Abstract
The present study examined the effects of foods containing lipopolysaccharides from Pantoea agglomerans (LPSp) on eye–nose allergic symptoms using a double-blind, placebo-controlled, randomized, parallel-group comparative research design. Sixty-three Japanese individuals aged 20–65 years with eye–nose allergic symptoms were included in this study and assigned to the LPS (480 μg/day)-containing food and placebo groups. Data on the subjective eye–nose allergic symptoms and antiallergic medication during the 8-week period were evaluated. The immunoglobulin E (IgE) and eosinophil counts were measured as indicators that may be correlated with allergy. No significant group differences were found in the change in eye–nose allergic symptoms from baseline. However, the LPS group showed a significantly shorter duration of antiallergic medication use and lower total antiallergic drug score than the placebo group. The corrected nasal allergy score calculated by taking into account the antiallergic drug score at week 8 was predominantly lower in the LPS group. The IgE to house dust and cedar pollen and eosinophil counts tended to be lower in the LPS group, and the total IgE and eosinophil counts were significantly lower in the LPS group at week 4. In conclusion, our results indicate that LPS-containing foods alleviate eye–nose allergic symptoms and consequently lower the use of antiallergic drugs (UMIN000049974).
1. Introduction
Allergies are classified as types I, II, III, and IV, of which the incidence of type I allergies involving IgE-type antibodies, i.e., bronchial asthma, hay fever, atopic dermatitis, urticaria, food allergy, and others, has been increasing recently. For example, the “Hay Fever Environmental Health Manual 2022” published by the Ministry of the Environment in Japan reports that hay fever occurred in 19.6% of the population in 1998, in 29.8% in 2008, and in 42.5% in 2019, an increase of almost 10% every 10 years. According to the “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020 [1],” the prevalence of hay fever may further increase in the future. Additionally, the “Japanese Consumer Affairs Agency’s 2022 Report on Research and Study Project on Food Labeling Related to Food Allergy” reported 6080 immediate food allergy cases in 2020, following an increase in the 2017 survey (4851 cases).
Regarding the increase in allergy incidence, Strachan in 1989 reported that “Over the past century, declining family size, improvements in household amenities, and higher standards of personal cleanliness have reduced the opportunity for cross infection in young families. This may have resulted in a more widespread clinical expression of atopic disease, emerging earlier in wealthier people, similar to that seen for hay fever”, which was referred to as the hygiene hypothesis [2].
Later, in 2002, Braun-Fahrländer et al.’s European epidemiological study demonstrated that children living in rural areas with a high natural exposure dose of the Gram-negative bacterial component lipopolysaccharide (LPS) had a lower incidence of hay fever and asthma than children living in sanitary urban areas, thereby proving the hygiene hypothesis [3]. This previous study revealed that a hygienic environment is correlated with the development of allergies, and that the bacterial component LPS, particularly, is key to allergy suppression.
In Inagawa et al.’s study, the intradermal administration of LPS from Pantoea agglomerans (LPSp) suppressed IgE-dependent allergies in mice [4]. Yoshioka et al. also found in their human double-blind study that foods containing acetic acid bacteria extract reduced the nasal discomfort caused by cedar pollen [5,6]. Given that acetic acid bacteria are Gram-negative bacteria, acetic acid bacteria extract contains LPS. These results indicate that LPS intake may contribute to the alleviation of type I allergic symptoms.
LPS has been known to be a substance that induces strong inflammation when infected or artificially introduced into the body [7,8,9,10,11,12,13,14,15,16,17,18,19,20]. However, it is ubiquitous in the environment and does not show toxicity by oral or dermal ingestion. Somacy-FP100, a food ingredient containing LPSp as an active ingredient, has been manufactured and marketed by Macrophi Inc. (Kagawa, Japan) since 2007, and healthy foods containing Somacy-FP100 have been marketed since then, but no damage to health has been confirmed so far.
Although the standard intake of Somacy-FP100 is 1 mg/kg/day (10 μg/kg/day as LPSp), a high safety level of 4500 mg/kg/day (45,000 μg/kg/day as LPSp), known as a Non-Observed Adverse Effect Level, has been confirmed in a 90-day repeated dose study using rats based on the Organization for Economic Cooperation and Development standards [21].
Furthermore, four double-blind human clinical trials using food products containing Somacy-FP100 have been conducted to date; all have shown no abnormal laboratory values or adverse events, thereby confirming the product’s safety [22,23,24,25].
Moreover, in a double-blind 30-patient pilot study on immunity using a food product containing Somacy-FP100 with LPSp as the active ingredient, LPSp tended to suppress the participants’ eye–nose allergic symptoms [22]. When the analysis was limited to only those with symptom onset, a statistically significant reduction in symptoms was observed in the LPSp group. This indicates that eye–nose allergies may be prevented and improved by consuming foods containing LPSp. However, to date, no human study has been conducted on a sufficient number of individuals to examine the effects of LPSp ingestion on eye–nose allergies.
Therefore, the present study was conducted to confirm whether LPS-containing foods would reduce eye–nose allergic symptoms in healthy individuals (no specific chronic disease, not receiving any kind of hospital treatment, and not interfering with daily life). To examine the effects on nasal allergic reactions, the main physical outcome was a subjective survey of eye–nose allergic symptoms and the degree of interference with daily life according to the Japanese Rhinoconjunctivitis Quality of Life (QOL) questionnaire (JRQLQ). Additionally, the intake of antiallergic drugs during the study period was monitored using a daily diary, and blood tests were performed to examine the IgE and eosinophil counts, which are known to increase during allergic episodes.
