Abstract
This comprehensive review explores two antiretroviral drugs, Etravirine (ETV) and Darunavir (DRV), a non-nucleoside reverse transcriptase inhibitor and a protease inhibitor, that are commonly used in human immunodeficiency virus (HIV) infection treatment, often in combination with each other. The pharmacokinetic properties of these drugs are covered as well as the clinical trials of these two drugs combined. This paper also delves into the possible repurposing of these two drugs for other diseases, with drug repurposing being a significant factor in addressing global health challenges. DRV was extensively studied for treating COVID-19, as well as other infections, such as candidiasis and cryptococcosis, while ETV proved to be efficient in hampering Zika virus brain infection. The focus on cancer repurposing is also explored, with the results revealing that ETV has a particular inhibitory effect on ovarian cancer in vitro and on cancer molecules, such as anterior gradient protein 2 homolog (AGR2) and casein kinase 1 (CK1ε), and that DRV has an in silico inhibitory effect on human lactate dehydrogenase A (LDHA) and induces the in vitro and in vivo inhibition of pepsin, consequent laryngopharyngeal reflux, and possible laryngeal and hypopharyngeal carcinomas. The significance of fresh methods of drug development is emphasized in this work, as is the enormous potential for new therapeutic uses of the antiretroviral drugs ETV and DRV in viral and non-viral disorders.
1. Introduction
The human immunodeficiency virus (HIV) is a retrovirus comprising two types, with HIV-1 being the primary cause of acquired immunodeficiency syndrome (AIDS) globally given its higher infectivity compared to the other type [1]. As of 2022, UNAIDS reported that approximately 39 million individuals were living with HIV globally, with 1.3 million new cases in 2022 alone and with 630.000 deaths related to this disease, mainly occurring in Eastern and Southern Africa [2]. AIDS leads to a severe acquired deficiency of immune function mediated by cells, rendering the affected individuals susceptible to opportunistic infections and neoplasms [3].
HIV accumulates in lymphoid tissues, establishing viral reservoirs, with memory CD4+ T cells serving as its primary cellular targets. Upon infection, these cells enter a state of latent resting and their overall numbers decrease. Additionally, HIV dysregulates other cell types, including B cells, CD8+ T cells, nonlymphoid cells, and natural killer cells. The depletion and dysregulation of CD4+ T cells, along with the disruption of other cell populations, result in an impaired immune response against HIV and other pathogens [4]. This virus contains two copies of single-stranded RNA molecules, and, among its essential genes, the pol gene plays a crucial role as it encodes two enzymes vital for viral infection: (i) a reverse transcriptase (RT) that converts RNA into DNA, which becomes integrated into the host’s DNA, (ii) and a protease responsible for cleaving protein precursors [5].
In modern times, HIV treatment enables patients to maintain immunological function via reducing viral replication, which not only leads to improved life quality and expectancy but also contributes to a significant reduction in HIV transmission rates, with sexual transmission declining by over 90%. The standard treatment for HIV infection involves a combination of three drugs: two nucleoside analog RT inhibitors (NRTIs) along with either a non-nucleoside RT inhibitor (NNRTIs), a protease inhibitor (PI), or an integrase inhibitor, taken orally daily. Adjustment of this regimen can occur in two scenarios: (i) if the current treatment proves ineffective, leading to virologic failure (the detection of high amounts of HIV RNA), prompting a reevaluation of the treatment plan, (ii) or when the current treatment has been effective and resulted in virologic suppression, allowing patients to switch to a less potent regimen to maintain viral suppression [6].
2. Pharmacokinetics and Physicochemical Properties
Etravirine (ETV) and Darunavir (DRV) are antiretrovirals used in the treatment of HIV-1 infections, usually given in a multidrug fashion. ETV is an NNRTI, while DRV is a PI. These two drugs were chosen for this review as they present several indications in the literature that they are relevant for combination and repurposing, representing information that is scattered and that the authors aim to consolidate. Furthermore, both these drugs have passed the required amount of time for a patent and already have generics, an advantage when developing a repurposing strategy. They have also been on the market for several years, and their safety profile is well known. Their use in combination with each other, a topic that will be explored in this review, was also a point considered in the development of this review along with the aggrupation of these two drugs.
2.1. Etravirine
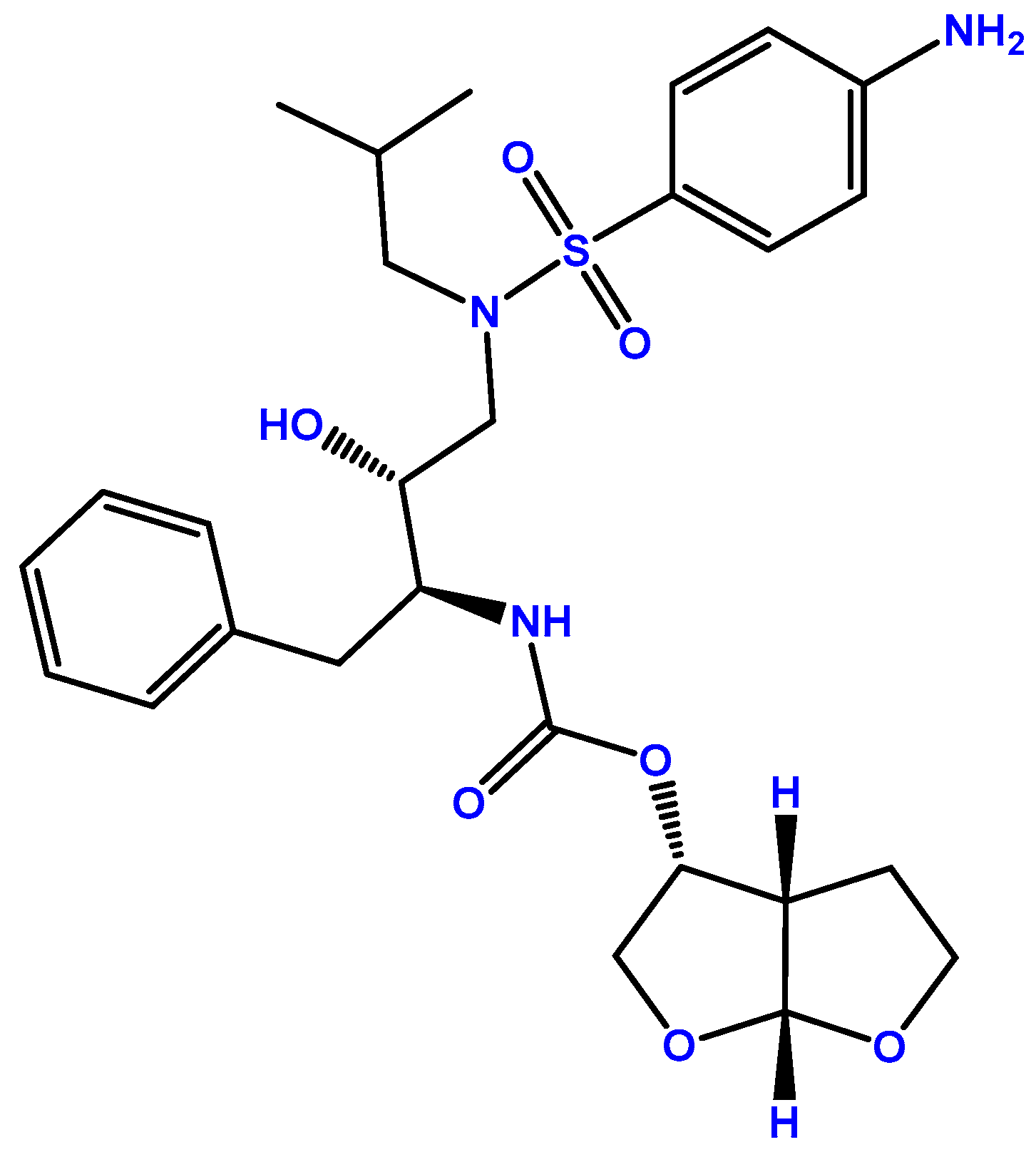
ETV (Figure 1) was first approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) in 2008 under the commercial name Intelence® (Janssen Pharmaceuticals, Beerse, Belgium) [7,8]. Figure 1 represents the chemical structure of ETV. Its route of administration is oral, through 25, 100, and 200 mg tablets administered to people 6 years of age and older, and is indicated for treatment-experienced patients and those with HIV-1 strains resistant to other antiretroviral agents [8]. This drug is a diarylpyrimidine that binds to the HIV-1 RT allosteric site in a flexible manner, which allows ETV to adjust to mutations that alter the binding pocket of the RT [9]. RT is crucial for the replication of the HIV-1 retrovirus since it catalyzes the reverse transcription of its RNA into cDNA, which can then be inserted into the host cell genome and be used to produce proteins required for the replication of the HIV-1 virus [10].
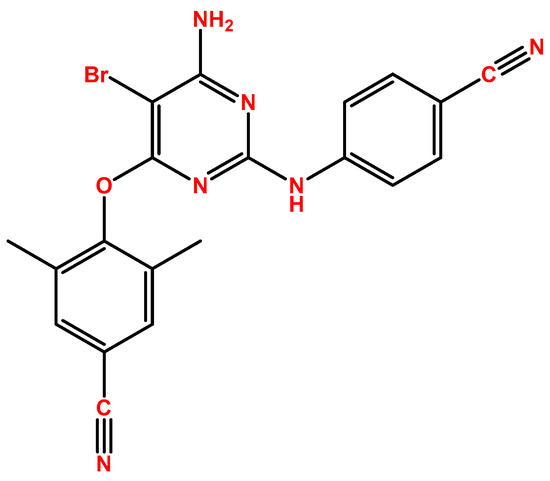
Figure 1.
