Trigeminal Neuralgia Treatment Outcomes Following Gamma Knife Stereotactic Radiosurgery
Abstract
1. Introduction
2. Materials and Methods
3. Results
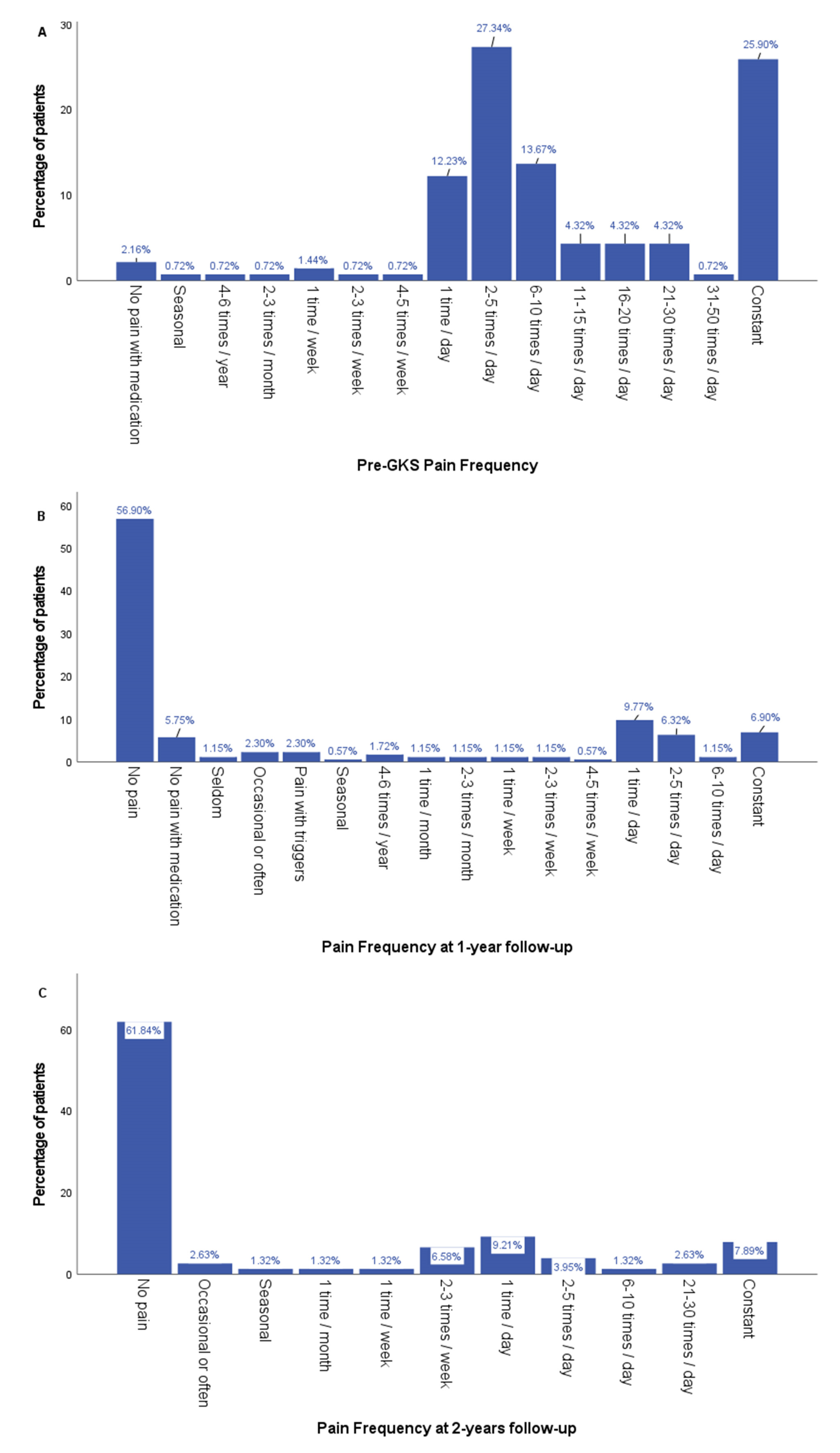
3.1. Clinical Outcomes
3.2. Factors Associated with Good GKRSR Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameli, N.O. Avicenna and trigeminal neuralgia. J. Neurol. Sci. 1965, 2, 105–107. [Google Scholar] [CrossRef]
- Love, S.; Patel, N.K. Trigeminal Neuralgia In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 1173–1177. [Google Scholar]
- Minervini, G.; Del Mondo, D.; Russo, D.; Cervino, G.; D’Amico, C.; Fiorillo, L. Stem Cells in Temporomandibular Joint Engineering: State of Art and Future Persectives. J. Craniofac. Surg. 2022, 33, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Fiorillo, L.; Russo, D.; Lanza, A.; D’Amico, C.; Cervino, G.; Meto, A.; Di Francesco, F. Treatment in Patients with Temporomandibular Disorders and Orofacial Pain and/or Bruxism: A Review of the Literature. Prosthesis 2022, 4, 253–262. [Google Scholar] [CrossRef]
- Minervini, G.; Russo, D.; Herford, A.S.; Gorassini, F.; Meto, A.; D’Amico, C.; Cervino, G.; Cicciu, M.; Fiorillo, L. Teledentistry in the Management of Patients with Dental and Temporomandibular Disorders. Biomed. Res. Int. 2022, 2022, 7091153. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; D’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. CRANIO® 2022, 1–17. [Google Scholar] [CrossRef]
- Burchiel, K.J. A new classification for facial pain. Neurosurgery 2003, 53, 1164–1166. [Google Scholar] [CrossRef]
- Nurmikko, T.J.; Eldridge, P.R. Trigeminal neuralgia—Pathophysiology, diagnosis and current treatment. Br. J. Anaesth. 2001, 87, 117–132. [Google Scholar] [CrossRef]
- Sheehan, J.; Pan, H.-C.; Stroila, M.; Steiner, L. Gamma knife surgery for trigeminal neuralgia: Outcomes and prognostic factors. J. Neurosurg. 2005, 102, 434–441. [Google Scholar] [CrossRef]
- Barzaghi, L.R.; Albano, L.; Scudieri, C.; Gigliotti, C.R.; del Vecchio, A.; Mortini, P. Factors affecting long-lasting pain relief after Gamma Knife radiosurgery for trigeminal neuralgia: A single institutional analysis and literature review. Neurosurg. Rev. 2021, 44, 2797–2808. [Google Scholar] [CrossRef]
