A Review Concerning the Use of Etravirine and Darunavir in Translational Medicine
Abstract
:1. Introduction
2. Pharmacokinetics and Physicochemical Properties
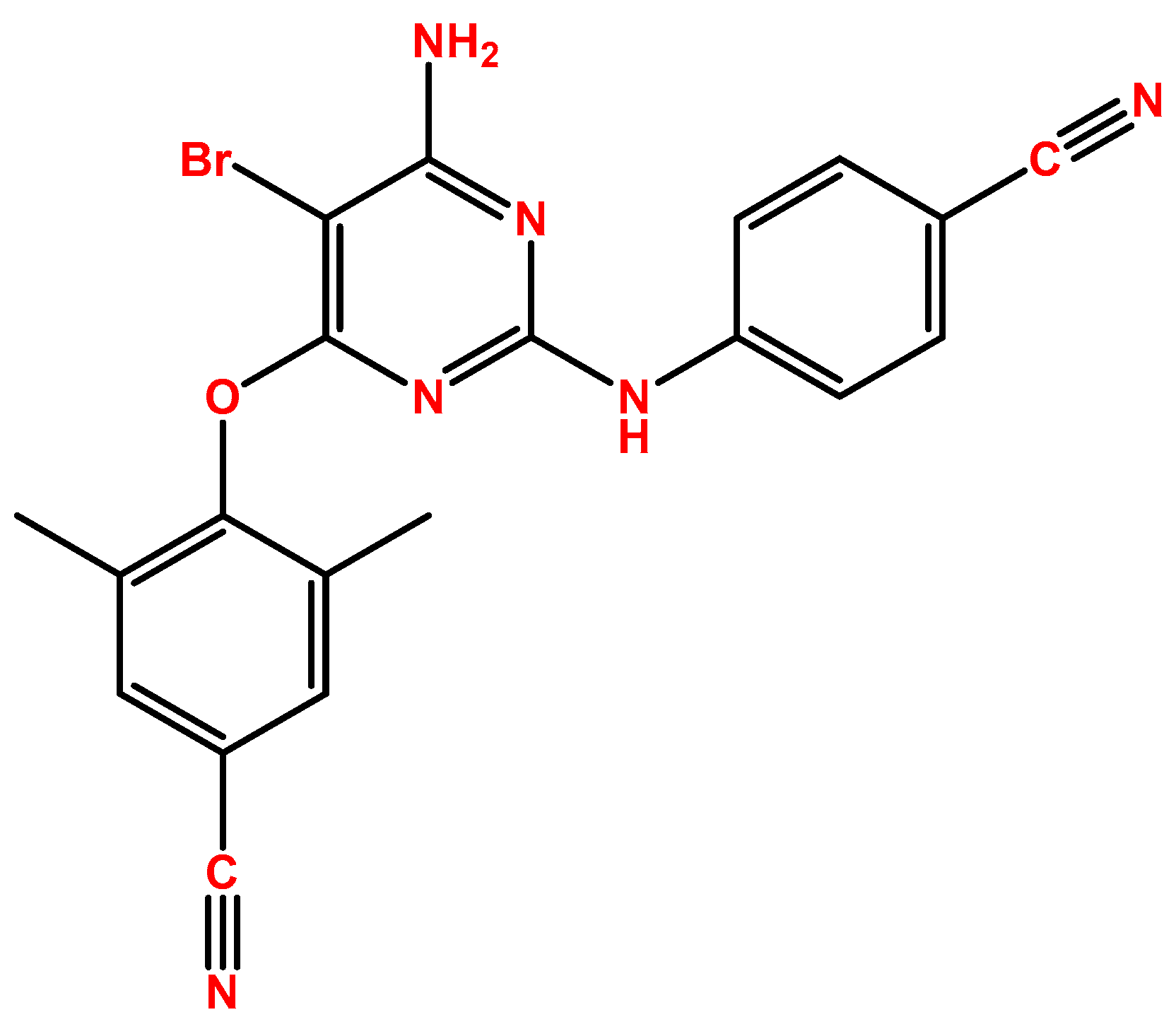
2.1. Etravirine
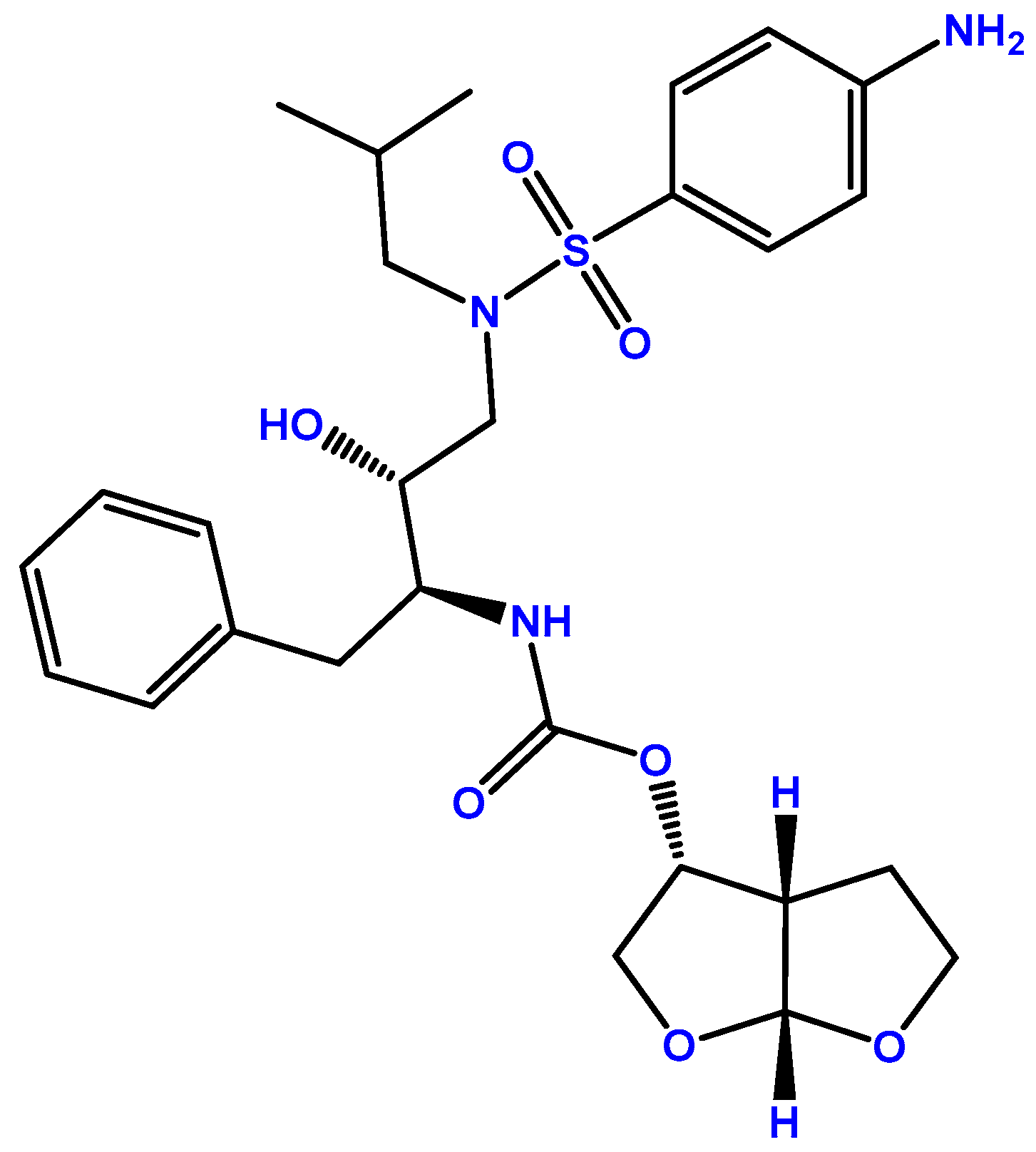
2.2. Darunavir
3. Combination of ETV and DRV
4. Repurposing of ETV and DRV
4.1. Repurposing for Other Pathogens
4.2. Repurposing for Cancer Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, S.; Nelson, A.M. HIV and the spectrum of human disease. J. Pathol. 2015, 235, 229–241. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 17 July 2023).
- Patton, L.L. HIV disease. Dent. Clin. N. Am. 2003, 47, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Fanales-Belasio, E.; Raimondo, M.; Suligoi, B.; Buttò, S. HIV virology and pathogenetic mechanisms of infection: A brief overview. Ann. Ist. Super. Sanita 2010, 46, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef]
- EMA. Intelence. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/intelence (accessed on 18 July 2023).
- FDA. INTELENCE® (Etravirine) Tablets for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022187s009lbl.pdf (accessed on 18 July 2023).
- Schöller-Gyüre, M.; Kakuda, T.N.; Raoof, A.; De Smedt, G.; Hoetelmans, R.M. Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin. Pharmacokinet. 2009, 48, 561–574. [Google Scholar] [CrossRef]
- Müller, T.G.; Zila, V.; Peters, K.; Schifferdecker, S.; Stanic, M.; Lucic, B.; Laketa, V.; Lusic, M.; Müller, B.; Kräusslich, H.G. HIV-1 uncoating by release of viral cDNA from capsid-like structures in the nucleus of infected cells. Elife 2021, 10, e64776. [Google Scholar] [CrossRef] [PubMed]
- Yanakakis, L.J.; Bumpus, N.N. Biotransformation of the antiretroviral drug etravirine: Metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab. Dispos. 2012, 40, 803–814. [Google Scholar] [CrossRef]
- Havens, J.P.; Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine: An Updated Review. Clin. Pharmacokinet. 2020, 59, 137–154. [Google Scholar] [CrossRef]
- Bank, D. Etravirine. Available online: https://go.drugbank.com/drugs/DB06414 (accessed on 18 July 2023).