Chemical structure of ETV (developed using ChemDraw®. A Chemical Drawing Software. Available online: https://chemdrawdirect.perkinelmer.cloud/js/sample/index.html (accessed on 30 July 2023)).
The posology of ETV is 200 mg administered twice daily, always after a meal since systemic exposure decreases in fasting conditions, reaching a maximum plasma concentration (Cmax) after 4 h, at which point it extensively binds with plasma proteins, mainly albumin and α1-acid glycoprotein [7]. This drug has an area under the curve at 12 h (AUC12h) of around 4522 ng·h/mL and a Cmax of 297 ng/mL, inducing RT inhibition at a half maximal effective concentration (EC50) of around 0.9–21.7 nM, depending on the HIV-1 strain [8]. TV is mainly metabolized by CYP2C19, CYP3A4, and CYP2C9 through the hydroxylation of the methyl groups of the dimethylbenzonitrile moiety into mono- and dimethylhydroxylated metabolites. Afterward, metabolites originating from CYP3A4 are glucuronidated by UGT1A3 and 1A8, forming an O-glucuronide metabolite [11]. This metabolism occurs in the liver, and the metabolites are more than 90% less effective in inhibiting RT than the parent drug [12]. In total, 93.7% of ETV is eliminated through feces, of which 81–86% is unchanged ETV. Only 1.2% is eliminated renally, mostly consisting of metabolites, and this drug is dialyzable and has a terminal elimination half-life of 41 h [13].
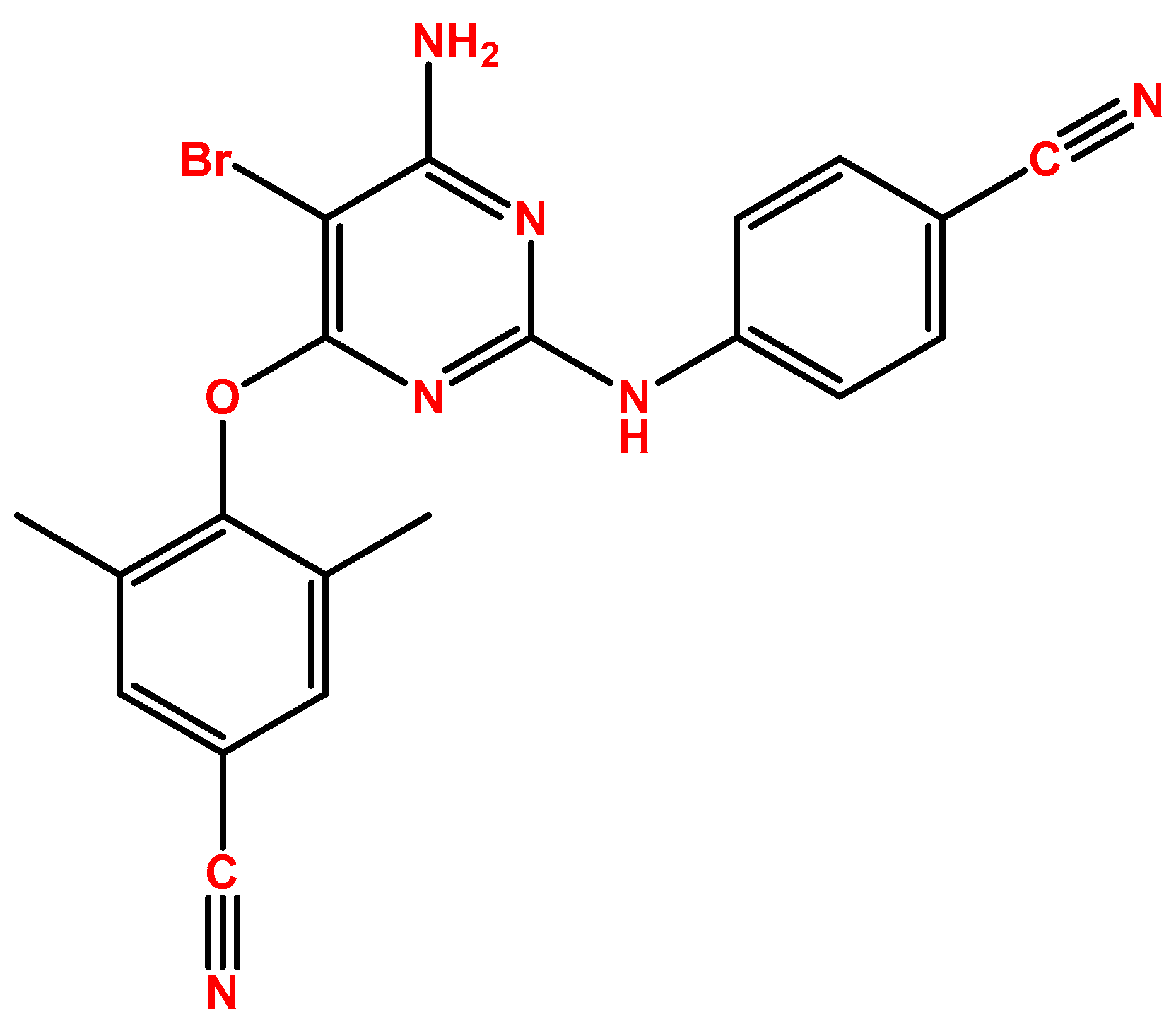
2.2. Darunavir
DRV (Figure 2), first introduced by Janssen-Cilag International as Prezista®, was approved in 2006 by the FDA [14] and in 2007 by the EMA [15], and, as mentioned above, this drug is a protease inhibitor. The HIV-1 protease performs the posttranslational processing of the gag and gag-pol polyproteins, which is required to produce structural proteins of the viral core critical for the maturation of viral particles and viral infectivity [16]. Figure 2 demonstrates the chemical structure of this compound. DRV develops strong hydrogen bonds with amino acids in the main chains of the HIV-1 protease active site, and it also binds to the surface of a flexible flap in the protease dimer, which, overall, gives DRV the ability to adapt to changes in the protease structure. This maintains the inhibition of the protease (even in resistant HIV types), which will then halt the processing of the gag and gag-pol proteins and virus maturation, with an EC50 of 1–5 nM [17]. DRV is indicated for patients older than 3 years of age and heavier than 15 kg, and it is always given in combination, mostly with low-dose ritonavir but also with cobicistat. Treatment-naïve patients’ posology is 800 mg of DRV with 100 mg of ritonavir or 150 mg of cobicistat, and for treatment-experienced patients, it is 600 mg DRV with 100 mg of ritonavir, both administered twice daily and always in a fasted state [15]. When in the plasma, DRV is 95% bound to proteins, primarily α1-acid glycoprotein, with an AUC24h of around 93,026–124,698 ng·h/mL and a Cmax of 2282–3578 ng/mL, depending on the dosage. DRV is subject to hepatic oxidative metabolism via CYP family enzymes, mainly CYP3A4, and the oxidative metabolites are 90% less effective than DRV.
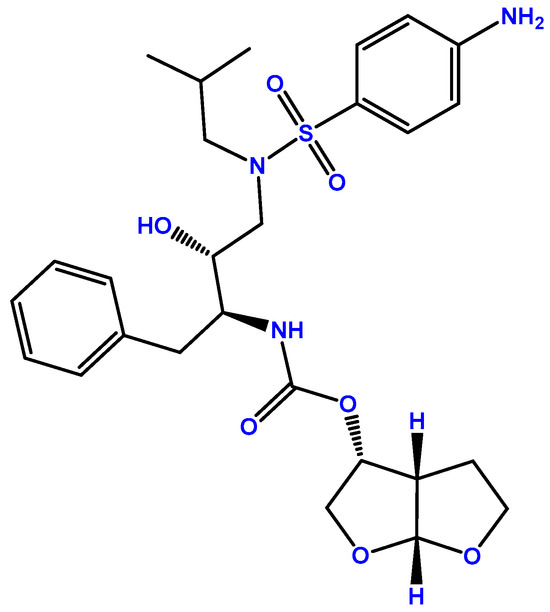
Figure 2.
Chemical structure of DRV (developed using ChemDraw®. A Chemical Drawing Software. Available online: https://chemdrawdirect.perkinelmer.cloud/js/sample/index.html (accessed on 30 July 2023)).
Concomitant administration with ritonavir is carried out specifically because it is a CYP3A4 inhibitor, and when used in combination with DRV, it suppresses this drug’s ability to be metabolized, increasing the parent drug concentration in the plasma and, consequently, its systemic availability [14]. The main metabolic pathways described in the literature were aniline aromatic hydroxylation, carbamate hydrolysis, and isobutyl aliphatic hydroxylation, processes presumably induced by CYP3A4, and all pathways were inhibited via ritonavir boosting and, to a lesser extent, glucuronidation. When boosted with ritonavir, 79.5% of the DRV dosage is eliminated through feces, of which 41.2% is unchanged DRV. In urine, the percentage is lower, at 13.9%, of which 7.7% is unchanged DRV, and this drug has a terminal elimination half-life of 15 h when used in combination [18].