- Tuleasca, C.; Régis, J.; Sahgal, A.; De Salles, A.; Hayashi, M.; Ma, L.; Martínez-Álvarez, R.; Paddick, I.; Ryu, S.; Slotman, B.J.; et al. Stereotactic radiosurgery for trigeminal neuralgia: A systematic review. J. Neurosurg. 2019, 130, 733–757. [Google Scholar] [CrossRef]
- Rogers, C.L.; Shetter, A.G.; Fiedler, J.A.; Smith, K.A.; Han, P.P.; Speiser, B.L. Gamma knife radiosurgery for trigeminal neuralgia: The initial experience of the Barrow Neurological Institute. Int. J. Radiat. Oncol. 2000, 47, 1013–1019. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Riesenburger, R.I.; Hwang, S.W.; Schirmer, C.M.; Zerris, V.; Wu, J.K.; Mahn, K.; Klimo, P., Jr.; Mignano, J.; Thompson, C.J.; Yao, K.C. Outcomes following single-treatment Gamma Knife surgery for trigeminal neuralgia with a minimum 3-year follow-up. J. Neurosurg. 2010, 112, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Chang, J.W. Gamma Knife Radiosurgery on the Trigeminal Root Entry Zone for Idiopathic Trigeminal Neuralgia: Results and a Review of the Literature. Yonsei Med. J. 2020, 61, 111–119. [Google Scholar] [CrossRef]
- Fountas, K.N.; Smith, J.R.; Lee, G.P.; Jenkins, P.D.; Cantrell, R.R.; Sheils, W.C. Gamma Knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: Long-term outcome and complications. Neurosurg. Focus 2007, 23, E8. [Google Scholar] [CrossRef]
- Hayashi, M.; Chernov, M.; Tamura, N.; Taira, T.; Izawa, M.; Yomo, S.; Nagai, M.; Chang, C.S.; Ivanov, P.; Tamura, M.; et al. Stereotactic radiosurgery of essential trigeminal neuralgia using Leksell Gamma Knife model C with automatic positioning system: Technical nuances and evaluation of outcome in 130 patients with at least 2 years follow-up after treatment. Neurosurg. Rev. 2011, 34, 497–508. [Google Scholar] [CrossRef]
- Young, R.F.; Vermulen, S.; Posewitz, A. Gamma knife radiosurgery for the treatment of trigeminal neuralgia. Stereotact. Funct. Neurosurg. 1998, 70, 192–199. [Google Scholar] [CrossRef]
- Maesawa, S.; Salame, C.; Flickinger, J.C.; Pirris, S.; Kondziolka, D.; Lunsford, L.D. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J. Neurosurg. 2001, 94, 14–20. [Google Scholar] [CrossRef]
- Pollock, B.E.; Phuong, L.K.; Gorman, D.A.; Foote, R.L.; Stafford, S.L. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J. Neurosurg. 2002, 97, 347–353. [Google Scholar] [CrossRef]