- FDA. PREZISTA (Darunavir). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021976s021lbl.pdf (accessed on 4 July 2023).
- EMA. Prezista. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prezista (accessed on 19 July 2023).
- Gulnik, S.; Erickson, J.W.; Xie, D. HIV protease: Enzyme function and drug resistance. Vitam. Horm. 2000, 58, 213–256. [Google Scholar] [CrossRef]
- De Meyer, S.; Azijn, H.; Surleraux, D.; Jochmans, D.; Tahri, A.; Pauwels, R.; Wigerinck, P.; de Béthune, M.P. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 2005, 49, 2314–2321. [Google Scholar] [CrossRef]
- Vermeir, M.; Lachau-Durand, S.; Mannens, G.; Cuyckens, F.; van Hoof, B.; Raoof, A. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 2009, 37, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.W.; Montaner, J.S. Etravirine in combination with darunavir/ritonavir and optimized background regimen results in suppression of HIV replication in treatment-experienced patients. Expert Opin. Pharmacother. 2010, 11, 1433–1437. [Google Scholar] [CrossRef]
- Schöller-Gyüre, M.; Kakuda, T.N.; Sekar, V.; Woodfall, B.; De Smedt, G.; Lefebvre, E.; Peeters, M.; Hoetelmans, R.M. Pharmacokinetics of darunavir/ritonavir and TMC125 alone and coadministered in HIV-negative volunteers. Antivir. Ther. 2007, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Granneman, G.R.; Bertz, R.J. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 1998, 35, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Madruga, J.V.; Cahn, P.; Grinsztejn, B.; Haubrich, R.; Lalezari, J.; Mills, A.; Pialoux, G.; Wilkin, T.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, A.; Campbell, T.; Clotet, B.; Johnson, M.; Katlama, C.; Moll, A.; Towner, W.; Trottier, B.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 39–48. [Google Scholar] [CrossRef]
- Katlama, C.; Haubrich, R.; Lalezari, J.; Lazzarin, A.; Madruga, J.V.; Molina, J.M.; Schechter, M.; Peeters, M.; Picchio, G.; Vingerhoets, J.; et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: Pooled 48 week analysis of two randomized, controlled trials. Aids 2009, 23, 2289–2300. [Google Scholar] [CrossRef]
- Katlama, C.; Clotet, B.; Mills, A.; Trottier, B.; Molina, J.M.; Grinsztejn, B.; Towner, W.; Haubrich, R.; Nijs, S.; Vingerhoets, J.; et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir. Ther. 2010, 15, 1045–1052. [Google Scholar] [CrossRef]
- Gazzola, L.; Cicconi, P.; Ripamonti, D.; Di Filippo, E.; Gustinetti, G.; Di Biagio, A.; Marchetti, G.; Bini, T.; d’Arminio Monforte, A. Efficacy and safety of darunavir/ritonavir plus etravirine dual regimen in antiretroviral therapy-experienced patients: A multicenter clinical experience. HIV Clin. Trials 2014, 15, 140–150. [Google Scholar] [CrossRef]
- Ruane, P.J.; Brinson, C.; Ramgopal, M.; Ryan, R.; Coate, B.; Cho, M.; Kakuda, T.N.; Anderson, D. The Intelence aNd pRezista Once A Day Study (INROADS): A multicentre, single-arm, open-label study of etravirine and darunavir/ritonavir as dual therapy in HIV-1-infected early treatment-experienced subjects. HIV Med. 2015, 16, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Moltó, J.; Curran, A.; Miranda, C.; Challenger, E.; Santos, J.R.; Ribera, E.; Khoo, S.; Valle, M.; Clotet, B. Pharmacokinetics of darunavir/cobicistat and etravirine alone and co-administered in HIV-infected patients. J. Antimicrob. Chemother. 2018, 73, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, R.H.; van der Heiden, I.P.; van den Anker, J.N.; Lindemans, J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin. Chem. 2002, 48, 1668–1671. [Google Scholar] [CrossRef]
- Belkhir, L.; Elens, L.; Zech, F.; Panin, N.; Vincent, A.; Yombi, J.C.; Vandercam, B.; Haufroid, V. Interaction between Darunavir and Etravirine Is Partly Mediated by CYP3A5 Polymorphism. PLoS ONE 2016, 11, e0165631. [Google Scholar] [CrossRef]
- HIV.gov. Pre-Exposure Prophylaxis. Available online: https://www.hiv.gov/hiv-basics/hiv-prevention/using-hiv-medication-to-reduce-risk/pre-exposure-prophylaxis/ (accessed on 25 July 2023).