3. Combination of ETV and DRV
ETV combined with DRV boosted with ritonavir is used in the treatment of experienced patients that have drug-resistant HIV infections [19], and several clinical trials preceded this approach.
One of the first trials was a phase I trial in which two treatments were tested, 600/100 mg of DRV/ritonavir administered twice daily with either 100 or 200 mg of ETV supplied twice daily, and the corresponding pharmacokinetic parameters were registered. In terms of DRV pharmacokinetics, no alterations were observed. For ETV, when only 100 mg was administered twice daily, a decrease in AUC12h by 37% as well as decreases in Cmax and Cmin of 32% and 49%, respectively, were observed [20]. These decreases could be related to the fact that ritonavir is an inducer of CYP2C9, CYP2C19, and glucuronosyl transferase [21], which are known metabolizers of ETV. This, however, was altered when 200 mg of ETV was given to patients, with increases in AUC12h, Cmax,, and Cmin of 80, 81, and 67% in comparison with a 100 mg dose [20].
The DUET-1 (NCT00254046) and DUET-2 (NCT00255099) clinical trials were phase III double-blind randomized trials carried out in several different countries in which patients received either a placebo or 200 mg of ETV twice daily with 600/100 mg DRV/ritonavir twice daily. These patients were treatment-experienced individuals that showed virological failure with standard NNRTIs, and confirmation of the success of this new treatment was established as below 50 copies of HIV-1 RNA per mL present at week 24. By this time, 56% and 62% of the patients administered the treatment achieved a viral load of less than 50 copies per mL, which can be compared to the values of 39% and 44% for the placebo groups [22,23]. Generally, the types of adverse effects (AEs) were the same in the treatment and placebo groups, with only a small increase in rashes in the treatment groups in the DUET-1 trial and, overall, some cases of neuropsychiatric and hepatic AEs [22]. This result demonstrated that the combination of ETV with DRV/ritonavir was more effective than the drugs alone and that ETV had a higher genetic barrier to resistance than other NNRTIs [22,23]. The patients from these studies continued being monitored for a 48-week and 96-week analysis of the long-term effects of ETV [24,25]. More than 80% and 90% of the patients that had a viral load of fewer than 50 copies/mL at 24 weeks and 48 weeks maintained this state at up to 96 weeks, with sustained safety and tolerability of ETV [25]. No new AEs were reported after 48 weeks besides those previously mentioned, and the onset of a rash after week 48 was rare [24].
A multicenter clinical experience trial of three Italian departments of infectious diseases with treatment-experienced patients treated with 200 mg of ETV and 800/100 mg or 600/100 mg of DRV/ritonavir was also performed. Virological efficacy was achieved, with safety even in highly penetrated patients [26]. Another treatment regimen was also tested in the INROADS Intelence aNd pRezista Once a Day Study (NCT01199939), in which 400 mg of ETV with 800/100 mg of DRV/ritonavir administered once a day was tested in treatment-naïve patients with transmitted resistance and in treatment-resistant patients for 48 weeks. The main outcome of this research, an overall effectiveness of 89% (HIV-1 RNA < 50 copies/mL) at week 48, showed that this combination was a successful nucleoside-sparing regimen. The once-daily combination of ETV and DRV/ritonavir was well tolerated, with a low incidence of major AEs and AEs associated with the course of therapy. Rash, which occurred for two participants, was the most frequent AE of therapy. Limb fat, bone density, or bone markers did not significantly alter under the nucleoside-sparing regimen. In patients with transmitted resistance who had received therapy before or had never had it, this regimen was virologically effective and well tolerated, with modest to moderate lipid increases, no changes in the distribution of fat in the limbs, and no clinically significant alterations in glucose metabolism [27].
Besides DRV boosted with ritonavir, ETV was also tested in combination with DRV boosted with cobicistat. The pharmacokinetics of the drugs were evaluated in a phase I open clinical trial in which 800/150 mg of DRV/cobicistat and 400 mg of ETV administered once daily were tested alone and in combination for 14 days (NCT02818348). A decrease in cobicistat plasma levels was noted, with decreases of 30%, 14%, and 66% for AUC24h, Cmax, and C24h, and DRV showed a decrease only at C24h (56%). Meanwhile, ETV kinetics were unchanged in combination. These results showed that there was a drug–drug interaction between ETV and cobicistat and that perhaps the combination of ETV is better for DRV/ritonavir than for DRV/cobicistat [28]. Table 1 gives a summary of all the clinical trials involving ETV and DRV combined.

Table 1.
Clinical trials of the combination between ETV and DRV.
The combination of ETV with DRV could be a problem for CYP3A5-expressing people. CYP3A5 is one of the most abundant cytochrome P450s, but there is a common variant that has a single-nucleotide polymorphism, CYP3A5*3, that leads to the loss of function of this enzyme, and individuals with homozygosity for this polymorphism are CYP3A5 non-expressors [29,30]. A total of 91–95% of Caucasians have this polymorphism, while only 27% of the African population has it, so functional CYP3A5 is mainly encountered in the latter population [30,31]. A study that included CYP3A5 expressors tested DRV/ritonavir alone and in combination with ETV to see if there would be an alteration in the plasma concentration of DRV. While the polymorphism did not alter the concentrations of DRV when alone, when combined with ETV, there was a significantly lower DRV plasma concentration in CYP3A5 expressors than in those with the polymorphism. This demonstrates a potential induction of functional CYP3A5 by ETV, which puts CYP3A5 expressor patients, mainly those of African descent, at risk of receiving sub-therapeutic concentrations of DRV. This combination is, therefore, not indicated for these patients [32].
Besides the treatment of already-infected people, pre-exposure prophylaxis, or PrEP, is a method of preventing HIV infection or its spread through the body among people with a higher risk of HIV either through sexual intercourse or due to drug injection [33]. This treatment usually consists of a combination of 300 mg of tenofovir and 200 mg of emtricitabine taken once daily orally. It has been approved under the name Truvada and is associated with high costs [34]. Several studies demonstrated that, when the regimen is adhered to, it has 99% effectiveness in preventing sexually acquired HIV [35,36,37,38,39], and for drug injection, there is 74–84% effectiveness through a regimen of only tenofovir [40,41]. The effectiveness of PrEP is connected to adherence to daily or at least 4-times-a-week ingestion [42], and the failure of PrEP has been demonstrated in individuals who had late access to PrEP or had either not initiated treatment or had not adhered to PrEP care [43]. In 2021, a new injectable PrEP, named Apretude, was approved by the FDA. It is a long-release injectable suspension of cabotegravir given every two months after two initial injection doses one month apart, with a cost of USD 4000 per dose as of March 2023 [44]. This formulation is more effective than tenofovir/emtricitabine at preventing HIV infection through sexual intercourse [45,46]. Overall, both treatments are very expensive, and failure to adhere to the treatments impacts their effectiveness. While Apretude has solved the adherence problem, its cost is still quite high.
A study decided to focus on patent-expired antivirals as new targets for PrEP using an in silico simulation approach to the investigation. This study accounted for the modes of action of the drugs, their pharmacokinetics and pharmacodynamics, and the viral exposure that occurs during sexual intercourse to establish correlations between different drugs’ in vitro potency and PrEP efficacy. This study’s main objective was to develop a screening tool to identify suitable candidates for PrEP based on the pharmacokinetic and pharmacodynamic characteristics of antiretroviral drugs. By analyzing various treatment-approved antiviral drugs, the researchers identified ETV and DRV as potential candidates for effective HIV prophylaxis. Both drugs were predicted to fully prevent HIV infection, even when administered orally and in cases of poor adherence, due to their favorable inhibitory quotients, meaning that their concentrations achieved in clinical settings significantly exceeded their EC50 (in vitro potency) [47]. Furthermore, ETV’s long elimination half-life of 40 h [48] suggested that it could maintain beneficial inhibitory levels, supporting the possibility of sustained prophylactic efficacy. However, this study warned of potential safety concerns for ETV, particularly regarding liver toxicity and skin reactions. PIs also had a steep concentration–prophylaxis profile. This indicated that the efficacy of PIs like DRV could switch between zero and complete protection, something that could be mitigated by using administration forms of DRV that do not rely on daily dosing, such as slow-release or nanoparticle formulations. In summary, both ETV and DRV demonstrated promise as potential candidates for PrEP against HIV. Their favorable inhibitory quotients and predicted efficacy in preventing infection, allied with the fact that they are both patent-free, make them noteworthy options for further investigation for a more affordable PrEP. However, before considering their use in PrEP applications, a thorough evaluation of safety concerns and the implementation of appropriate dosing strategies to maintain optimal drug concentrations are essential [47]. Biological information that points to the combination of ETR and DRV boosted with RTV and their accumulation in semen plasma and rectal tissue further indicates the possible effectiveness of these drugs in preventing HIV acquisition through rectal contact and suppressing virus replication in the male genital tract. This demonstrates that this drug combination can potentially be used for HIV prevention among both infected and uninfected individuals [49].
4. Repurposing of ETV and DRV
Drug repurposing, which involves using already-approved medications for one condition to treat an altogether different one, is an emerging methodology in the hunt for novel disease treatment approaches. In this process, the repurposed medications have already undergone the necessary risk assessment processes and been deemed safe for human use, thereby helping to avoid the high costs and sluggish speeds that are fundamentally connected with the creation of new pharmaceuticals [50]. Herein, we will focus on some possible new uses for ETV and DRV that were found in the literature.
4.1. Repurposing for Other Pathogens
The repurposing of drugs usually starts by testing them against similar diseases, which, in the case of the drugs focused on in this study, are caused by other pathogens.