2. Materials and Methods
2.1. Test Foods and Intake
Tablets containing Somacy-FP100 (Macrophi Inc., Kagawa, Japan) with LPSp as the active ingredient and placebo tablets with Somacy-FP100 replaced by dextrin (Matsutani Chemical Industry Co., Ltd., Hyogo, Japan) were used as the test foods. Both the LPS and placebo foods were manufactured by Umeken Co., Ltd. (Osaka, Japan) and were identical in taste, appearance, and nutritional composition. Table 1 shows the nutritional composition of the LPS and placebo foods. The actual LPS tablet contained 240 μg (actual value) LPSp.

Table 1.
Nutritional composition of test foods.
2.2. Participants
The participants were recruited by notifying the registered monitors of the Non-profit Organization Innate Immune Network and through public announcements (posters or newspapers).
The inclusion criteria were as follows: Japanese healthy individuals aged between 20 and 65 years, who have no specific chronic diseases, are not receiving any kind of treatment at a hospital and are healthy enough not to have any problems in their daily lives. Individuals whose eye–nose allergic symptoms by JRQLQ were 2–10 scores or the specific IgE for both house dust and cedar pollens was ≥0.35 UA/mL and who had not used any antiallergic drugs for 4 weeks prior to the start of the study were screened.
The exclusion criteria were as follows: (1) patients taking medications or visiting the hospital for any disease; (2) under the supervision of a physician, exercising, or on a diet; (3) may be allergic to any of the tested foods; (4) pregnant or lactating women; (5) smokers; (6) using drugs (antibiotics, immunosuppressive drugs, anti-inflammatory drugs, antirheumatic drugs, antihistamines, antiallergic drugs, lactic acid bacteria preparations, and so on) that may affect study participation; (7) unable to stop eating foods that may affect study participation (functional foods that may affect immune function or eye–nose discomfort); (8) participating in another clinical trial; (9) donated at least 400 mL of blood at least 3 months prior to the date of consent or at least 200 mL of blood at least 1 month prior to the date of consent; and (10) persons who have been determined by the principal investigator to be ineligible to participate in this study.
Based on a previous preliminary pilot study’s results, we calculated a reasonable number of participants for a statistical study of eye–nose allergic symptoms using G*Power software (Ver. 3.1.9.6. Universität Düsseldorf, Düsseldorf, Germany), which was 54. Assuming a 10% dropout rate, testing with 60 participants was thought to enable the verification of efficacy. Therefore, the study targeted a total of 60 participants in the placebo and LPS groups. The final number of participants was 63 (32 in the placebo group and 31 in the LPS group). Of the 63 subjects, 47 started during January and 16 started in February. There were no significant differences regarding the number of people in the two groups by month of start.
2.3. Test Design
The present investigation was conducted as a single-center, double-blind, randomized, placebo-controlled, parallel-group study from January to April 2023 at the Non-Profit Organization Innate Immune Network, a clinical trial organization (Kagawa, Japan). The participants were randomly assigned to the following two groups using Mujinwari (IRUKA System K.K, Tokyo, Japan) to ensure no significant differences between the groups in terms of sex and age: an LPS-containing food group (LPS group) and a placebo group. Both groups were coded as Groups A and B. The allocation information was blinded to all participants, intervention providers, and analysis providers from the completion of allocation to the end of the analysis of the study results. This allocation was performed by the registration allocation manager. During the study period, the participants took 2 tablets per day (for the LPS group, daily intake of 480 μg LPS) for 8 weeks continuously. The intake or non-intake of the test food was noted in the daily diary. The timing of the test food intake was basically after breakfast, but if the participants forgot to consume it after breakfast, they were asked to consume it at other times and note this in their daily diary. Participants were asked to maintain their previous diet, drinking, and exercise habits as much as possible during the study period and to note in their daily diary whether or not they differed from their normal routine. Participants were not restricted from taking their medications and were asked to note in their daily diary if they used any medications.
No follow-up was conducted after the study was completed.
2.4. Assessment of Subjective Symptoms of Eye–Nose Allergy
As the primary endpoints, the following questionnaires were administered and evaluated according to the JRQLQ before, 4 weeks after, and 8 weeks after intake of the test foods: (1) nasal (watery, sneezing, stuffy, and itchy nose) and eye (itchy and watery eyes) symptoms were rated on a 5-point scale (0: no symptom, 1: mild, 2: somewhat severe, 3: severe, 4: very severe) based on the most severe symptoms in the last 1 to 2 weeks; (2) in the QOL questionnaire, 17 items were rated on a 5-point scale (0: none, 1: mild, 2: somewhat severe, 3: severe, 4: very severe); and (3) the summative state was evaluated on a 5-point scale (0: fine, 1: mild, 2: somewhat severe, 3: severe, 4: very severe) on the Face Scale (cartoons of five different facial expressions, each representing a condition including symptoms, life, and feelings) for the participant’s summative condition (including symptoms, life, and feelings) in the last 1–2 weeks.
2.5. IgE and Eosinophil Counts
As secondary endpoints, total and specific IgE (to house dust and cedar pollen) were measured using a Fluorescence Enzyme Immunoassay, and eosinophil was counted using flow cytometry under at least 12 h of fasting before, 4 weeks after, and 8 weeks after the consumption of the test food. The measurements were contracted to Shikoku Chuken Inc. (Kagawa, Japan).
2.6. Questionnaire Survey on Daily Life and Medication (Daily Diary)
The participants were asked to keep a daily diary throughout the study period to confirm their intake of test foods, body temperature, bowel movements, unusual diet, alcohol consumption, exercise, presence and degree of symptoms of cold/influenza/new coronavirus infection, and special notes (e.g., taking unusual medications, influenza/new coronavirus vaccination).