- Coutinho, B.; Prasad, R. Emtricitabine/tenofovir (Truvada) for HIV prophylaxis. Am. Fam. Physician 2013, 88, 535–540. [Google Scholar]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef]
- Grant, R.M.; Anderson, P.L.; McMahan, V.; Liu, A.; Amico, K.R.; Mehrotra, M.; Hosek, S.; Mosquera, C.; Casapia, M.; Montoya, O.; et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect. Dis. 2014, 14, 820–829. [Google Scholar] [CrossRef]
- Volk, J.E.; Marcus, J.L.; Phengrasamy, T.; Blechinger, D.; Nguyen, D.P.; Follansbee, S.; Hare, C.B. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin. Infect. Dis. 2015, 61, 1601–1603. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, S.E.; Vittinghoff, E.; Anderson, P.L.; Doblecki-Lewis, S.; Bacon, O.; Chege, W.; Postle, B.S.; Matheson, T.; Amico, K.R.; et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern. Med. 2016, 176, 75–84. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Dunn, D.T.; Desai, M.; Dolling, D.I.; Gafos, M.; Gilson, R.; Sullivan, A.K.; Clarke, A.; Reeves, I.; Schembri, G.; et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016, 387, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Choopanya, K.; Martin, M.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Kitisin, P.; Natrujirote, P.; Kittimunkong, S.; et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013, 381, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Vanichseni, S.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Curlin, M.E.; Na-Pompet, S.; Warapronmongkholkul, A.; et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. Aids 2015, 29, 819–824. [Google Scholar] [CrossRef]
- Riddell, J.t.; Amico, K.R.; Mayer, K.H. HIV Preexposure Prophylaxis: A Review. JAMA 2018, 319, 1261–1268. [Google Scholar] [CrossRef]
- Marcus, J.L.; Hurley, L.B.; Nguyen, D.P.; Silverberg, M.J.; Volk, J.E. Redefining Human Immunodeficiency Virus (HIV) Preexposure Prophylaxis Failures. Clin. Infect. Dis. 2017, 65, 1768–1769. [Google Scholar] [CrossRef]
- El-Haddad, A.; Erlich, D. Cabotegravir (Apretude) for Pre-exposure Prophylaxis for HIV Type 1 Infection. Am. Fam. Physician 2023, 107, 545–546. [Google Scholar]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I.; et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N. Engl. J. Med. 2021, 385, 595–608. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Hughes, J.P.; Bock, P.; Ouma, S.G.; Hunidzarira, P.; Kalonji, D.; Kayange, N.; Makhema, J.; Mandima, P.; Mathew, C.; et al. Cabotegravir for the prevention of HIV-1 in women: Results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022, 399, 1779–1789. [Google Scholar] [CrossRef]
- Duwal, S.; Dickinson, L.; Khoo, S.; von Kleist, M. Mechanistic framework predicts drug-class specific utility of antiretrovirals for HIV prophylaxis. PLoS Comput. Biol. 2019, 15, e1006740. [Google Scholar] [CrossRef]
- Tsibris, A.M.N.; Hirsch, M.S. 130—Antiretroviral Therapy for Human Immunodeficiency Virus Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition); Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 1622–1641.e1626. [Google Scholar]
- Brown, K.C.; Patterson, K.B.; Jennings, S.H.; Malone, S.A.; Shaheen, N.J.; Asher Prince, H.M.; Spacek, M.; Cohen, M.S.; Kashuba, A.D. Single- and multiple-dose pharmacokinetics of darunavir plus ritonavir and etravirine in semen and rectal tissue of HIV-negative men. J. Acquir. Immune Defic. Syndr. 2012, 61, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Taverne-Ghadwal, L.; Kuhns, M.; Buhl, T.; Schulze, M.H.; Mbaitolum, W.J.; Kersch, L.; Weig, M.; Bader, O.; Groß, U. Epidemiology and Prevalence of Oral Candidiasis in HIV Patients From Chad in the Post-HAART Era. Front. Microbiol. 2022, 13, 844069. [Google Scholar] [CrossRef] [PubMed]
- Fenley, J.C.; de Barros, P.P.; Carmo, P.; Garcia, M.T.; Rossoni, R.D.; Junqueira, J.C. Repurposing HIV Protease Inhibitors Atazanavir and Darunavir as Antifungal Treatments against Candida albicans Infections: An In Vitro and In Vivo Study. Curr. Issues Mol. Biol. 2022, 44, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. Available online: https://www.who.int/publications/i/item/9789241550277 (accessed on 18 July 2023).
