Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer
Abstract
:1. Introduction
2. Results
2.1. Study Population
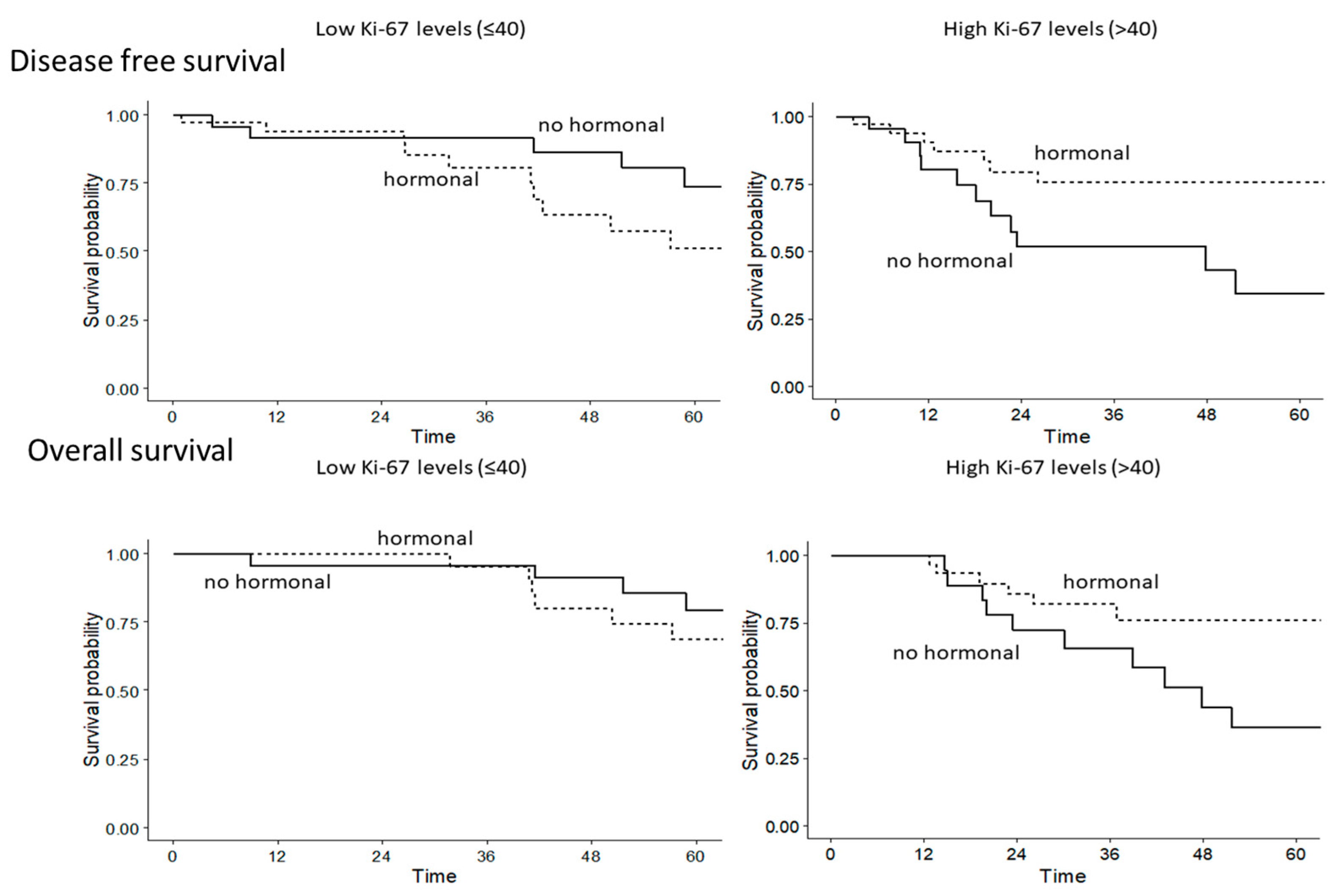
2.2. Univariate and Multivariate Analysis of Disease-Free Survival and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patients
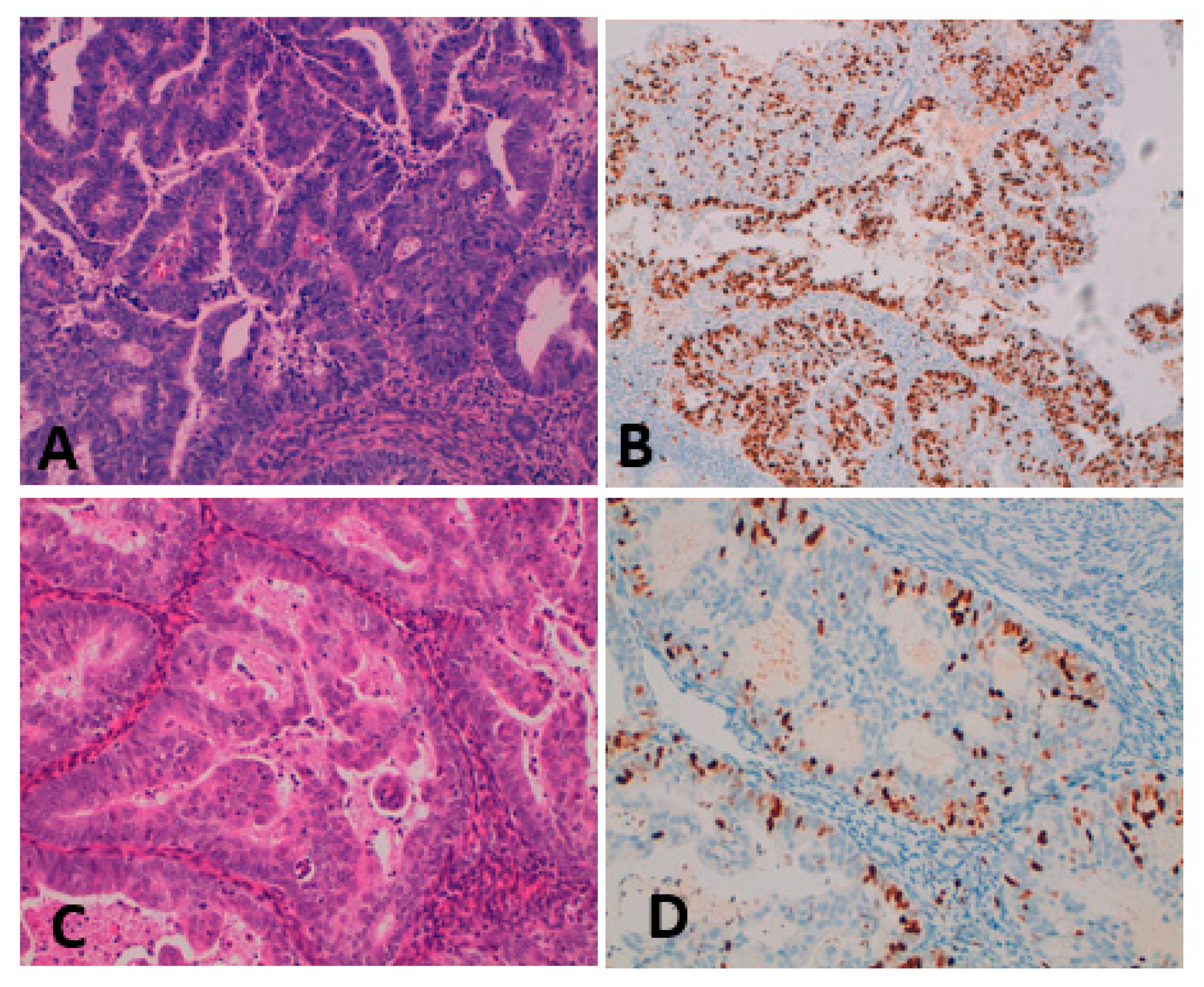
4.2. Tissue Sampling, Histopathological Analysis, and Ki-67 Determination
4.3. Evaluation of Ki-67 Value
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society, Cancer Statistics for USA. 2022. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html (accessed on 6 July 2022).
- “I numeri del Cancro in Italia 2022”, AIOM—AIRTUM. Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf (accessed on 21 January 2023).