Antiallergic drugs were scored (drug score) according to the method used for nasal allergy diagnosis and treatment, based on the “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020” [1] published by the Japan Society of Immunology Allergology and Infection in Otorhinolaryngology. Additionally, the corrected nasal allergy score (similar to the “symptom-drug score” in the “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020” [1]), which is the nasal allergy score (change from baseline) plus drug score, was also evaluated.
2.7. Safety Assessment (Hematology and Blood Chemistry Tests)
Hematological (white blood cell count, red blood cell count, hemoglobin, hematocrit, platelet count, neutrophil count, lymphocyte count, monocyte count, eosinophil count, and basophil count) and blood biochemical (AST, ALT, creatinine, and CRP) tests were performed before and 4 and 8 weeks after the intake of test foods under at least 12 h of fasting. The measurements were outsourced to Shikoku Chuken Inc.
2.8. Statistical Analysis
The total score of the eye–nose allergic symptoms and each sub-score were compared between the two groups, as well as the change in IgE and eosinophil counts. For the antiallergic medication during the study period, the number of people taking the medication, number of days on the medication, drug score, and total corrected nasal allergy score were compared between the two groups. For safety evaluation, the values at baseline and at 8 weeks after intake were compared within counties.
The data analysts were blinded, and the analyses were conducted using BellCurve for Excel (ver. 4.05, Social survey research information), with Mann–Whitney’s U test for comparison between the two groups and Wilcoxon’s signed rank test for comparison within groups. The participants’ basic characteristics are shown as mean ± standard deviation (SD), whereas the other test results are shown as mean ± standard error (SE). A p-value of <5% is considered to indicate a significant difference.
2.9. Pollen Dispersal Status
Pollen dispersal conditions in Kagawa Prefecture, the test site, measured using the Durham method [26], which measures the number of pollens falling per 1 cm2, by Kagawa University, were referred.
3. Results
3.1. Participants’ Basic Characteristics
Testing was conducted from 16 January 2023 to 22 April 2023. Sixty-three individuals (17 men and 46 women, mean age: 46.4 ± 9.8 years) participated in this study.
The flow of participant selection for the analysis is shown in Figure 1, and the participants’ basic characteristics are presented in Table 2. The number of study participants was 32 and 31 in the placebo and LPS groups, respectively. The present investigation was conducted in compliance with the study plan. LPS intake status was verified by the daily diary. Of the 63 participants, 62 had an intake rate of 90% or higher (including 43 with 100% intake), and only 1 had an intake rate of 73.2%. There were no reported adverse events or dropouts during the study period, and 63 patients were included in the intention-to-treat (ITT) analysis. However, a per protocol set (PPS) analysis was also performed, which excluded six participants with factors possibly affecting the study results (three participants with cold symptoms for >10 days during the study period, one participant who consumed <80% of the test food, one participant who lost the daily diary for the first half of the study period, and one participant whose survey date was ≥7 days behind schedule). In the PPS, the number of participants was 29 and 28 in the placebo and LPS groups, respectively.
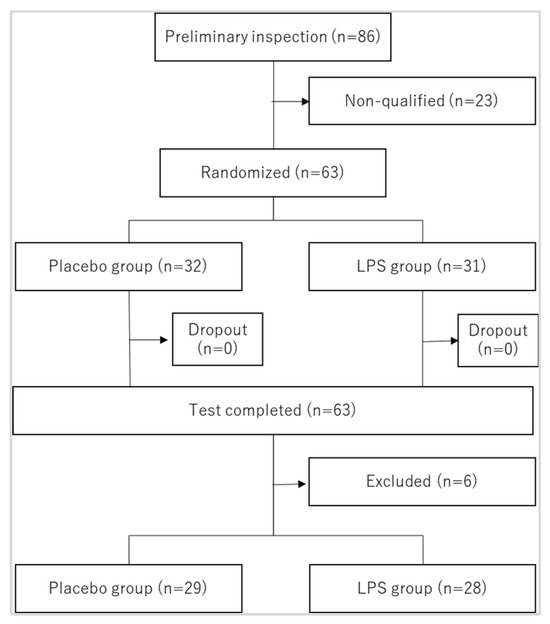
Figure 1.
Flow of subject selection. Excluded persons were as follows: (1) those who had cold symptoms for more than 10 days during the study period; (2) those whose intake rate of the test food was less than 80%; (3) those who lost the logbook for the first half of the study period; (4) those whose survey date deviated from the schedule by more than 7 days.

Table 2.
Participants’ basic characteristics.
There were no significant differences in sex or age between the two groups. There were also no significant differences in the overall nasal symptom score at study entry, IgE to house dust, or IgE to cedar pollen between the two groups.
3.2. Eye–Nose Allergic Symptoms
Changes in eye–nose allergic symptoms, QOL, and summative status score from baseline by JRQLQ and those after 4 and 8 weeks of intake are shown in Table 3. Figure 2 shows the scattering of pollen in Kagawa Prefecture, the test site, during the study period. Cedar and cypress pollens were prominent in early and late March, respectively. Correspondingly, the eye–nose allergy scores increased in most participants from baseline [Table 3]. The ITT analysis of all eye–nose allergic symptoms, overall QOL, and summative status at 4 and 8 weeks after the start of test food intake, which surveyed the status over the past 1–2 weeks, showed no statistically significant differences between the placebo and LPS groups. There were no statistically significant differences when the eye and nasal allergic symptoms were analyzed separately. This result was also the same for the PPS analysis.

Table 3.
Eye–nose allergy scores, QOL, and summative status score.