- Sidrim, J.J.; Perdigão-Neto, L.V.; Cordeiro, R.A.; Brilhante, R.S.; Leite, J.J.; Teixeira, C.E.; Monteiro, A.J.; Freitas, R.M.; Ribeiro, J.F.; Mesquita, J.R.; et al. Viral protease inhibitors affect the production of virulence factors in Cryptococcus neoformans. Can. J. Microbiol. 2012, 58, 932–936. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Silva, J.A.T.; Araújo, G.D.S.; Pereira, V.S.; Gotay, W.J.P.; Oliveira, J.S.; Guedes, G.M.M.; Pereira-Neto, W.A.; Castelo-Branco, D.; Cordeiro, R.A.; et al. Darunavir inhibits Cryptococcus neoformans/Cryptococcus gattii species complex growth and increases the susceptibility of biofilms to antifungal drugs. J. Med. Microbiol. 2020, 69, 830–837. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological Alert, Neurological Syndrome, Congenital Malformations, and Zika Virus Infection. Implications for Public Health in the Americas (1 December 2015). Available online: https://iris.paho.org/handle/10665.2/50697?show=full (accessed on 25 July 2023).
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika Virus Infection in the Brain by the Antiretroviral Drug Rilpivirine. Mol. Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Song, H.; Wang, H.; Chai, Y.; Su, C.; Qi, J.; Shi, Y.; Gao, G.F. The crystal structure of Zika virus NS5 reveals conserved drug targets. Embo J. 2017, 36, 919–933. [Google Scholar] [CrossRef]
- Noble, C.G.; Lim, S.P.; Arora, R.; Yokokawa, F.; Nilar, S.; Seh, C.C.; Wright, S.K.; Benson, T.E.; Smith, P.W.; Shi, P.Y. A Conserved Pocket in the Dengue Virus Polymerase Identified through Fragment-based Screening. J. Biol. Chem. 2016, 291, 8541–8548. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020, 18, 784–790. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Lei, L.; Egli, M. Label-Free Electrophoretic Mobility Shift Assay (EMSA) for Measuring Dissociation Constants of Protein-RNA Complexes. Curr. Protoc. Nucleic Acid. Chem. 2019, 76, e70. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Singh, M.; Ravichandiran, V.; Murty, U.S.N.; Srivastava, H.K. Peptide-like and small-molecule inhibitors against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 2904–2913. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S. Molecular Docking and Virtual Screening Based Prediction of Drugs for COVID-19. Comb. Chem. High. Throughput Screen. 2021, 24, 716–728. [Google Scholar] [CrossRef]
- Khan, S.A.; Zia, K.; Ashraf, S.; Uddin, R.; Ul-Haq, Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2021, 39, 2607–2616. [Google Scholar] [CrossRef]
- Silva, J.R.A.; Kruger, H.G.; Molfetta, F.A. Drug repurposing and computational modeling for discovery of inhibitors of the main protease (M(pro)) of SARS-CoV-2. RSC Adv. 2021, 11, 23450–23458. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Hegazy, M.F. In-silico drug repurposing and molecular dynamics puzzled out potential SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2021, 39, 5756–5767. [Google Scholar] [CrossRef]
- Velagacherla, V.; Suresh, A.; Mehta, C.H.; Nayak, U.Y.; Nayak, Y. Multi-Targeting Approach in Selection of Potential Molecule for COVID-19 Treatment. Viruses 2023, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Banik, A.; Chowdhury, I.M.; Sajib, E.H.; Sarkar, S. Identification of potential antivirals against SARS-CoV-2 using virtual screening method. Inform. Med. Unlocked 2021, 23, 100531. [Google Scholar] [CrossRef] [PubMed]