- Petit, J.H.; Herman, J.M.; Nagda, S.; DiBiase, S.J.; Chin, L.S. Radiosurgical treatment of trigeminal neuralgia: Evaluating quality of life and treatment outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1147–1153. [Google Scholar] [CrossRef]
- Urgosik, D.; Liscak, R.; Novotny, J., Jr.; Vymazal, J.; Vladyka, V. Treatment of essential trigeminal neuralgia with gamma knife surgery. J. Neurosurg. 2005, 102, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Longhi, M.; Rizzo, P.; Nicolato, A.; Foroni, R.; Reggio, M.; Gerosa, M. Gamma knife radiosurgery for trigeminal neuralgia: Results and potentially predictive parameters—Part I: Idiopathic trigeminal neuralgia. Neurosurgery 2007, 61, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Zorro, O.; Lobato-Polo, J.; Kano, H.; Flannery, T.J.; Flickinger, J.C.; Lunsford, L.D. Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J. Neurosurg. 2010, 112, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Chan, M.D.; McCoy, T.P.; Aubuchon, A.C.; Bourland, J.D.; McMullen, K.P.; Deguzman, A.F.; Munley, M.T.; Shaw, E.G.; Tatter, S.B.; et al. Predictive variables for the successful treatment of trigeminal neuralgia with gamma knife radiosurgery. Neurosurgery 2012, 70, 566–572; discussion 572–563. [Google Scholar] [CrossRef]
- Young, B.; Shivazad, A.; Kryscio, R.J.; Clair, W.S.; Bush, H.M. Long-term outcome of high-dose gamma knife surgery in treatment of trigeminal neuralgia. J. Neurosurg. 2013, 119, 1166–1175. [Google Scholar] [CrossRef]
- Lucas, J.T., Jr.; Nida, A.M.; Isom, S.; Marshall, K.; Bourland, J.D.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Predictive nomogram for the durability of pain relief from gamma knife radiation surgery in the treatment of trigeminal neuralgia. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 120–126. [Google Scholar] [CrossRef]
- Regis, J.; Tuleasca, C.; Resseguier, N.; Carron, R.; Donnet, A.; Gaudart, J.; Levivier, M. Long-term safety and efficacy of Gamma Knife surgery in classical trigeminal neuralgia: A 497-patient historical cohort study. J. Neurosurg. 2016, 124, 1079–1087. [Google Scholar] [CrossRef]
- Taich, Z.J.; Goetsch, S.J.; Monaco, E.; Carter, B.S.; Ott, K.; Alksne, J.F.; Chen, C.C. Stereotactic Radiosurgery Treatment of Trigeminal Neuralgia: Clinical Outcomes and Prognostic Factors. World Neurosurg. 2016, 90, 604–612. [Google Scholar] [CrossRef]