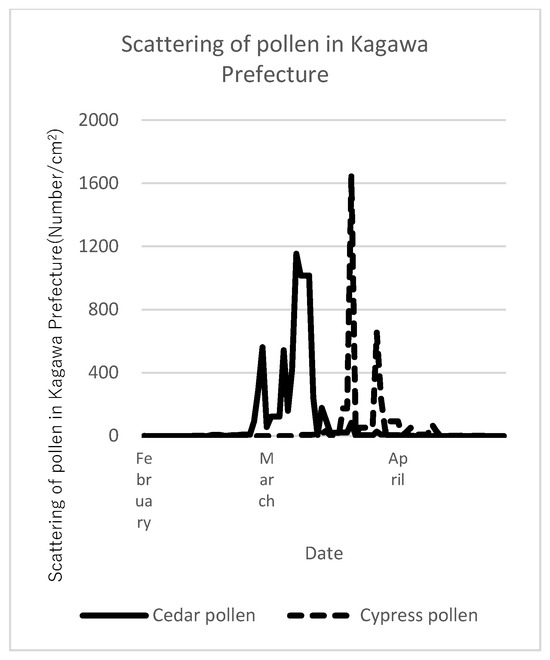
Figure 2.
Scattering of pollen during the study period. Pollen dispersal conditions in Kagawa Prefecture (the test site) measured by Kagawa University.
The analysis of nasal allergic symptoms was stratified by age >/= 40 years, sex, body mass index >/= 25 kg/m2, IgE score at screening >/= 10 UA/mL (mean IgE to cedar pollen of subjects at screening was 10.39), nasal allergy score at screening >/= 5 (mean score of participants at screening was 5.02), and study start date. No statistically significant difference in the overall eye–nose allergy score was found between the placebo and LPS groups. This result was also the same for the PPS analysis.
3.3. Analysis of Eye–Nose Allergic Symptoms Considering the Use of Antiallergic Drugs
In this study, the condition for participation was the absence of the use of antiallergic drugs in the 4 weeks prior to the start of the study, but the use of antiallergic drugs during the study period was not restricted. Thus, although there was no significant difference between the two groups, in terms of eye–nasal allergy symptoms after the start of pollen dispersal, the effect of antiallergic medications should be taken into account (Table 3).
In the ITT analysis, among the individuals analyzed, 16 and 6 participants in the placebo and LPS groups, respectively, used any kind of antiallergic medication during the study period (Table 4). The number of days on antiallergic medications during the study period was significantly lower in the LPS group than in the placebo group (Figure 3A). This result was also the same for the PPS analysis.

Table 4.
Number of people taking antiallergic medication.
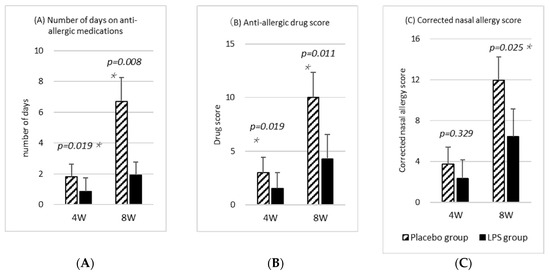
Figure 3.
Antiallergic medication. (A) Number of days on antiallergic medication. (B) Drug score. Antiallergic drugs were scored based on “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020“, published by the Japan Society of Immunology Allergology and Infection in Otorhinolaryngology. (C) Corrected allergy sore. The corrected nasal allergy score was calculated by adding the antiallergic drug score to the nasal allergy score, based on the “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020” [1]. Statistical comparisons between the placebo and LPS groups were made using the Mann–Whitney’s U test. Results are shown as mean ± standard error (SE), and p-value of <5% is considered a significant difference. Asterisks (*) indicate significant difference.
Additionally, Figure 3B shows the drug scores. The medications were scored according to the “Practical Guideline for the Management of Allergic Rhinitis in Japan 2020” [1] as follows: (1) steroid-free nasal drops: 1 point; (2) steroid-free eye drops: 1 point; (3) oral antihistamines and release inhibitors: 1 point; (4) steroid-containing nasal drops: 2 points; (5) steroid-containing eye drops: 2 points; and (6) oral steroid-containing drugs: 3 points. The drug score was the sum of the different or similarly effective drugs used in a day. The results showed that the LPS group had significantly lower scores than the placebo group throughout the study period (Figure 3B). This result was also the same for the PPS analysis.
For the nasal allergic symptoms, Figure 3C shows the corrected nasal allergy score, which is the change in the symptom score relative to baseline plus the drug score. Given that the corrected nasal allergy score is used in the diagnostic treatment of nasal allergy, the scores for the four types of nasal allergic symptoms among the six types of eye–nose allergic symptoms were used (Table 3). The corrected nasal allergy score at week 4 was calculated by adding the drug score from the start of intake to week 4 to the nasal allergy score change at week 4, and the corrected nasal allergy score at week 8 was calculated by adding the drug score from week 5 to week 8 to the nasal allergy score change at week 8. Given that none of the participants had used medication in the 4 weeks prior to the study, the drug score added to the pre-intake nasal allergy score was set to zero. The corrected nasal allergy score, which takes into account the effect of antiallergic drugs, was significantly lower in the LPS group at week 8 [Figure 3C]. This result was also the same for the PPS analysis. Furthermore, ITT analysis of the corrected allergy score, which was calculated using the eye–nose allergy score instead of the nasal allergy score and adding the drug score, also showed that the LPS group had a significantly lower score at week 8 (p = 0.045).
3.4. Changes in the IgE and Eosinophil Counts
Figure 4 shows the changes in the IgE and eosinophil counts, which were correlated with the allergic symptoms, for 4 and 8 weeks after the start of intake compared to the baseline values. The IgE to house dust, IgE to cedar pollen, and eosinophil counts tended to be lower in the LPS group than in the placebo group throughout the study period, whereas the total IgE and eosinophil counts were significantly lower in the LPS group at 4 weeks after the start of test food intake.