Infection by Candida albicans is one of the most widely spread fungal infections in the world. It is an opportunistic fungus that can proliferate rapidly and invade tissues, causing various diseases, with oral and vaginal candidiasis being very common examples [51]. Oral candidiasis is often present in HIV-positive patients and is a precursor of esophageal candidiasis, an AIDS-defining disease, and a study demonstrated that HAART therapy decreased the oral colonization of C. albicans [52]. In this setting, the focus is on HIV-PIs, and one study focused on studying the impact of second-generation PIs, including DRV, on strains of C. albicans in vitro and in G. mellonella larvae. This study demonstrated that DRV had antifungal activity, inducing a decrease in virulence-related gene expression, thus protecting a larval model from death via this disease. This was achieved with a minimum inhibitory concentration of 512 µg/mL, which is a high value and suggests that DRV can be used as an adjuvant for the treatment of this disease [53]. Another often opportunistic infection often associated with AIDS is cryptococcosis, caused by the Cryptococcus neoformans and Cryptococcus gattii fungi, which causes high mortality in low-to-middle-income countries due to its entry into the central nervous system [54,55]. DRV was shown to decrease the capsule production and proteolytic activity of C. neoformans in one study [56]. It also had an inhibitory effect on planktonic growth, metabolic activity, and the biofilm formation of C. gattii and C. neoformans alone and even more so when used in combination with antifungals, having a synergistic effect with amphotericin B [57].
Meanwhile, ETV has been shown to inhibit the ability of Zika virus (ZIKV) to infect brain cells. This is a flavivirus that is transmitted via mosquito bites and is widespread throughout Brazil and the rest of the Americas [58]. This disease, which had previously only been associated with mild fever, rashes, and conjunctivitis [59], is associated with severe neurological complications in adults, with a high incidence of Guillain–Barré Syndrome (weakness of the limbs and nerve-innervated muscles) [60,61], as well as neonatal complications, wherein a correlation between microcephaly and infection with ZIKV during pregnancy has been established [62,63]. The RNA genome of this virus has a positive strand that codes for the NS5 protein, which is essential for viral replication [64]. Researchers have discovered possible regions of ETV in a computational analysis of the ZIKV NS5 protein, which may hinder a successful ZIKV infection cycle in the brain [65]. NS5 has two druggable sites that are suitable for small-molecule ligands like ETV to bind to. The binding pocket for SAM, the methyl donor in the 5′ RNA cap structure, is shared by the first site, the SAM site, which is found on the MTase domain of NS5 (N terminus). Small compounds like ETV that bind to this location can allosterically hinder viral replication [66]. The palm site, which is the second druggable location, is shared by DENV NS5 and other flaviviruses. It is found on the NS5’s RNA-dependent RNA polymerase (RdRp) domain (C terminus). The enzymatic activity of DENV RdRp is inhibited by competitive binding at the palm site [67]. Researchers have hypothesized that ETV may operate similarly by binding to ZIKV NS5’s palm site, and they have experimentally mutated several amino acid residues with possible ETV binding affinity to confirm their findings. Surprisingly, the outcomes demonstrated that ETV displayed a preference for binding to the NS5 protein’s RdRp domain (C terminus) as opposed to the MTase domain (N terminus), as was previously thought to be the case. These interactions were drastically reduced when these residues (mutant-14A) were introduced, proving that one or more of the altered residues are responsible for ETV binding. Furthermore, polymerase activity experiments confirmed these binding results, indicating that ETV binds to the RdRp domain and inhibits NS5 polymerase activity. The IFN−/− mouse model, which is prone to ZIKV replication in the brain and is widely used for the study of this disease [68], was used by researchers to evaluate the in vivo effects of ETV on ZIKV infection. ETV treatment prevented viral multiplication, decreased brain inflammation, and shielded neurons from apoptosis and necrosis in ZIKV-infected IFN−/− mice. Notably, ETV reduced the mortality caused by ZIKV. These results revealed that the in vivo spread of ZIKV in the brain was adversely affected by ETV’s direct interaction with the viral protein NS5 and that this drug could have the potential to be used for repurposing in ZIKV treatment [65].
Recently, the COVID-19 epidemic started and spread through the world rapidly, and drug repurposing was one of the first resources used by investigators to establish a treatment for this disease. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China at the end of 2019 and is the pathogen behind this pneumonic disease [69]. This virus enters the cell by binding its spike S protein to the cellular receptor angiotensin-converting enzyme 2 (ACE2), and the S protein has to be cleaved into S1/S2 units by cellular protease TMPRSS2 for S2 to bind to the cellular membrane and enter the cell [70,71]. The DNA of the SARS-CoV-2 virus contains two open reading frames that, when broken down, yield the 16 nonstructural proteins (NSP) required for this virus to complete its life cycle: polyproteins 1a and 1b. The chymotrypsin-like protease (3CLpro also known as Mpro) and, to a lesser extent, the papain-like protease (PLpro), are responsible for this process in betacoronaviruses like SARS-CoV 2 [72]. These are all possible targets of COVID-19 treatment with repurposing drugs, and antiretroviral drugs were one of the first groups studied, among which DRV has been extensively studied.
One of the first indications that DRV could be used for SARS-CoV-2 inhibition was uncovered in March of 2020 when a group of investigators used a molecule transformer–drug target interaction prediction model to assess the binding parameters of several drugs with some of the virus’ proteins [73], like the proteases as well as the helicase, which form part of the replication machinery [74]. The dissociation constants (kd), that is, the concentrations of the drug at which half of it is bonded to the target, were assessed, and the lower the kd, the higher the affinity of the drug with the target [75]. The drug Prezcobix, which combines DRV and cobicistat, had a kd of 90.38 nM with the SARS-CoV-2 helicase, which demonstrates the moderate affinity of DRV with this enzyme [73].
The Mpro enzyme has been one of the most targeted for inhibition, and the initial studies used in silico models to assess several types of drugs for this purpose. Molecular docking, molecular dynamic simulations, and molecular-mechanics-generalized born surface area calculations were used to obtain the docked poses, biding energy values, and evaluate the stability of interactions [76]. All these studies demonstrated that DRV is among the drugs with the highest affinity for SARS-CoV-2 Mpro and can effectively inhibit this virus’s activity [76,77,78,79,80,81]. Other in silico investigations also demonstrated that DRV has a relatively strong affinity with other SARS-CoV-2 proteins, as shown in a study of the S protein and the NSP9 RNA binding protein [82], and another one demonstrated that DRV targeted various binding motifs of every NSP protein tested except, interestingly and in contrast with the previous study, Nsp9 [83]. DRV was also shown to have synergy in silico with other compounds that were thought to be capable of being repurposed for treating COVID-19, namely, vitamin C derivates [84] and the marine alkaloid 8-hydroxymanzamine [85].
However, in vitro testing of DRV and other antiretrovirals was performed as well, using HEK-239 T cells transfected with SARS-CoV-2 Mpro, and the results regarding DRV in the study indicated that it, along with other HIV protease inhibitors, did not significantly inhibit the Mpro of SARS-CoV-2 in vitro. The conducted in vitro enzymatic assay revealed that DRV showed an IC50 value of 36.1 µM, which is considered suboptimal for inhibiting the viral enzyme in question [86]. Additionally, previous reports on the minimum concentrations of DRV in patient serum during antiretroviral treatment indicated a value of 3.3 µM [87], further highlighting the challenge of achieving the high plasma levels of DRV required to effectively block viral replication via inhibiting Mpro [86]. Some other studies highlighted the limitations of DRV repurposing for COVID-19. One aimed to investigate the population pharmacokinetics of DRV among patients infected with SARS-CoV-2 compared to patients with HIV. The researchers found that the clearance of DRV was significantly lower in SARS-CoV-2 patients compared to that of HIV patients, which was related to the levels of the cytokine IL-6 [88]. This cytokine is known to be involved in inflammation and may downregulate the activity of enzymes responsible for drug metabolism, including CYP3A4 [89], which metabolizes DRV, and dosing adjustment could be required for patients with severe SARS-CoV-2 infections [88]. Distribution to tissues is also a major agent in drug efficacy for this disease, mainly with respect to the lung and intestine, as they are the tissues with higher expression of ACE2 and TMPR22. DRV accumulates in the intestine but not in the lung and has failed to reduce viral loads in nasopharyngeal swabs, which makes DRV not ideal for treating COVID-19 [90].