- Moreno, N.E.M.; Gutiérrez-Sárraga, J.; Rey-Portolés, G.; Jiménez-Huete, A.; Álvarez, R.M. Long-Term Outcomes in the Treatment of Classical Trigeminal Neuralgia by Gamma Knife Radiosurgery. Neurosurgery 2016, 79, 879–888. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, Y.; Yao, D.; Xiong, N.; Abdelmaksoud, A.; Wang, H. Outcomes of Two-Isocenter Gamma Knife Radiosurgery for Patients with Typical Trigeminal Neuralgia: Pain Response and Quality of Life. World Neurosurg. 2018, 109, e531–e538. [Google Scholar] [CrossRef]
- Gagliardi, F.; Spina, A.; Bailo, M.; Boari, N.; Cavalli, A.; Franzin, A.; Fava, A.; Del Vecchio, A.; Bolognesi, A.; Mortini, P. Effectiveness of Gamma Knife Radiosurgery in Improving Psychophysical Performance and Patient’s Quality of Life in Idiopathic Trigeminal Neuralgia. World Neurosurg. 2018, 110, e776–e785. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Chen, C.-J.; Chong, S.T.; Hung, S.-C.; Yang, H.-C.; Lin, C.J.; Wu, C.-C.; Chung, W.-Y.; Guo, W.-Y.; Pan, D.H.-C.; et al. Early Stereotactic Radiosurgery for Medically Refractory Trigeminal Neuralgia. World Neurosurg. 2018, 112, e569–e575. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.D.; Tai, A.; Wooster, M.; Rashid, A.; Chen, R.; Baig, N.; Jay, A.; Harter, K.W.; Randolph-Jackson, P.; Omogbehin, A.; et al. Trigeminal neuralgia treatment outcomes following Gamma Knife radiosurgery with a minimum 3-year follow-up. J. Radiat. Oncol. 2014, 3, 125–130. [Google Scholar] [CrossRef]
- Reinard, K.; Nerenz, D.R.; Basheer, A.; Tahir, R.; Jelsema, T.; Schultz, L.; Malik, G.; Air, E.L.; Schwalb, J.M. Racial disparities in the diagnosis and management of trigeminal neuralgia. J. Neurosurg. 2017, 126, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.H.; Sherry, A.D.; Kim, E.; Anderson, J.; Luo, G.; Yu, H.; Englot, D.J.; Chambless, L.B.; Cmelak, A.J.; Attia, A. Body mass index and response to stereotactic radiosurgery in the treatment of refractory trigeminal neuralgia: A retrospective cohort study. J. Radiosurg. SBRT 2020, 6, 253–261. [Google Scholar]
- Hozumi, J.; Sumitani, M.; Matsubayashi, Y.; Abe, H.; Oshima, Y.; Chikuda, H.; Takeshita, K.; Yamada, Y. Relationship between Neuropathic Pain and Obesity. Pain Res. Manag. 2016, 2016, 2487924. [Google Scholar] [CrossRef]