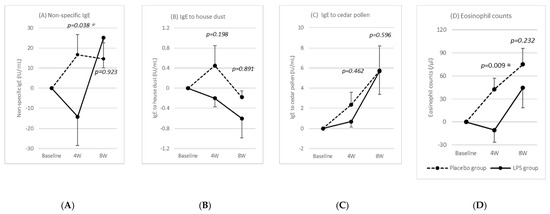
Figure 4.
Changes in IgE and eosinophil counts. Values are the change from baseline. Statistical comparisons between the placebo and LPS groups were made using the Mann–Whitney’s U test. Results are shown as mean ± standard error (SE), and p-value of <5% is considered a significant difference. Asterisks (*) indicate significant difference.
3.5. Safety Evaluation
No adverse events were reported during the study period, and none of the participants dropped out. The changes in blood test values at baseline and after 8 weeks of intake are shown in Table 5. The results of all hematology and blood biochemistry tests showed variations within the reference values in both the placebo and LPS groups. This result was also the same for the PPS analysis. Continuous consumption of LPS-containing foods for 8 weeks was considered safe by the principal investigator.

Table 5.
Changes in blood test values.
4. Discussion
The purpose of this study was to investigate the usefulness of LPS as a food material. Therefore, the study was conducted using the method of ingestion as a food. The present 8-week, parallel-group, randomized, double-blind study on eye–nose allergic symptoms, antiallergic medication use, and changes in IgE and eosinophil counts was conducted in healthy participants (no specific chronic disease, not receiving any kind of hospital treatment, and not interfering with daily life) to examine the effect of LPS intake on eye–nose allergic symptoms. No adverse events, dropouts, or changes in hematology and blood biochemistry test results were observed in this study.
We previously investigated the effects of LPS-containing foods on the immune system in a 30-patient pilot study. In this pilot study, we examined eye–nose allergic symptoms. Given that this pilot study did not screen for eye–nose allergic symptoms, the actual incidence of eye–nose allergic symptoms was 37% in the LPS and placebo groups combined. When analyzing only the data of patients with the onset of symptoms, the LPS group showed a statistically significant reduction in symptom severity [22]. Therefore, in the present study, the participants whose eye–nose allergic symptoms by JRQLQ were 2–10 scores or the specific IgE for both house dust and cedar pollens was ≥0.35 UA/mL were screened. The JRQLQ is an eye–nose allergy questionnaire commonly used in Japan and recommended by nasal allergy practice guidelines as an assessment of quality of life. The final number of participants was 32 in the placebo group and 31 in the LPS group.
Our food test was conducted from mid-January to late April, which is the same time period as the pilot study. During this period, the scattering of cedar and cypress pollens became more pronounced from early to late March, and the participants’ eye–nose allergic symptoms also worsened.
The analysis of eye–nose allergic symptoms investigated at 4 and 8 weeks after intake showed no significant differences between the LPS and placebo groups. As previously mentioned, in previous pilot studies, the LPS group was significantly less symptomatic than the placebo group when the analysis was restricted to those who developed symptoms. Contrarily, in the present study, we screened for allergies, and, although the incidence of eye–nose allergic symptoms during the study period was 100%, there was no significant difference between the two groups in terms of symptoms. This difference may be due to the fact that the questionnaire items were not exactly the same between the preliminary study and the present study, as well as the fact that the previous pilot study used a method of recording symptoms on a daily basis.
On the other hand, in a comparison of the number of days the two groups used antiallergic drugs, the LPS group showed a significantly shorter duration than the placebo group. This suggests that the participants used antiallergic drugs according to their symptoms to control their eye–nose allergic symptoms, and as a result, no differences in symptoms may have been observed. In fact, the corrected nasal allergy score, calculated by adding “the nasal allergy score” and “the anti-allergic drug score measured and tabulated according to the type of active ingredient,” a method used in the diagnostic treatment of nasal allergy, was significantly lower in the LPS group, suggesting that the oral intake of LPS at least reduces nasal allergic symptoms.
Additionally, we investigated the IgE and eosinophil counts, which are thought to be correlated with allergic symptoms. With the exception of IgE to house dust, total IgE, IgE to cedar pollen, and eosinophil counts increased as the pollen dispersal increased. However, the IgE to house dust and cedar pollen and eosinophil counts tended to be lower in the LPS group than in the placebo group throughout the study period, with the LPS group showing significantly lower total IgE and eosinophil counts at week 4. This suggests that LPS may inhibit IgE production and act as an anti-inflammatory agent. However, the correlation between LPS intake and fluctuations in IgE and eosinophils needs further confirmation.
LPS, also known as endotoxin, has not been shown to be toxic orally or dermally [27,28,29]. Moreover, the oral intake of LPS was considered a factor in suppressing allergies, with their incidence increasing in recent years [3,30,31,32,33,34,35].
In hay fever, as a typical example of type I allergy, contact with or inhalation of pollen causes rhinitis, such as sneezing, runny nose, and stuffy nose, and eye symptoms, such as itching and running tears. This occurs when specific IgE antibodies against pollen antigens are produced by B cells and bind to mast cells, and, when pollen antigens bind to the IgE antibodies on the mast cells, chemical transmitters, including histamine and leukotrienes, are released from the mast cells, which stimulate the nerves and blood vessels.