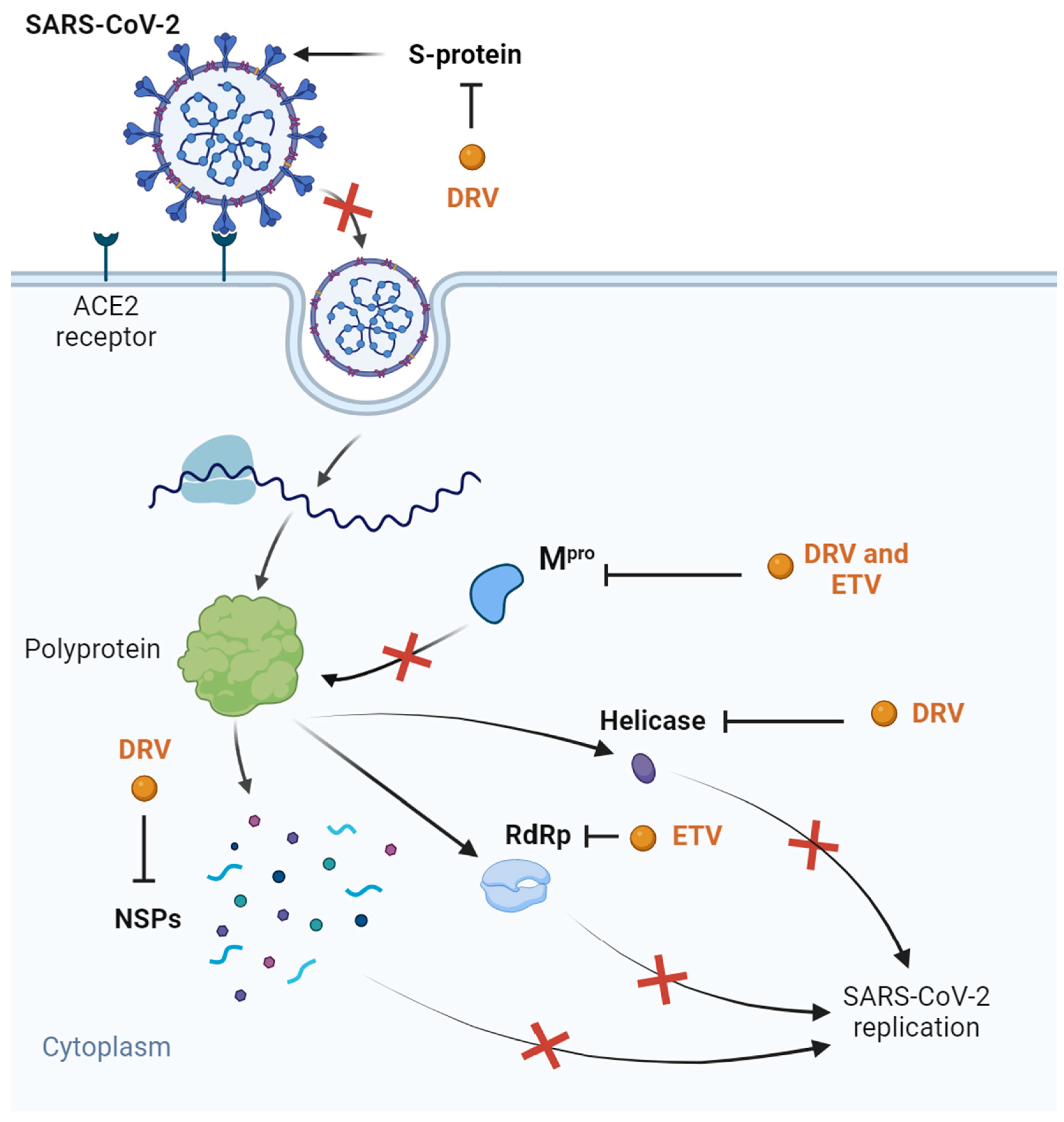
Some clinical trials were started with this drug, either in combination with ritonavir or cobicistat: NCT04252274; NCT04425382; NCT04304053. The first one was performed to access the safety of DRV/cobicistat in COVID-19 patients, with the results showing that the drugs were well tolerated [91]. We could not find results for the remaining trials. Overall, DRV showed some interesting properties in relation to COVID-19 treatment, but after the development of the COVID-19 vaccines and the release of some unfavorable in vitro results, this drug was forgotten for this purpose. ETV was also briefly studied in silico for COVID-19 treatment due to its high bioavailability value and binding energy with Mpro and the RdRp [92], but it was not studied further. Figure 3 demonstrates all the possible mechanisms of the DRV- and ETV-based inhibition of SARS-CoV-2 replication.
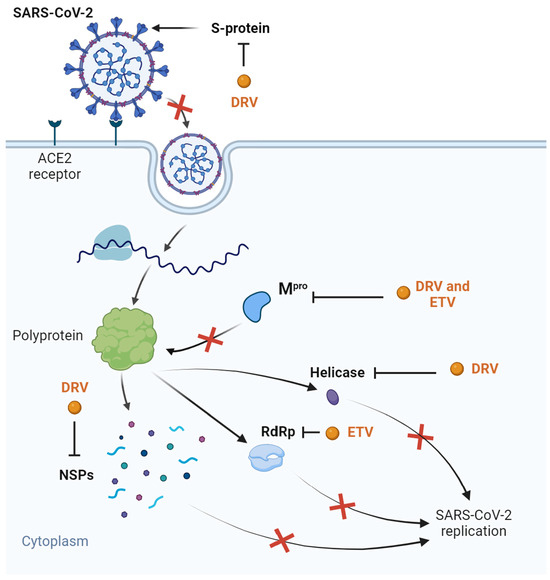
Figure 3.
Suggested inhibitory targets of DRV and ETV for SARS-CoV-2 determined through several in silico studies. DRV was shown to exhibit affinity with S-protein, inhibiting entrance in the cell host, Mpro, thereby inhibiting polyprotein processing, helicase, and several NSPs, inhibiting virus replication. ETV showed promise for Mpro and RdRp binding, inhibiting further SARS-CoV-2 replication. The red “x” represent the inhibitory effects of ETV and DRV. Adapted from “COVID-19 Drug Mechanism of Action (Layout)” created by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 30 July 2023).
4.2. Repurposing for Cancer Treatment
In terms of experimental studies, ETV has been studied in relation to ovarian cancer in an extensive study on the effects of several antiretrovirals on the alteration the of the cell cycle, differentiation, and DNA damage and the SKOV3 cell line. Applied at a concentration of 20 µM for 48 h., ETV caused cell damage, and it also stopped cells from entering into the cell cycle by blocking the passage of G0 to G1 as well as via decreasing the quantity of proteins associated with the cell cycle, like cyclin D1 and phosphorylated retinoblastoma (Rb) [93]. There was also an increase in p21, which is anti-proliferative and a cell cycle blocker since it inhibits cyclin-dependent kinases, which normally work in conjunction with cyclins to manage the cell cycle [94]. The drug also increased levels of E-cadherin, a tumor suppressor protein that, in healthy cells, is responsible for epithelial and cell–cell adhesion and is downregulated in cancer cells, allowing for invasion and metastasis [95]. An ETV-mediated increase in E-cadherin caused the differentiation of SKOV3 cells into less-invasive cells. Overall, ETV demonstrated the best effect on the ovarian cancer cells out of all the antiretrovirals tested, with the highest effects on cell cycle arrest and DNA damage, and it was the only one that caused cell differentiation [93].
ETV’s ability to reduce the survival of ovarian cancer cells may also result from its ability to inhibit the human anterior gradient protein 2 homolog (AGR2). This is a disulfide isomerase that regulates protein folding, is expressed in the endoplasmic reticulum, and is linked to the beginning of carcinogenesis, its progression, and resistance to therapy [30]. Normally, AGR2 regulates the total protein counts of cells, and it is essential for milk production during lactation [96], for the production of epithelial mucins for the respiratory system [97], and, overall, in the differentiation of epithelial goblet cells, i.e., cells that secrete mucus for some tissues, such as reproductive and gastrointestinal tissues [98]. The role of AGR2 in cancer cells is twofold, as there is intracellular AGR2, which maintains endoplasmic reticulum proteostasis required by the increased secretory demands of cancer cells, and there is extracellular AGR2, which causes inflammation, angiogenesis, and oncogenic signaling related to epithelial tumorigenesis [99]. The ability of AGR2 to induce angiogenesis has been linked to its interaction with vascular endothelial growth factor-A (VEGF-A), which enhances the VEGF-VEGFR2 pathway, which is often augmented in cancer, such as in prostate cancer, and is associated with angiogenesis [100]. The authors of one study decided to assess the effect of ETV on AGR2 inhibition using the ovarian cancer cell lines SKOV3, A2780 normal and Adriamycin-resistant, and OVCAR8, using concentrations ranging from 1.25 to 10 µM at different time points ranging from 12 to 72 h, performing several experiments. An in vivo study using an orthotopic ovarian cancer mouse model was also performed, with 100 mg/kg of ETV administered three times a week for three weeks. The drug was capable of decreasing the cell viability of all the cell lines as well as colony formation, migration, and invasion, with an IC50 of around 7.5 µM, and it inhibited tumor growth and the weight of tumor metastasis in vivo. Furthermore, ETV decreased AGR2 levels, both in the cells and in the mouse model, as well as the formation of HUVEC (human umbilical vein endothelial cells) tubes, which are associated with angiogenesis. Synergism with paclitaxel was also observed, both in vivo and in vitro, and no toxic effects were observed in the vital organs. Overall, this study demonstrates the capacity of ETV to inhibit ovarian cancer via the inhibition of AGR2, showing that it is promising for repurposing with respect to this cancer and potentially others where AGR2 overexpression is prominent, possibly via administration with paclitaxel due to their synergic effect [101].