- Paleari, L.; Pesce, S.; Rutigliani, M.; Greppi, M.; Obino, V.; Gorlero, F.; Vellone, V.G.; Marcenaro, E. New Insights into Endometrial Cancer. Cancers 2021, 13, 1496. [Google Scholar] [CrossRef] [PubMed]
- Onstand, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Tian, W.; Zhu, Y.; Xue, F. The therapeutic significance of aromatase inhibitors in endometrial carcinoma. Gynecol Oncol. 2014, 134, 190–195. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- Vermij, L.; Smit, V.; Nout, D.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Bosse, T.; Creutzberg, C.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 10 July 2022).
- Paleari, L.; Rutigliani, M.; Siri, G.; Provinciali, N.; Colombo, N.; DeCensi, A. Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study. Int. J. Mol. Sci. 2020, 21, 2227. [Google Scholar] [CrossRef]
- Gerdes, J.; Li, L.; Schlueter, C.; Duchrow, M.; Wohlenberg, C.; Gerlach, C.; Stahmer, I.; Kloth, S.; Brandt, E.; Flad, H.D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991, 138, 867–873. [Google Scholar]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumor proliferation marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lelle, R.J.; Pickartz, H.; Heidenreich, W.; Schwarting, R.; Kurtsiefer, L.; Stauch, G.; Stein, H. Growth fractions in breast cancers determined in situ with monoclonal antibody Ki-67. J. Clin. Pathol. 1986, 39, 977–980. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Hashmi, K.A.; Irfan, M.; Khan, S.M.; Edhi, M.M.; Ali, J.P.; Hashmi, S.K.; Asif, H.; Faridi, N.; Khan, A. Ki67 index in intrinsic breast cancer subtypes and its association with prognostic parameters. BMC Res. Notes 2019, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Smith, S.; Nielsen, T.O.; Parpia, S.; Fyles, A.W.; Bane, A.; Liu, F.-F.; Grimard, L.; Stevens, C.; Bowen, J.; et al. LUMINA: A prospective trial omitting radiotherapy (RT) following breast conserving surgery (BCS) in T1N0 luminal A breast cancer (BC). J. Clin. Oncol. 2022, 40, LBA501. [Google Scholar] [CrossRef]
- Salama, A.; Arafa, M.; ElZahaf, E.; Shebl, A.M.; Awad, A.A.E.; Ashamallah, S.A.; Hemida, R.; Gamal, A.; Foda, A.A.; Zalata, K.; et al. Potential Role for a Panel of Immunohistochemical Markers in the Management of Endometrial Carcinoma. J. Pathol. Transl. Med. 2019, 53, 164–172. [Google Scholar] [CrossRef]
- Zhu, C.; Luo, J.; Shi, H.; Xie, X.; Ding, Z. Expression of tubulin, p53, ki67, receptors for estrogen, and progesterone in endometrial cancer. Eur. J. Gynaecol. Oncol. 2009, 30, 514–517. [Google Scholar]
- Horrée, N.; van Diest, P.J.; van der Groep, P.; Sie-Go, D.M.; Heintz, A.P. Progressive derailment of cell cycle regulators in endometrial carcinogenesis. J. Clin. Pathol. 2008, 61, 36–42. [Google Scholar] [CrossRef]
- Stefansson, I.M.; Salvesen, H.B.; Immervoll, H.; Akslen, L.A. Prognostic impact of histological grade and vascular invasion compared with tumor cell proliferation in endometrial carcinoma of endometroid type. Histopathology 2004, 44, 472–479. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; RGelber, D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of woman with early breast cancer: Highlights of the St gallen international expert consensus on the primary therapy of early breast cancer. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Kitson, S.; Sivalingam, V.N.; Bolton, J.; McVey, R.; Nickkho-Amiry, M.; Powell, M.E.; Leary, A.; Nijman, H.W.; Nout, R.A.; Bosse, T. Ki-67 in endometrial cancer: Scoring optimization and prognostic relevance for window studies. Modern Pathol. 