- Halder, U.C. Predicted antiviral drugs Darunavir, Amprenavir, Rimantadine and Saquinavir can potentially bind to neutralize SARS-CoV-2 conserved proteins. J. Biol. Res. 2021, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Swain, S.S.; Paital, B.; Panda, M. Combinatorial approach of vitamin C derivative and anti-HIV drug-darunavir against SARS-CoV-2. Front. Biosci. 2022, 27, 10. [Google Scholar] [CrossRef]
- Swain, S.S.; Singh, S.R.; Sahoo, A.; Panda, P.K.; Hussain, T.; Pati, S. Integrated bioinformatics-cheminformatics approach toward locating pseudo-potential antiviral marine alkaloids against SARS-CoV-2-Mpro. Proteins 2022, 90, 1617–1633. [Google Scholar] [CrossRef]
- Mahdi, M.; Mótyán, J.A.; Szojka, Z.I.; Golda, M.; Miczi, M.; Tőzsér, J. Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2’s main protease. Virol. J. 2020, 17, 190. [Google Scholar] [CrossRef]
- Gutierrez-Valencia, A.; Torres-Cornejo, A.; BenMarzouk-Hidalgo, O.J.; Ruiz-Valderas, R.; Lluch, A.; Viciana, P.; López-Cortés, L.F. Darunavir minimum plasma concentration and ritonavir-boosted darunavir monotherapy outcome in HIV-infected patients. Antivir. Ther. 2014, 19, 443–447. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Londero, A.; Della Siega, P.; Givone, F.; Fabris, M.; Biasizzo, J.; Tascini, C.; Pea, F. Comparative Population Pharmacokinetics of Darunavir in SARS-CoV-2 Patients vs. HIV Patients: The Role of Interleukin-6. Clin. Pharmacokinet. 2020, 59, 1251–1260. [Google Scholar] [CrossRef]
- Machavaram, K.K.; Almond, L.M.; Rostami-Hodjegan, A.; Gardner, I.; Jamei, M.; Tay, S.; Wong, S.; Joshi, A.; Kenny, J.R. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: Suppression of CYP3A by IL-6. Clin. Pharmacol. Ther. 2013, 94, 260–268. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment. Eur. J. Pharmacol. 2020, 889, 173634. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Liu, L.; Xu, Q.; Ling, Y.; Huang, D.; Huang, W.; Song, S.; Xu, S.; Shen, Y.; et al. Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa241. [Google Scholar] [CrossRef] [PubMed]
- Indu, P.; Rameshkumar, M.R.; Arunagirinathan, N.; Al-Dhabi, N.A.; Valan Arasu, M.; Ignacimuthu, S. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach. J. Infect. Public. Health 2020, 13, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Lucariello, A.; Sellitto, C.; Agliata, I.; Carleo, M.A.; Sangiovanni, V.; Esposito, V.; Guerra, G.; Cobellis, L.; De Luca, A. Different Cell Cycle Modulation in SKOV-3 Ovarian Cancer Cell Line by Anti-HIV Drugs. Oncol. Res. 2017, 25, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, B.; Usluer, S. p21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef]
- Pećina-Slaus, N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003, 3, 17. [Google Scholar] [CrossRef]
- Verma, S.; Salmans, M.L.; Geyfman, M.; Wang, H.; Yu, Z.; Lu, Z.; Zhao, F.; Lipkin, S.M.; Andersen, B. The estrogen-responsive Agr2 gene regulates mammary epithelial proliferation and facilitates lobuloalveolar development. Dev. Biol. 2012, 369, 249–260. [Google Scholar] [CrossRef]
- Schroeder, B.W.; Verhaeghe, C.; Park, S.W.; Nguyenvu, L.T.; Huang, X.; Zhen, G.; Erle, D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 178–185. [Google Scholar] [CrossRef]
- Zheng, W.; Rosenstiel, P.; Huse, K.; Sina, C.; Valentonyte, R.; Mah, N.; Zeitlmann, L.; Grosse, J.; Ruf, N.; Nürnberg, P.; et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes. Immun. 2006, 7, 11–18. [Google Scholar] [CrossRef]