Inhibiting casein kinase 1 (CK1ε), an enzyme belonging to a family of enzymes implicated in signal transduction pathways, has been investigated as a possible method of action of ETV [102]. The Dishevelled protein, which protects the stability of β-catenin by blocking its degradation complex, is phosphorylated by CK1ε, which is a positive regulator of the WNT/β-catenin pathway and activated by WNT [103]. With the buildup of β-catenin in the nucleus, which triggers cell proliferation, this pathway’s deregulation is linked to the emergence of early cancer [104]. Since CK1ε inhibition has been investigated, it has been shown to cause cancer cells to undergo cell cycle arrest and apoptosis, and ETV has been suggested to be a CK1ε inhibitor. In this in silico study, the investigators developed a pharmacophore model of binding modes utilizing the well-characterized catalytic pocket of CK1ε and known inhibitors. This model was then used to perform a virtual screening of an FDA-approved drugs library, and two drugs were identified, one of which was ETV. This drug stably bound to CK1ε through hydrogen bonds, with a calculated theoretical binding affinity similar to that of an already-approved CK1ε inhibitor, umbralisib, whose ability was better than the other drug (abacavir), so this study concluded that ETV could be repurposed for the inhibition of this enzyme [102].
As for DRV, an in silico study aimed to discover new inhibitors to target human lactate dehydrogenase A (LDHA) [105]. This enzyme, which turns pyruvate into lactate, is critical for glycolysis, but it is also important as a single-stranded DNA-binding protein when present in the nucleus. LDHA levels are frequently elevated in cancer patients due to the shift from aerobic phosphorylation to anaerobic glycolysis, and LDHA promotes several of the disease’s well-known characteristics, including increased proliferation, immune escape, cell invasion, metastasis, and angiogenesis [106], so the inhibition of LDHA activity is a potential target for cancer treatment. As such, one study developed a pharmacophore model of LDHA, and model docking was used to screen several drugs. DRV was shown to bind with the catalytic site of the enzyme with high stability (similar to known inhibitors) using hydrophobic interactions and hydrogen bonds, hampering the conformational changes needed for catalytic activity [105].
Other potential repurposing pathways of DRV are laryngeal and hypopharyngeal carcinomas. These carcinomas are associated with laryngopharyngeal reflux (LPR), in which substances like pepsin, hydrochloric acid, bile acids, and salts and bacteria suffer reflux in the larynx, oropharynx, and nasopharynx. Pepsin can cause a loss of epithelium of the mucous membranes of the larynx and hypopharynx, and it can also cause the carcinomas previously mentioned [107]. One study developed a pepsin-binding scheme and a mouse model of LPR to study several drugs, their inhibitory effects on pepsin, and their ability to protect against in vivo damage. The results of the study suggest that DRV demonstrated potential as an anti-peptic agent in preventing laryngeal damage caused by pepsin exposure in the mouse model. DRV was found to bind and inhibit pepsin effectively, with the lowest IC50 (0.06 µM) in the in vitro assays. In the in vivo model, DRV, when administered locally via inhalation, preserved normal laryngeal histology despite pepsin exposure, preventing pepsin-mediated laryngeal damage. This study provides an initial proof of concept that a pepsin-targeting therapeutic, such as DRV, may reduce mucosal damage like that seen in LPR patients [108]. Since pepsin damage and laryngeal and hypopharyngeal carcinomas have also been connected, DRV can potentially also be used for the treatment of those carcinomas in addition to LPR treatment.
Table 2 summarizes all the potential repurposing pathways for DRV and ETV mentioned in this article.

Table 2.
Studies on repurposing of DRV and ETV for different diseases.
Other antiretrovirals have also been studied in relation to cancer. Other PIs like DRV that have been shown to exhibit effects include ritonavir (in treating cervical cancer) [109] and nelfinavir (in treating pancreatic cancer) [110]. These drugs provide direct evidence both through in silico methods and in cancer cells, while DRV’s effect is still only theoretical. Regarding NNRTIs like ETV, efavirenz has shown extensive repurposing possibilities, with even some clinical trials being underway for cancers like pancreatic and prostate cancer [111]. One of the studies that evaluated ETV but also included efavirenz and another NNRTI called rilpivirine showed that the last two drugs had a lower EC50 in treating pancreatic cancer than ETV (31.5 and 24.4 μmol/L vs. 89.9 μmol/L). While these NNRTIs show more promise with regard to this type of cancer, ETV was still one of the drugs with the strongest cytotoxic effects, and it is still very much worth pursuing [112].
This comprehensive review sheds light on the untapped potential of DRV and ETV that extends far beyond their original use. While fragments of this potential have been suggested in separate articles, this review consolidates the evidence, showcasing the substantial opportunities for drug repurposing with these agents. It is the belief of the authors that the adaptability of ETV and DRV exemplifies the exciting possibilities within translational medicine, urging further exploration. These antiretroviral drugs, with their expanding horizons, dictate a future in medicine where drug repurposing and innovation are the driving forces behind progress. This review also aims to raise public awareness about the potential of drug repurposing and its implications, both positive and negative, fostering support for innovative approaches in medicine.
Looking ahead, the in-depth exploration of these drugs’ mechanisms of action in various diseases is crucial, as this area remains relatively uninvestigated. Clear definitions of these mechanisms and effective pathways for their efficacy are necessary to harness their full potential and target them more precisely for specific diseases. Additionally, defining appropriate dosages is crucial. To achieve these goals, comprehensive research strategies are indispensable. This entails conducting meticulous in vitro studies using diverse models and harnessing the power of big-data-driven research conducted using in silico tools, streamlining the approach to this subject. Subsequently, advancing to clinical trials, possibly in conjunction with other relevant drugs or even in combination with each other, is the logical progression. For this vision to materialize, collaboration between the pharmaceutical industry and regulatory bodies is indispensable. While drug repurposing can be cost-effective, it still requires financial backing and regulatory approval. The success of this approach, particularly with respect to ETV and DRV, hinges on fostering interdisciplinary research and the exchange of knowledge and ideas among experts in virology, oncology, pharmacology, and related fields. This collaboration will unlock the full potential of these two versatile antiretroviral drugs, potentially revolutionizing the way we approach disease treatment in the future.
5. Conclusions
The usage of ETV and DRV, two antiretroviral medications frequently used to treat HIV-1 infections, is discussed in this paper along with an examination of their pharmacokinetics, physicochemical characteristics, and potential for use in other medical procedures. These drugs have demonstrated strong outcomes in sustaining viral suppression and have been widely utilized in combination therapy to treat drug-resistant HIV infections, with several clinical trials proving these capacities.
This article also examined the possibilities for repurposing ETV and DRV for other medicinal uses, including preventing the growth of diseases like candidiasis, cryptococcosis, and the ZIKV. DRV also demonstrated an initial potential for COVID-19 treatment, but further studies did not support this and so its application is still uncertain. According to the in vitro results combined with its inhibition of AGR2 and CK1ε, ETV also showed promise in the treatment of ovarian cancer, and DRV demonstrated promise as an LDHA and pepsin inhibitor in the fight against cancer and LPR.
The significance of repurposing existing drugs for brand-new therapeutic uses is emphasized in the analyzed studies. While both ETV and DRV have shown promise in a variety of settings outside of HIV therapy, further preclinical and clinical research is required to confirm their effectiveness and safety in these novel uses. Drug repurposing offers a time- and cost-efficient method for discovering novel medicines for a range of ailments, possibly helping people all over the world. To guarantee the effective and secure repurposing of these antiretroviral medications, however, a thorough evaluation of safety and pharmacokinetic interactions is essential.
Author Contributions
Conceptualization, N.V.; methodology, M.P.; formal analysis, M.P. and N.V.; investigation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020 Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT) in the framework of projects IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
M.P. acknowledges FCT for funding her Ph.D. grant (2021.07450.BD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucas, S.; Nelson, A.M. HIV and the spectrum of human disease. J. Pathol. 2015, 235, 229–241. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 17 July 2023).
- Patton, L.L. HIV disease. Dent. Clin. N. Am. 2003, 47, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Fanales-Belasio, E.; Raimondo, M.; Suligoi, B.; Buttò, S. HIV virology and pathogenetic mechanisms of infection: A brief overview. Ann. Ist. Super. Sanita 2010, 46, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef]
- EMA. Intelence. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/intelence (accessed on 18 July 2023).
- FDA. INTELENCE® (Etravirine) Tablets for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022187s009lbl.pdf (accessed on 18 July 2023).
- Schöller-Gyüre, M.; Kakuda, T.N.; Raoof, A.; De Smedt, G.; Hoetelmans, R.M. Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin. Pharmacokinet. 2009, 48, 561–574. [Google Scholar] [CrossRef]
- Müller, T.G.; Zila, V.; Peters, K.; Schifferdecker, S.; Stanic, M.; Lucic, B.; Laketa, V.; Lusic, M.; Müller, B.; Kräusslich, H.G. HIV-1 uncoating by release of viral cDNA from capsid-like structures in the nucleus of infected cells. Elife 2021, 10, e64776. [Google Scholar] [CrossRef] [PubMed]
- Yanakakis, L.J.; Bumpus, N.N. Biotransformation of the antiretroviral drug etravirine: Metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab. Dispos. 2012, 40, 803–814. [Google Scholar] [CrossRef]
- Havens, J.P.; Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine: An Updated Review. Clin. Pharmacokinet. 2020, 59, 137–154. [Google Scholar] [CrossRef]
- Bank, D. Etravirine. Available online: https://go.drugbank.com/drugs/DB06414 (accessed on 18 July 2023).
- FDA. PREZISTA (Darunavir). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021976s021lbl.pdf (accessed on 4 July 2023).
- EMA. Prezista. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prezista (accessed on 19 July 2023).