The mechanism by which orally ingested LPS suppresses allergies is not yet clear, but the following possibilities can be considered. A possible mechanism of LPS suppression of IgE antibody-dependent type I allergy is the normalization of Th1/Th2 balance [27]. Helper T cells (Th cells) differentiate into Th1 or Th2 cells in response to stimulation with different types of cytokines. Th1 cells induce cellular immunity, whereas Th2 cells induce liquid immunity, particularly inducing B cells to produce IgE antibodies [36,37,38,39]. Th1 and Th2 cells suppress each other and maintain a balance, but in type I allergies, Th2 cells become dominant for some reason and overreact to antigens that should not attack them [40,41,42,43,44,45,46,47]. LPS binds to the LPS receptor “Toll-like receptor 4 (TLR4)” expressed on macrophages, dendritic cells, intestinal epithelial cells, and other cells in the mucosa and causes them to secrete cytokines (IL12 and so on) that promote Th1 differentiation, thereby returning Th2 dominance to a normal state with Th1/Th2 balance and possibly improving allergic symptoms [48].
Another possibility is the activation of regulatory T cells (Treg cells) by LPS. Treg cells directly inhibit allergen-specific Th2 cell activation, minimize the production of Th2-promoting cytokines IL-4, IL-5, IL-13, and IL-9, and suppress allergic inflammation through direct effects on mast cells, basophils, and eosinophils [49,50]. These Treg cells express TLR4, reportedly stimulated by LPS proliferate, and further enhance their anti-inflammatory function [51,52,53]. Thus, LPS may contribute to the alleviation of allergic symptoms through the activation of Treg cells.
Although Braun-Fahrländer et al. have shown that the intake of LPS during early childhood reduces the subsequent development of atopic allergic disease [3], the present results suggest that LPS intake may also be effective in alleviating allergies in adults. Braun-Fahrländer et al. mentioned that early childhood LPS exposure may lead to immune tolerance to LPS and subsequent suppression of the development of acquired immunity. However, since orally ingested LPS is not directly absorbed by the body, the immune tolerance observed in vitro in innate immune cells to high concentrations of LPS stimulation cannot be directly applied to orally administered LPS. Even though LPS intake may affect the development of acquired immunity in early childhood, the suppression of allergic symptoms in adults in the present study suggests that LPS is involved in regulating acquired immunity.
The limitations of the present study include the lack of significant differences in eye–nose allergic symptoms, with respect to the amount of change relative to the start of food intake. However, significant differences were observed in the number of days on antiallergic medications and drug scores at weeks 4 and 8 and in the corrected nasal allergy score at week 8. Significant differences in total IgE and eosinophil counts were also observed at week 4, although these were not significant at week 8.
The study began 1 month before the start of pollen dispersal. However, the start of intake of the test foods differed by up to 1 month among the participants. Therefore, the amount of pollen in the air and the corresponding symptoms during the period when the questionnaires were filled out (the 4th and 8th week after the start of the intake) varied per person. This may have led to a lack of significant differences in eye–nose allergic symptoms. In future trials, it may be important to align the timing of trial starts as much as possible.
In the past, human studies on LPS-containing foods have established a daily intake of 400–600 µg LPS. In the current study, the measured intake was also 480 μg. In all cases, positive effects of LPS have been observed, but further investigation is needed to determine if this amount is adequate to fully demonstrate the efficacy of LPS. The timing and duration of LPS intake to be effective may also be worth considering.
Regarding the incidence of allergic diseases, according to the “Rheumatism and Allergy Task Force Report” issued by the Ministry of Health, Labour, and Welfare in Japan as of 2011, "Approximately one in two people in the total population of Japan experience some form of allergic disease, and the number of such cases is rapidly increasing. " More people can be expected to experience eye and nasal allergic symptoms that may not fall into the realm of disease, but which may cause a seasonal decline in QOL. Although antiallergic drugs temporarily suppress symptoms, they can induce drowsiness as a side effect; thus, safe foods that contribute to the alleviation of allergic symptoms are meaningful. In the future, the development of food products containing LPS that have allergy-relieving effects is expected.
5. Conclusions
The oral intake of LPS decreased the dose of antiallergic medication for eye–nose allergic symptoms. The fact that there was no difference in eye–nose allergic symptoms despite the decrease in the dose of antiallergic medication is thought to be the result of LPS alleviating eye–nose allergic symptoms, suggesting that the oral administration of LPS has an alleviating effect on eye–nose allergic symptoms.
Author Contributions
G.-I.S., H.I., and C.K. conceived and designed the test. M.U. analyzed the data. T.F. and N.W. provided advice on the study design and statistical analysis. C.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was not externally funded and was conducted at the expense of Macrophi Inc.
Institutional Review Board Statement
This study was conducted in compliance with the Declaration of Helsinki (1964) and the Ethical Guidelines for Life Science and Medical Research Involving Human Subjects (23 March 2021) by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labour, and Welfare, and the Ministry of Economy, Trade and Industry. The present study was approved by the Ethics Committee of the Non-Profit Organization Innate Immune Network (approval number LSIN 2022-001, approval date November 28, 2022). The study protocol was pre-registered in the clinical trial registration system (UMIN-CTR: UMIN000049974).
Informed Consent Statement
The study protocol was fully explained to the participants, and written informed consent was obtained from them. Written informed consent for the publication of this paper was also obtained from the participants.
Data Availability Statement
Data are available from the corresponding author upon request. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The cooperation of all those who participated in this study is gratefully acknowledged. This study was supported by the Non-Profit Organization Innate Immune Network and the Medical Corporation Natsumekai Bijutsukan Clinic (Kagawa, Japan).
Conflicts of Interest
Soma, Inagawa, Uehiro, and Kohchi are affiliated with Macrophi Inc. Fukaya and Watanabe are affiliated with Tashikani-plus. Co. Ltd. All authors declare no competing interests.