- Moidu, N.A.; Rahman, N.S.A.; Syafruddin, S.E.; Low, T.Y.; Mohtar, M.A. Secretion of pro-oncogenic AGR2 protein in cancer. Heliyon 2020, 6, e05000. [Google Scholar] [CrossRef]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M.; et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef]
- Ly, T.T.G.; Yun, J.; Ha, J.S.; Kim, Y.J.; Jang, W.B.; Van Le, T.H.; Rethineswaran, V.K.; Choi, J.; Kim, J.H.; Min, S.H.; et al. Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 944. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Bahena, L.; Sánchez-Álvarez, A.A.; Ruiz-Moreno, A.J.; Velasco-Velázquez, M.A. Repositioning of Etravirine as a Potential CK1ε Inhibitor by Virtual Screening. Pharmaceuticals 2021, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Klimowski, L.K.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Virshup, D.M. Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. Febs J. 2006, 273, 4594–4602. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Dutta Chowdhury, K.; Dey, S.R.; Paul, A.; Haldar, R. Exploring the possibility of drug repurposing for cancer therapy targeting human lactate dehydrogenase A: A computational approach. J. Biomol. Struct. Dyn. 2022, 1–10. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Yin, C.Y.; Zhang, S.S.; Zhong, J.T.; Zhou, S.H. Pepsin and Laryngeal and Hypopharyngeal Carcinomas. Clin. Exp. Otorhinolaryngol. 2021, 14, 159–168. [Google Scholar] [CrossRef]
- Johnston, N.; Samuels, T.L.; Goetz, C.J.; Arnold, L.A.; Smith, B.C.; Seabloom, D.; Wuertz, B.; Ondrey, F.; Wiedmann, T.S.; Vuksanovic, N.; et al. Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model. Laryngoscope 2023, 133 (Suppl. S1), S1–S11. [Google Scholar] [CrossRef]
- Swami, D.; Mudaliar, P.; Bichu, Y.S.; Kumar Sahu, V.; Devarajan, S.; Basu, S.; Aich, J. Synergistic combination of ritonavir and cisplatin as an efficacious therapy in human cervical cancer cells: A computational drug discovery and in vitro insight. J. Biomol. Struct. Dyn. 2023, 41, 5802–5816. [Google Scholar] [CrossRef]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Efavirenz: History, Development and Future. Biomolecules 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Erber, S.; Harrer, T.; Klinker, H.; Roth, T.; Parsch, H.; Fiebig, N.; Fietkau, R.; Distel, L.V. Efavirenz Has the Highest Anti-Proliferative Effect of Non-Nucleoside Reverse Transcriptase Inhibitors against Pancreatic Cancer Cells. PLoS ONE 2015, 10, e0130277. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial | Regimen | Time | Main Results | Ref. |
|---|---|---|---|---|
| Pharmacokinetic study | 600/100 mg DRV/r (t.d) + 100 or 200 mg ETV (t.d) | 16 days | 49% Cmin in comparison with ETV alone 49% Cmin in comparison with 100 mg ETV Ritonavir decreased ETV exposure, which was resolved with increased ETV dosage | [20] |
| DUET-1 (NCT00254046) | 600/100 mg DRV/r (t.d) + 100 or 200 mg ETV (t.d) | 24 weeks | 56% and 62% of patients achieved viral loads <50 copies/mL No serious AEs were noted; only rashes and some cases of neuropsychiatric and hepatic AEs were observed Effective combination | [22,23] |
| DUET-2 (NCT00255099) | 48 weeks | Continued efficient inhibition of virus replication | [24] | |
| 96 weeks | Sustained virus replication (>80%), high safety and tolerability and no new AEs | [25] | ||
| Multicenter Italian clinical experience | 600/100 mg or 800/100 mg DRV/r (t.d) + 200 mg ETV (t.d) | 24 months | Virological efficacy with safety even in highly penetrated patients | [26] |