- Gulnik, S.; Erickson, J.W.; Xie, D. HIV protease: Enzyme function and drug resistance. Vitam. Horm. 2000, 58, 213–256. [Google Scholar] [CrossRef]
- De Meyer, S.; Azijn, H.; Surleraux, D.; Jochmans, D.; Tahri, A.; Pauwels, R.; Wigerinck, P.; de Béthune, M.P. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 2005, 49, 2314–2321. [Google Scholar] [CrossRef]
- Vermeir, M.; Lachau-Durand, S.; Mannens, G.; Cuyckens, F.; van Hoof, B.; Raoof, A. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 2009, 37, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.W.; Montaner, J.S. Etravirine in combination with darunavir/ritonavir and optimized background regimen results in suppression of HIV replication in treatment-experienced patients. Expert Opin. Pharmacother. 2010, 11, 1433–1437. [Google Scholar] [CrossRef]
- Schöller-Gyüre, M.; Kakuda, T.N.; Sekar, V.; Woodfall, B.; De Smedt, G.; Lefebvre, E.; Peeters, M.; Hoetelmans, R.M. Pharmacokinetics of darunavir/ritonavir and TMC125 alone and coadministered in HIV-negative volunteers. Antivir. Ther. 2007, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Granneman, G.R.; Bertz, R.J. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 1998, 35, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Madruga, J.V.; Cahn, P.; Grinsztejn, B.; Haubrich, R.; Lalezari, J.; Mills, A.; Pialoux, G.; Wilkin, T.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, A.; Campbell, T.; Clotet, B.; Johnson, M.; Katlama, C.; Moll, A.; Towner, W.; Trottier, B.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 39–48. [Google Scholar] [CrossRef]
- Katlama, C.; Haubrich, R.; Lalezari, J.; Lazzarin, A.; Madruga, J.V.; Molina, J.M.; Schechter, M.; Peeters, M.; Picchio, G.; Vingerhoets, J.; et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: Pooled 48 week analysis of two randomized, controlled trials. Aids 2009, 23, 2289–2300. [Google Scholar] [CrossRef]
- Katlama, C.; Clotet, B.; Mills, A.; Trottier, B.; Molina, J.M.; Grinsztejn, B.; Towner, W.; Haubrich, R.; Nijs, S.; Vingerhoets, J.; et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir. Ther. 2010, 15, 1045–1052. [Google Scholar] [CrossRef]
- Gazzola, L.; Cicconi, P.; Ripamonti, D.; Di Filippo, E.; Gustinetti, G.; Di Biagio, A.; Marchetti, G.; Bini, T.; d’Arminio Monforte, A. Efficacy and safety of darunavir/ritonavir plus etravirine dual regimen in antiretroviral therapy-experienced patients: A multicenter clinical experience. HIV Clin. Trials 2014, 15, 140–150. [Google Scholar] [CrossRef]
- Ruane, P.J.; Brinson, C.; Ramgopal, M.; Ryan, R.; Coate, B.; Cho, M.; Kakuda, T.N.; Anderson, D. The Intelence aNd pRezista Once A Day Study (INROADS): A multicentre, single-arm, open-label study of etravirine and darunavir/ritonavir as dual therapy in HIV-1-infected early treatment-experienced subjects. HIV Med. 2015, 16, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Moltó, J.; Curran, A.; Miranda, C.; Challenger, E.; Santos, J.R.; Ribera, E.; Khoo, S.; Valle, M.; Clotet, B. Pharmacokinetics of darunavir/cobicistat and etravirine alone and co-administered in HIV-infected patients. J. Antimicrob. Chemother. 2018, 73, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, R.H.; van der Heiden, I.P.; van den Anker, J.N.; Lindemans, J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin. Chem. 2002, 48, 1668–1671. [Google Scholar] [CrossRef]
- Belkhir, L.; Elens, L.; Zech, F.; Panin, N.; Vincent, A.; Yombi, J.C.; Vandercam, B.; Haufroid, V. Interaction between Darunavir and Etravirine Is Partly Mediated by CYP3A5 Polymorphism. PLoS ONE 2016, 11, e0165631. [Google Scholar] [CrossRef]
- HIV.gov. Pre-Exposure Prophylaxis. Available online: https://www.hiv.gov/hiv-basics/hiv-prevention/using-hiv-medication-to-reduce-risk/pre-exposure-prophylaxis/ (accessed on 25 July 2023).
- Coutinho, B.; Prasad, R. Emtricitabine/tenofovir (Truvada) for HIV prophylaxis. Am. Fam. Physician 2013, 88, 535–540. [Google Scholar]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef]
- Grant, R.M.; Anderson, P.L.; McMahan, V.; Liu, A.; Amico, K.R.; Mehrotra, M.; Hosek, S.; Mosquera, C.; Casapia, M.; Montoya, O.; et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect. Dis. 2014, 14, 820–829. [Google Scholar] [CrossRef]
- Volk, J.E.; Marcus, J.L.; Phengrasamy, T.; Blechinger, D.; Nguyen, D.P.; Follansbee, S.; Hare, C.B. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin. Infect. Dis. 2015, 61, 1601–1603. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, S.E.; Vittinghoff, E.; Anderson, P.L.; Doblecki-Lewis, S.; Bacon, O.; Chege, W.; Postle, B.S.; Matheson, T.; Amico, K.R.; et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern. Med. 2016, 176, 75–84. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Dunn, D.T.; Desai, M.; Dolling, D.I.; Gafos, M.; Gilson, R.; Sullivan, A.K.; Clarke, A.; Reeves, I.; Schembri, G.; et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016, 387, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Choopanya, K.; Martin, M.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Kitisin, P.; Natrujirote, P.; Kittimunkong, S.; et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013, 381, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Vanichseni, S.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Curlin, M.E.; Na-Pompet, S.; Warapronmongkholkul, A.; et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. Aids 2015, 29, 819–824. [Google Scholar] [CrossRef]
- Riddell, J.t.; Amico, K.R.; Mayer, K.H. HIV Preexposure Prophylaxis: A Review. JAMA 2018, 319, 1261–1268. [Google Scholar] [CrossRef]
- Marcus, J.L.; Hurley, L.B.; Nguyen, D.P.; Silverberg, M.J.; Volk, J.E. Redefining Human Immunodeficiency Virus (HIV) Preexposure Prophylaxis Failures. Clin. Infect. Dis. 2017, 65, 1768–1769. [Google Scholar] [CrossRef]
- El-Haddad, A.; Erlich, D. Cabotegravir (Apretude) for Pre-exposure Prophylaxis for HIV Type 1 Infection. Am. Fam. Physician 2023, 107, 545–546. [Google Scholar]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I.; et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N. Engl. J. Med. 2021, 385, 595–608. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Hughes, J.P.; Bock, P.; Ouma, S.G.; Hunidzarira, P.; Kalonji, D.; Kayange, N.; Makhema, J.; Mandima, P.; Mathew, C.; et al. Cabotegravir for the prevention of HIV-1 in women: Results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022, 399, 1779–1789. [Google Scholar] [CrossRef]
- Duwal, S.; Dickinson, L.; Khoo, S.; von Kleist, M. Mechanistic framework predicts drug-class specific utility of antiretrovirals for HIV prophylaxis. PLoS Comput. Biol. 2019, 15, e1006740. [Google Scholar] [CrossRef]
- Tsibris, A.M.N.; Hirsch, M.S. 130—Antiretroviral Therapy for Human Immunodeficiency Virus Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition); Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 1622–1641.e1626. [Google Scholar]
- Brown, K.C.; Patterson, K.B.; Jennings, S.H.; Malone, S.A.; Shaheen, N.J.; Asher Prince, H.M.; Spacek, M.; Cohen, M.S.; Kashuba, A.D. Single- and multiple-dose pharmacokinetics of darunavir plus ritonavir and etravirine in semen and rectal tissue of HIV-negative men. J. Acquir. Immune Defic. Syndr. 2012, 61, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Taverne-Ghadwal, L.; Kuhns, M.; Buhl, T.; Schulze, M.H.; Mbaitolum, W.J.; Kersch, L.; Weig, M.; Bader, O.; Groß, U. Epidemiology and Prevalence of Oral Candidiasis in HIV Patients From Chad in the Post-HAART Era. Front. Microbiol. 2022, 13, 844069. [Google Scholar] [CrossRef] [PubMed]
- Fenley, J.C.; de Barros, P.P.; Carmo, P.; Garcia, M.T.; Rossoni, R.D.; Junqueira, J.C. Repurposing HIV Protease Inhibitors Atazanavir and Darunavir as Antifungal Treatments against Candida albicans Infections: An In Vitro and In Vivo Study. Curr. Issues Mol. Biol. 2022, 44, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. Available online: https://www.who.int/publications/i/item/9789241550277 (accessed on 18 July 2023).
- Sidrim, J.J.; Perdigão-Neto, L.V.; Cordeiro, R.A.; Brilhante, R.S.; Leite, J.J.; Teixeira, C.E.; Monteiro, A.J.; Freitas, R.M.; Ribeiro, J.F.; Mesquita, J.R.; et al. Viral protease inhibitors affect the production of virulence factors in Cryptococcus neoformans. Can. J. Microbiol. 2012, 58, 932–936. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Silva, J.A.T.; Araújo, G.D.S.; Pereira, V.S.; Gotay, W.J.P.; Oliveira, J.S.; Guedes, G.M.M.; Pereira-Neto, W.A.; Castelo-Branco, D.; Cordeiro, R.A.; et al. Darunavir inhibits Cryptococcus neoformans/Cryptococcus gattii species complex growth and increases the susceptibility of biofilms to antifungal drugs. J. Med. Microbiol. 2020, 69, 830–837. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological Alert, Neurological Syndrome, Congenital Malformations, and Zika Virus Infection. Implications for Public Health in the Americas (1 December 2015). Available online: https://iris.paho.org/handle/10665.2/50697?show=full (accessed on 25 July 2023).