References
- Asako, M.; Okubo, K.; Ota, N.; Okano, M.; Kamijo, A.; Goto, M.; Sakasita, M.; Sakurai, D.; Terada, T.; Nakamaru, Y.; et al. Practical Guideline for the Management of Allergic Rhinitis in Japan, 9th ed.; Life Scirence: Tokyo, Japan, 2020. [Google Scholar]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Braun-Fahrlander, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L.; Maisch, S.; Carr, D.; Gerlach, F.; Bufe, A.; et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002, 347, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, H.; Nishizawa, T.; Takagi, T.; Mizuno, D.-I.; Soma, G.-I. Protective effect by intradermal administration of Pantoea agglomerans LPS (LPSp) and oral administration of ONO-4007. Biotherapy 1997, 11, 464–466. [Google Scholar]
- Yoshioka, S.; Oe, M.; Kamijyo, F.; Shimada, K.; Okuyama, Y.; Nishiyama, H.; Matsuoka, R.; Masuda, Y.; Kanemitsu, T.; Enomoto, M. Acetic Acid Bacteria (Gluconacetobacter hansenii GK-1) Relieves Nasal Discomforts—A Randomized Double-blinded Placebo-controlled Study. Jpn. Pharmacol. Ther. 2019, 47, 461–467. [Google Scholar]
- Kamijyo, F.; Oe, M.; Yoshioka, S.; Kajiyama, D.; Shimada, K.; Okuyama, Y.; Matsuoka, R.; Masuda, Y.; Kanemitsu, T.; Enomoto, M. Acetic Acid Bacteria (Gluconacetobacter hansenii GK-1) Relieves Nasal Discomforts of Japanese Cedar Pollimpsis. Jpn. Pharmacol. Ther. 2019, 47, 1993–1999. [Google Scholar]
- Brigham, K.L.; Meyrick, B. Endotoxin and lung injury. Am. Rev. Respir. Dis. 1986, 133, 913–927. [Google Scholar] [PubMed]
- Daly, C.G.; Seymour, G.J.; Kieser, J.B. Bacterial endotoxin: A role in chronic inflammatory periodontal disease? J. Oral. Pathol. 1980, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Worgul, B.V.; Merriam, G.R., Jr. Endotoxin-induced uveitis in the rat. Albrecht Von. Graefes Arch. Klin. Exp. Ophthalmol. 1980, 213, 221–233. [Google Scholar] [CrossRef]
- Herrmann, E.C., Jr.; Engle, C.; Perlman, P.L. Action of antiinflammatory agents on endotoxin-induced lung inflammation in mice. Am. J. Physiol. 1959, 197, 803–807. [Google Scholar] [CrossRef]
- Hopkins, R.W.; Damewood, C.A. Septic shock: Hemodynamics of endotoxin and inflammation. Am. J. Surg. 1974, 127, 476–483. [Google Scholar] [CrossRef]
- Howes, E.L.; Cruse, V.K.; Kwok, M.T. Mononuclear cells in the corneal response to endotoxin. Investig. Ophthalmol. Vis. Sci. 1982, 22, 494–501. [Google Scholar]
- Issekutz, A.C.; Bhimji, S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect. Immun. 1982, 36, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Issekutz, A.C.; Megyeri, P.; Issekutz, T.B. Role for macrophage products in endotoxin-induced polymorphonuclear leukocyte accumulation during inflammation. Lab. Investig. 1987, 56, 49–59. [Google Scholar]
- Jacobs, D.R.; Cohen, H.B. The inflammatory role of endotoxin in rabbit gram-negative bacterial endophthalmitis. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1074–1079. [Google Scholar]
- Meyrick, B.; Brigham, K.L. Repeated Escherichia coli endotoxin-induced pulmonary inflammation causes chronic pulmonary hypertension in sheep. Structural and functional changes. Lab. Investig. 1986, 55, 164–176. [Google Scholar] [PubMed]
- Movat, H.Z.; Cybulsky, M.I.; Colditz, I.G.; Chan, M.K.; Dinarello, C.A. Acute inflammation in gram-negative infection: Endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed. Proc. 1987, 46, 97–104. [Google Scholar]
- Pitts, D.L.; Williams, B.L.; Morton, T.H., Jr. Investigation of the role of endotoxin in periapical inflammation. J. Endod. 1982, 8, 10–18. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Raymond, W. Monocyte chemotactic activity induced by intravitreal endotoxin. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1267–1273. [Google Scholar]
- Young, R.S.; Yagel, S.K.; Towfighi, J. Systemic and neuropathologic effects of E. coli endotoxin in neonatal dogs. Pediatr. Res. 1983, 17, 349–353. [Google Scholar] [CrossRef]
- Phipps, K.R.; Sulaiman, C.; Simon, R.; Holalagoudar, S.; Kohchi, C.; Nakata, Y. Subchronic (90-day) toxicity assessment of Somacy-FP100, a lipopolysaccharide-containing fermented wheat flour extract from Pantoea agglomerans. J. Appl. Toxicol. 2020, 40, 1342–1352. [Google Scholar] [CrossRef]
- Kohchi, C.; Uehiro, M.; Yamashita, M.; Inagawa, H.; Soma, G.-I. Foods Containing Pantoea agglomerans LPS Reduce Eye-Nose Allergies—A Double-Blind, Placebo-Controlled, Randomized, Parallel-Group Comparative Pilot Study. Int. J. Transl. Med. 2023, 3, 299–309. [Google Scholar] [CrossRef]
- Nakata, K.; Nakata, Y.; Inagawa, H.; Nakamoto, T.; Yoshimura, H.; Soma, G. Pantoea agglomerans lipopolysaccharide maintains bone density in premenopausal women: A randomized, double-blind, placebo-controlled trial. Food Sci. Nutr. 2014, 2, 638–646. [Google Scholar] [CrossRef]