| INROADS (NCT01199939) | 800/100 mg DRV/r (o.d) + 400 mg ETV (o.d) | 48 weeks | Virological efficacy of 89% Well tolerated Low incidence of AEs (mostly rash) No change in limb fate, bone density, or glucose metabolism | [27] |
| NCT02818348 | 800/150 DRV/cobicistat (o.d) + 400 mg ETV (o.d) | 14 days | 66% C24h of cobicistat 56% C24h of DRV Drug–drug interaction between ETV and cobicistat; combination not recommend | [28] |
| Drug | Target | Model | Main Results | Ref. |
|---|---|---|---|---|
| DRV | Candidiasis | Strains of C. albicans G. mellonella larvae | Antifungal activity Protection of in vivo model with inhibitory concentration of 512 µg/mL | [53] |
| Cryptococcosis | C. neoformans and C. gattii | capsule production and proteolytic activity of C. neoformans Inhibition of planktonic growth, metabolic activity, and biofilm production Synergistic with amphotericin B | [57] | |
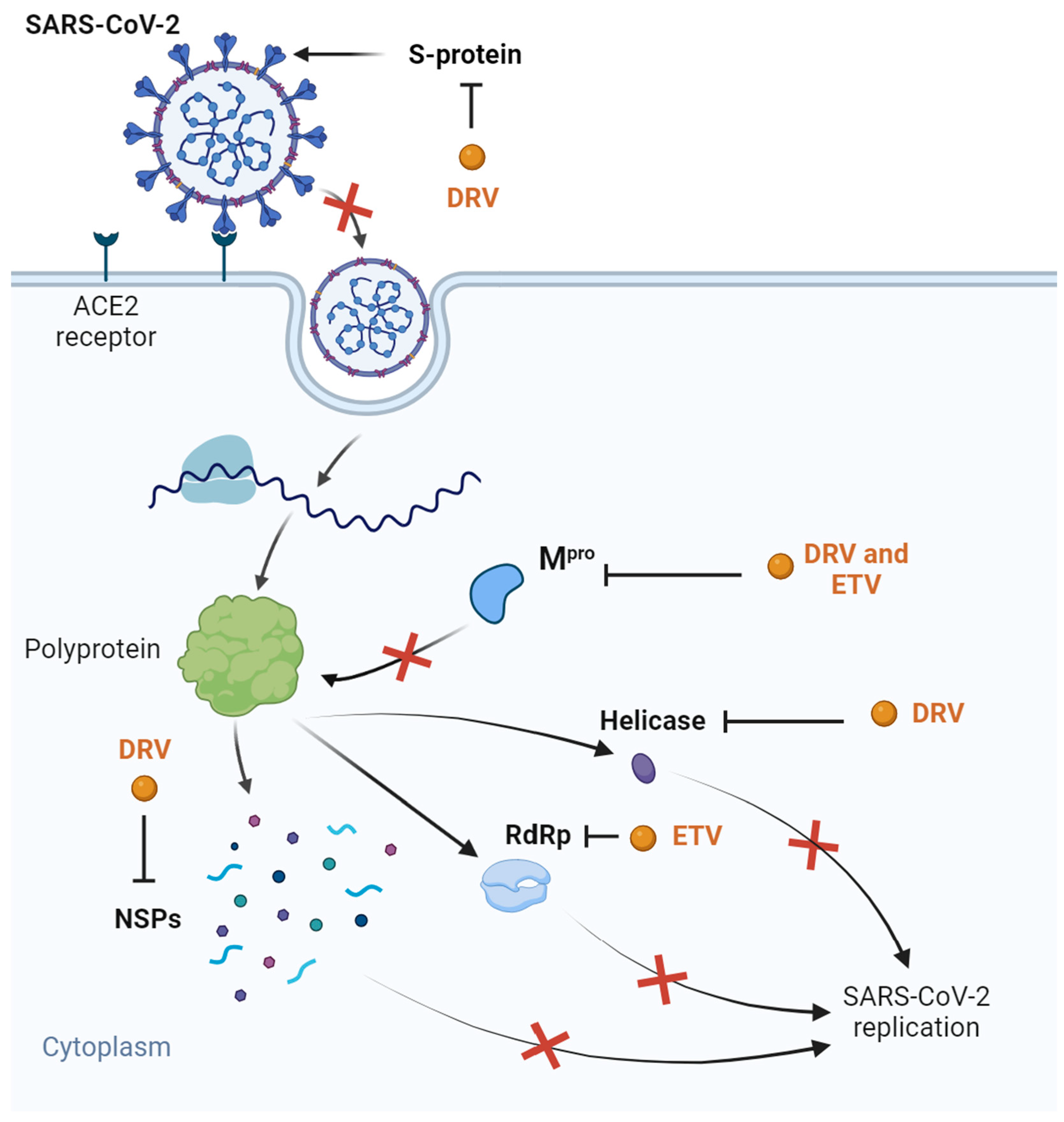
| COVID-19 | Molecular docking of several SARS-CoV-2 proteins Transfected HEK-239 T-cells Clinical trials | Binding affinity with SARS-CoV-2 Mpro, helicase, S ~ protein, and NSPs in silico In silico synergism with vitamin C and 8-hydroxymanzamine No significant inhibition of Mpro in vitro (IC50 of 36.1 µM) Clinical trials without results | [73,76,77,78,79,80,81,82,83,84,85,86,88,90,91] | |
| LDHA inhibition | Molecular docking of LDHA | Binds with catalytic sites similar to known inhibitors via hydrophobic interactions and hydrogen bonds Adapts to conformational changes Potential for inhibiting LDHA | [105] | |
| Laryngopharyngeal reflux Laryngeal and hypopharyngeal carcinomas | Pepsin protein Mouse model of LPR | Bound to and inhibited pepsin (IC50 of 0.06 µM) Prevented pepsin-mediated laryngeal damage | [108] | |
| ETV | Zika virus infection | Molecular docking of NS5 protein IFN−/− mouse model | High binding affinity with NS5 protein’s RdRp domain Inhibited NS5 activity virus replication, brain inflammation, neuron apoptosis, and necrosis mortality | [65] |
| COVID-19 | Molecular docking of Mpro and RdRp | High bioavailability and binding energy with enzymes | [92] | |
| Ovarian cancer | SKOV3 cell line | cycling D1 and Rb p21 and E-cadherin Blocked cell cycle and induced cell and DNA damage Loss of invasiveness differentiation | [93] | |
| SKOV3, A2790 and OVCAR8 cell lines Orthotopic ovarian cancer mouse model | cell viability, colony formation, migration, and invasion (IC50 of 7.5 µM) tumor growth and metastasis AGR2 levels and consequent angiogenesis Synergistic with paclitaxel | [101] | ||
| CK1ε inhibition | Molecular docking of CK1ε | Stably bound with CK1ε via hydrogen bonds Similar to known inhibitors Potential for CK1ε inhibition | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Vale, N. A Review Concerning the Use of Etravirine and Darunavir in Translational Medicine. Int. J. Transl. Med. 2023, 3, 461-478. https://doi.org/10.3390/ijtm3040032
Pereira M, Vale N. A Review Concerning the Use of Etravirine and Darunavir in Translational Medicine. International Journal of Translational Medicine. 2023; 3(4):461-478. https://doi.org/10.3390/ijtm3040032
Chicago/Turabian StylePereira, Mariana, and Nuno Vale. 2023. "A Review Concerning the Use of Etravirine and Darunavir in Translational Medicine" International Journal of Translational Medicine 3, no. 4: 461-478. https://doi.org/10.3390/ijtm3040032
APA StylePereira, M., & Vale, N. (2023). A Review Concerning the Use of Etravirine and Darunavir in Translational Medicine. International Journal of Translational Medicine, 3(4), 461-478. https://doi.org/10.3390/ijtm3040032