2017, 30, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jia, M.; Hu, J.; Huang, Z.; Deng, Y.; Lai, L.; Ding, S.; Hu, Z. Prognostic value of Ki67 in patients with stage 1–2 endometrial cancer: Validation of the cut-off value of Ki67 as a predictive factor. OncoTargets Ther. 2020, 13, 10841–10850. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Yardley, D.; Burris, H.A.; De Boer, R.; Amadori, D.; McIntyre, K.; Ejlertsen, B.; Gnant, M.; Jonat, W.; Pritchard, K.I.; et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: Final results of the randomized phase III Femara Versus Anastrozole Clinical Evaluation (FACE) trial. J. Clin. Oncol. 2017, 35, 1041–1048. [Google Scholar] [PubMed]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Ellis, M.J.; Sledge, G.W.; Budd, G.T.; Rabaglio, M.; Ansari, R.H.; Johnson, D.B.; Tozer, R.; et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—A randomized controlled phase III trial. J. Clin. Oncol. 2013, 31, 1398–1404. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Liu, X.; Ma, K.; Meng, Y.-T.; Yin, H.-F.; Wen, J.; Yang, J.-H.; Zhen, Z.; Feng, Z.-H.; et al. Clinical characteristics and prognostic characterization of endometrial carcinoma: A comparative analysis of molecular typing protocols. BMC Cancer 2023, 23, 243. [Google Scholar] [CrossRef]

| Overall (n = 158) | ||
|---|---|---|
| Age, median (IQR) | 75 (69–83) | |
| BMI, median (IQR) | 26.7 (23.6–31.0) | |
| Grade, n (%) | G1 | 9 (5.7) |
| G2 | 82 (52.2) | |
| G3 | 65 (41.4) | |
| I | 127 (81.4) | |
| Stage, n (%) | II | 13 (8.4) |
| III | 14 (9.0) | |
| Aromatase inhibitors | 92 (59.0) | |
| Therapy, n (%) | No adjuvant therapy | 49 (31.4) |
| Chemotherapy /Radiotherapy | 15 (9.6) | |
| ER, median (IQR) | 80 (60–90) | |
| PgR, median (IQR) | 80 (40–90) | |
| Ki-67, median (IQR) | 40 (30–70) |
| DFS HR (95% CI), p Value | OS HR (95% CI), p Value | |
|---|---|---|
| Age | 1.05 (1–01–1.08), 0.005 | 1.04 (1.00–1.09), 0.05 |
| Stage III/IV vs. Stage I/II | 7.60 (3.14–18.4), <0.001 | 1.85 (0.56–6.10), 0.31 |
| HT in high Ki-67 vs. no HT in high Ki-67 | 0.28 (0.12–0.69), 0.006 | 0.39 (0.14–1.08), 0.07 |
| No HT in high Ki-67 vs. no HT in low Ki-67 | 3.13 (1.35–7.14), 0.007 | 3.70 (1.69–8.33), 0.001 |
| HT in low Ki-67 vs. no HT in high Ki-67 | 0.45 (0.19–1.06), 0.068 | 0.26 (0.09–0.76), 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleari, L.; Rutigliani, M.; D’Ecclesiis, O.; Gandini, S.; Briata, I.M.; Webber, T.B.; Provinciali, N.; DeCensi, A. Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer. Int. J. Transl. Med. 2023, 3, 479-486. https://doi.org/10.3390/ijtm3040033
Paleari L, Rutigliani M, D’Ecclesiis O, Gandini S, Briata IM, Webber TB, Provinciali N, DeCensi A. Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer. International Journal of Translational Medicine. 2023; 3(4):479-486. https://doi.org/10.3390/ijtm3040033
Chicago/Turabian StylePaleari, Laura, Mariangela Rutigliani, Oriana D’Ecclesiis, Sara Gandini, Irene Maria Briata, Tania Buttiron Webber, Nicoletta Provinciali, and Andrea DeCensi. 2023. "Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer" International Journal of Translational Medicine 3, no. 4: 479-486. https://doi.org/10.3390/ijtm3040033
APA StylePaleari, L., Rutigliani, M., D’Ecclesiis, O., Gandini, S., Briata, I. M., Webber, T. B., Provinciali, N., & DeCensi, A. (2023). Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer. International Journal of Translational Medicine, 3(4), 479-486. https://doi.org/10.3390/ijtm3040033