- Nakata, K.; Taniguchi, Y.; Yoshioka, N.; Yoshida, A.; Inagawa, H.; Nakamoto, T.; Yoshimura, H.; Miyake, S.; Kohchi, C.; Kuroki, M.; et al. A mixture of Salacia oblonga extract and IP-PA1 reduces fasting plasma glucose (FPG) and low-density lipoprotein (LDL) cholesterol levels. Nutr. Res. Pract. 2011, 5, 435–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakata, Y.; Kohchi, C.; Ogawa, K.; Nakamoto, T.; Yoshimura, H.; Soma, G.I. Effects of 3 months continuous intake of supplement containing Pantoea agglomerans LPS to maintain normal bloodstream in adults: Parallel double-blind randomized controlled study. Food Sci. Nutr. 2018, 6, 197–206. [Google Scholar] [CrossRef]
- Durham, O.C. The volumetric incidence of atmospheric allergens; a proposed standard method of gravity sampling, counting, and volumetric interpolation of results. J. Allergy 1946, 17, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Yamaguchi, T.; Nagai, S.; Soma, G. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J. Biosci. Bioeng. 2006, 102, 485–496. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, N.; Nishizawa, T.; Inagawa, H.; Kohchi, C.; Soma, G. Utility and safety of LPS-based fermented flour extract as a macrophage activator. Anticancer Res. 2009, 29, 859–864. [Google Scholar]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]
- Chung, S.H.; Choi, S.H.; Cho, K.J.; Joo, C.K. Toll-like receptor 4 signalling attenuates experimental allergic conjunctivitis. Clin. Exp. Immunol. 2011, 164, 275–281. [Google Scholar] [CrossRef]
- Debarry, J.; Garn, H.; Hanuszkiewicz, A.; Dickgreber, N.; Blumer, N.; von Mutius, E.; Bufe, A.; Gatermann, S.; Renz, H.; Holst, O.; et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J. Allergy Clin. Immunol. 2007, 119, 1514–1521. [Google Scholar] [CrossRef]
- Looringh van Beeck, F.A.; Hoekstra, H.; Brunekreef, B.; Willemse, T. Inverse association between endotoxin exposure and canine atopic dermatitis. Vet. J. 2011, 190, 215–219. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Willart, M.A.; Vergote, K.; Gras, D.; Deswarte, K.; Ege, M.J.; Madeira, F.B.; Beyaert, R.; van Loo, G.; Bracher, F.; et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015, 349, 1106–1110. [Google Scholar] [CrossRef]
- Wang, N.; McKell, M.; Dang, A.; Yamani, A.; Waggoner, L.; Vanoni, S.; Noah, T.; Wu, D.; Kordowski, A.; Kohl, J.; et al. Lipopolysaccharide suppresses IgE-mast cell-mediated reactions. Clin. Exp. Allergy 2017, 47, 1574–1585. [Google Scholar] [CrossRef]
- Wang, Y.; McCusker, C. Neonatal exposure with LPS and/or allergen prevents experimental allergic airways disease: Development of tolerance using environmental antigens. J. Allergy Clin. Immunol. 2006, 118, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998, 3, 233–244. [Google Scholar] [CrossRef]
- Moverare, R.; Elfman, L.; Stalenheim, G.; Bjornsson, E. Study of the Th1/Th2 balance, including IL-10 production, in cultures of peripheral blood mononuclear cells from birch-pollen-allergic patients. Allergy 2000, 55, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000, 85, 9–18, quiz 18, 21. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar]
- Coyle, A.J.; Tsuyuki, S. Th2 cells and cytokine networks in allergic inflammation of the lung. Mediat. Inflamm. 1995, 4, 239–247. [Google Scholar] [CrossRef]
- Hamilos, D.L.; Leung, D.Y.; Wood, R.; Cunningham, L.; Bean, D.K.; Yasruel, Z.; Schotman, E.; Hamid, Q. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J. Allergy Clin. Immunol. 1995, 96, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Fukuda, T. Eosinophils and allergy in asthma. Allergy Proc. 1995, 16, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Bianco, A.; Catena, E.; De Palma, R.; Abbate, G.F. Th1/Th2 lymphocyte polarization in asthma. Allergy 2000, 55 (Suppl. S61), 6–9. [Google Scholar] [CrossRef] [PubMed]
- Mudde, G.C.; Bheekha, R.; Bruijnzeel-Koomen, C.A. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol. Today 1995, 16, 380–383. [Google Scholar] [CrossRef]
- Sampson, H.A. Mechanisms in adverse reactions to food. The skin. Allergy 1995, 50, 46–51. [Google Scholar] [CrossRef]
- Colavita, A.M.; Reinach, A.J.; Peters, S.P. Contributing factors to the pathobiology of asthma. The Th1/Th2 paradigm. Clin. Chest Med. 2000, 21, 263–277, viii. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, Y.; Matsuura, H.; Sato, A.; Kanzaki, H.; Katayama, H.; Arata, J. Hyperimmunoglobin E syndrome: A sign of TH1/TH2 imbalance? Eur. J. Dermatol. 1999, 9, 129–131. [Google Scholar]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef]
- Lewkowicz, N.; Klink, M.; Mycko, M.P.; Lewkowicz, P. Neutrophil--CD4+CD25+ T regulatory cell interactions: A possible new mechanism of infectious tolerance. Immunobiology 2013, 218, 455–464. [Google Scholar] [CrossRef]
- Caramalho, I.; Lopes-Carvalho, T.; Ostler, D.; Zelenay, S.; Haury, M.; Demengeot, J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003, 197, 403–411. [Google Scholar] [CrossRef]
- Lewkowicz, N.; Mycko, M.P.; Przygodzka, P.; Cwiklinska, H.; Cichalewska, M.; Matysiak, M.; Selmaj, K.; Lewkowicz, P. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016, 9, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, P.; Lewkowicz, N.; Sasiak, A.; Tchorzewski, H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J. Immunol. 2006, 177, 7155–7163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).