification for Gliomas and Implications on Imaging Diagnosis: Part 1-Key Points of the Fifth Edition and Summary of Imaging Findings on Adult-Type Diffuse Gliomas. J. Magn. Reson. Imaging 2023, 58, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Tesileanu, C.M.S.; Wick, W.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Erridge, S.; Vogel-baum, M.A.; Nowak, A.K.; Baurain, J.F.; et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): Second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 813–823. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).