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika Virus Infection in the Brain by the Antiretroviral Drug Rilpivirine. Mol. Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Song, H.; Wang, H.; Chai, Y.; Su, C.; Qi, J.; Shi, Y.; Gao, G.F. The crystal structure of Zika virus NS5 reveals conserved drug targets. Embo J. 2017, 36, 919–933. [Google Scholar] [CrossRef]
- Noble, C.G.; Lim, S.P.; Arora, R.; Yokokawa, F.; Nilar, S.; Seh, C.C.; Wright, S.K.; Benson, T.E.; Smith, P.W.; Shi, P.Y. A Conserved Pocket in the Dengue Virus Polymerase Identified through Fragment-based Screening. J. Biol. Chem. 2016, 291, 8541–8548. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020, 18, 784–790. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Lei, L.; Egli, M. Label-Free Electrophoretic Mobility Shift Assay (EMSA) for Measuring Dissociation Constants of Protein-RNA Complexes. Curr. Protoc. Nucleic Acid. Chem. 2019, 76, e70. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Singh, M.; Ravichandiran, V.; Murty, U.S.N.; Srivastava, H.K. Peptide-like and small-molecule inhibitors against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 2904–2913. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S. Molecular Docking and Virtual Screening Based Prediction of Drugs for COVID-19. Comb. Chem. High. Throughput Screen. 2021, 24, 716–728. [Google Scholar] [CrossRef]
- Khan, S.A.; Zia, K.; Ashraf, S.; Uddin, R.; Ul-Haq, Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2021, 39, 2607–2616. [Google Scholar] [CrossRef]
- Silva, J.R.A.; Kruger, H.G.; Molfetta, F.A. Drug repurposing and computational modeling for discovery of inhibitors of the main protease (M(pro)) of SARS-CoV-2. RSC Adv. 2021, 11, 23450–23458. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Hegazy, M.F. In-silico drug repurposing and molecular dynamics puzzled out potential SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2021, 39, 5756–5767. [Google Scholar] [CrossRef]
- Velagacherla, V.; Suresh, A.; Mehta, C.H.; Nayak, U.Y.; Nayak, Y. Multi-Targeting Approach in Selection of Potential Molecule for COVID-19 Treatment. Viruses 2023, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Banik, A.; Chowdhury, I.M.; Sajib, E.H.; Sarkar, S. Identification of potential antivirals against SARS-CoV-2 using virtual screening method. Inform. Med. Unlocked 2021, 23, 100531. [Google Scholar] [CrossRef] [PubMed]
- Halder, U.C. Predicted antiviral drugs Darunavir, Amprenavir, Rimantadine and Saquinavir can potentially bind to neutralize SARS-CoV-2 conserved proteins. J. Biol. Res. 2021, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Swain, S.S.; Paital, B.; Panda, M. Combinatorial approach of vitamin C derivative and anti-HIV drug-darunavir against SARS-CoV-2. Front. Biosci. 2022, 27, 10. [Google Scholar] [CrossRef]
- Swain, S.S.; Singh, S.R.; Sahoo, A.; Panda, P.K.; Hussain, T.; Pati, S. Integrated bioinformatics-cheminformatics approach toward locating pseudo-potential antiviral marine alkaloids against SARS-CoV-2-Mpro. Proteins 2022, 90, 1617–1633. [Google Scholar] [CrossRef]
- Mahdi, M.; Mótyán, J.A.; Szojka, Z.I.; Golda, M.; Miczi, M.; Tőzsér, J. Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2’s main protease. Virol. J. 2020, 17, 190. [Google Scholar] [CrossRef]
- Gutierrez-Valencia, A.; Torres-Cornejo, A.; BenMarzouk-Hidalgo, O.J.; Ruiz-Valderas, R.; Lluch, A.; Viciana, P.; López-Cortés, L.F. Darunavir minimum plasma concentration and ritonavir-boosted darunavir monotherapy outcome in HIV-infected patients. Antivir. Ther. 2014, 19, 443–447. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Londero, A.; Della Siega, P.; Givone, F.; Fabris, M.; Biasizzo, J.; Tascini, C.; Pea, F. Comparative Population Pharmacokinetics of Darunavir in SARS-CoV-2 Patients vs. HIV Patients: The Role of Interleukin-6. Clin. Pharmacokinet. 2020, 59, 1251–1260. [Google Scholar] [CrossRef]
- Machavaram, K.K.; Almond, L.M.; Rostami-Hodjegan, A.; Gardner, I.; Jamei, M.; Tay, S.; Wong, S.; Joshi, A.; Kenny, J.R. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: Suppression of CYP3A by IL-6. Clin. Pharmacol. Ther. 2013, 94, 260–268. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment. Eur. J. Pharmacol. 2020, 889, 173634. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Liu, L.; Xu, Q.; Ling, Y.; Huang, D.; Huang, W.; Song, S.; Xu, S.; Shen, Y.; et al. Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa241. [Google Scholar] [CrossRef] [PubMed]
- Indu, P.; Rameshkumar, M.R.; Arunagirinathan, N.; Al-Dhabi, N.A.; Valan Arasu, M.; Ignacimuthu, S. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach. J. Infect. Public. Health 2020, 13, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Lucariello, A.; Sellitto, C.; Agliata, I.; Carleo, M.A.; Sangiovanni, V.; Esposito, V.; Guerra, G.; Cobellis, L.; De Luca, A. Different Cell Cycle Modulation in SKOV-3 Ovarian Cancer Cell Line by Anti-HIV Drugs. Oncol. Res. 2017, 25, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, B.; Usluer, S. p21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef]
- Pećina-Slaus, N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003, 3, 17. [Google Scholar] [CrossRef]
- Verma, S.; Salmans, M.L.; Geyfman, M.; Wang, H.; Yu, Z.; Lu, Z.; Zhao, F.; Lipkin, S.M.; Andersen, B. The estrogen-responsive Agr2 gene regulates mammary epithelial proliferation and facilitates lobuloalveolar development. Dev. Biol. 2012, 369, 249–260. [Google Scholar] [CrossRef]
- Schroeder, B.W.; Verhaeghe, C.; Park, S.W.; Nguyenvu, L.T.; Huang, X.; Zhen, G.; Erle, D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 178–185. [Google Scholar] [CrossRef]
- Zheng, W.; Rosenstiel, P.; Huse, K.; Sina, C.; Valentonyte, R.; Mah, N.; Zeitlmann, L.; Grosse, J.; Ruf, N.; Nürnberg, P.; et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes. Immun. 2006, 7, 11–18. [Google Scholar] [CrossRef]
- Moidu, N.A.; Rahman, N.S.A.; Syafruddin, S.E.; Low, T.Y.; Mohtar, M.A. Secretion of pro-oncogenic AGR2 protein in cancer. Heliyon 2020, 6, e05000. [Google Scholar] [CrossRef]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M.; et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef]
- Ly, T.T.G.; Yun, J.; Ha, J.S.; Kim, Y.J.; Jang, W.B.; Van Le, T.H.; Rethineswaran, V.K.; Choi, J.; Kim, J.H.; Min, S.H.; et al. Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 944. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Bahena, L.; Sánchez-Álvarez, A.A.; Ruiz-Moreno, A.J.; Velasco-Velázquez, M.A. Repositioning of Etravirine as a Potential CK1ε Inhibitor by Virtual Screening. Pharmaceuticals 2021, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Klimowski, L.K.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Virshup, D.M. Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. Febs J. 2006, 273, 4594–4602. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Dutta Chowdhury, K.; Dey, S.R.; Paul, A.; Haldar, R. Exploring the possibility of drug repurposing for cancer therapy targeting human lactate dehydrogenase A: A computational approach. J. Biomol. Struct. Dyn. 2022, 1–10. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Yin, C.Y.; Zhang, S.S.; Zhong, J.T.; Zhou, S.H. Pepsin and Laryngeal and Hypopharyngeal Carcinomas. Clin. Exp. Otorhinolaryngol. 2021, 14, 159–168. [Google Scholar] [CrossRef]
- Johnston, N.; Samuels, T.L.; Goetz, C.J.; Arnold, L.A.; Smith, B.C.; Seabloom, D.; Wuertz, B.; Ondrey, F.; Wiedmann, T.S.; Vuksanovic, N.; et al. Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model. Laryngoscope 2023, 133 (Suppl. S1), S1–S11. [Google Scholar] [CrossRef]
- Swami, D.; Mudaliar, P.; Bichu, Y.S.; Kumar Sahu, V.; Devarajan, S.; Basu, S.; Aich, J. Synergistic combination of ritonavir and cisplatin as an efficacious therapy in human cervical cancer cells: A computational drug discovery and in vitro insight. J. Biomol. Struct. Dyn. 2023, 41, 5802–5816. [Google Scholar] [CrossRef]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Efavirenz: History, Development and Future. Biomolecules 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Erber, S.; Harrer, T.; Klinker, H.; Roth, T.; Parsch, H.; Fiebig, N.; Fietkau, R.; Distel, L.V. Efavirenz Has the Highest Anti-Proliferative Effect of Non-Nucleoside Reverse Transcriptase Inhibitors against Pancreatic Cancer Cells. PLoS ONE 2015, 10, e0130277. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).