- Rossi, H.L.; Broadhurst, K.A.; Luu, A.S.K.; Lara, O.; Kothari, S.D.; Mohapatra, D.P.; Recober, A. Abnormal trigeminal sensory processing in obese mice. Pain 2016, 157, 235–246. [Google Scholar] [CrossRef][Green Version]
- Arnone, G.D.; Esfahani, D.R.; Papastefan, S.; Rao, N.; Kumar, P.; Slavin, K.V.; Mehta, A.I. Diabetes and morbid obesity are associated with higher reoperation rates following microvascular decompression surgery: An ACS-NSQIP analysis. Surg. Neurol. Int. 2017, 8, 268. [Google Scholar] [CrossRef]
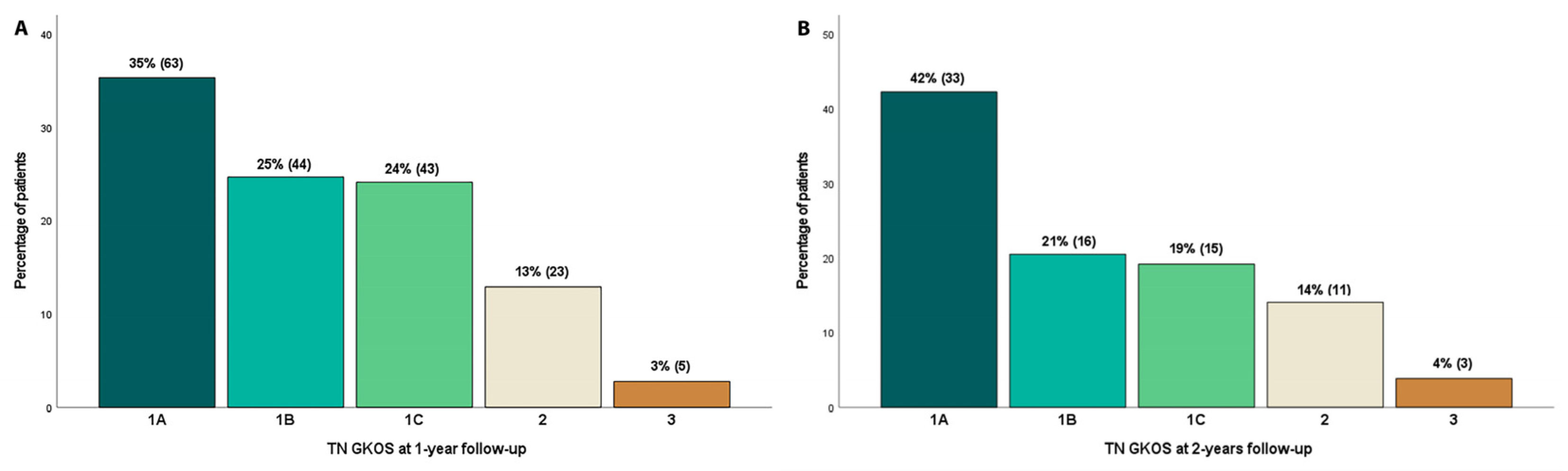
| Trigeminal Neuralgia Gamma Knife Outcome Scale (TN GKOS) | |
|---|---|
| Score | Description |
| 1A | Pain-free & off all pain medications |
| 1B | Pain-free on pain medications |
| 1C | Some pain, improved with GKSRS |
| 2 | Same as before GKSRS |
| 3 | Worse pain compared to before GKSRS |
| Variable | Category | Number Percentage % | TN GKOS at 1 Year | p-Value | TN GKOS at 2 Years | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 1C | 2 | 3 | 1A | 1B | 1C | 2 | 3 | |||||
| Age | Adult (18–60) | 48 27 | 10 20.8 | 8 16.7 | 17 35.4 | 10 20.8 | 3 6.3 | 0.005 | 5 25.0 | 4 20.0 | 6 30.0 | 3 15.0 | 2 10.0 | 0.185 |
| Geriatric (>60) | 130 73 | 53 40.8 | 36 27.7 | 26 20.0 | 13 10.0 | 2 1.5 | 28 48.3 | 12 20.7 | 9 15.5 | 8 13.8 | 1 1.7 | |||
| Gender | Male | 62 34.8 | 26 41.9 | 12 19.4 | 12 19.4 | 11 17.7 | 1 1.6 | 0.252 | 12 48.0 | 6 24.0 | 3 12.0 | 3 12.0 | 1 4.0 | 0.806 |
| Female | 116 65.2 | 37 31.9 | 32 27.6 | 31 26.7 | 12 10.3 | 4 3.4 | 21 39.6 | 10 18.9 | 12 22.6 | 8 15.1 | 2 3.8 | |||
| Race | African American | 21 11.8 | 4 19.0 | 2 9.5 | 9 42.9 | 6 28.6 | 0 0.0 | 0.013 | 3 30.0 | 0 0.0 | 5 50.0 | 2 20.0 | 0 0.0 | 0.180 |
| Caucasian | 153 86.0 | 58 37.9 | 42 27.5 | 31 20.3 | 17 11.1 | 5 3.3 | 29 43.9 | 16 24.2 | 9 13.6 | 9 13.6 | 3 4.5 | |||
| Hispanic | 4 2.2 | 1 25.0 | 0 0.0 | 3 75.0 | 0 0.0 | 0 0.0 | 1 50.0 | 0 0.0 | 1 50.0 | 0 0.0 | 0 0.0 | |||
| Pain side | Right | 80 50.0 | 29 36.3 | 20 25.0 | 16 20.0 | 13 16.3 | 2 2.5 | 0.038 | 18 54.5 | 5 15.2 | 4 12.1 | 6 18.2 | 0 0.0 | 0.014 |
| Left | 77 48.1 | 26 33.8 | 20 26.0 | 23 29.9 | 7 9.1 | 1 1.3 | 13 39.4 | 8 24.2 | 9 27.3 | 2 6.1 | 1 3.0 | |||
| Bilateral | 3 1.9 | 1 33.3 | 0 0.0 | 1 33.3 | 0 0.0 | 1 33.3 | 0 0.0 | 0 0.0 | 1 33.3 | 1 33.3 | 1 33.3 | |||
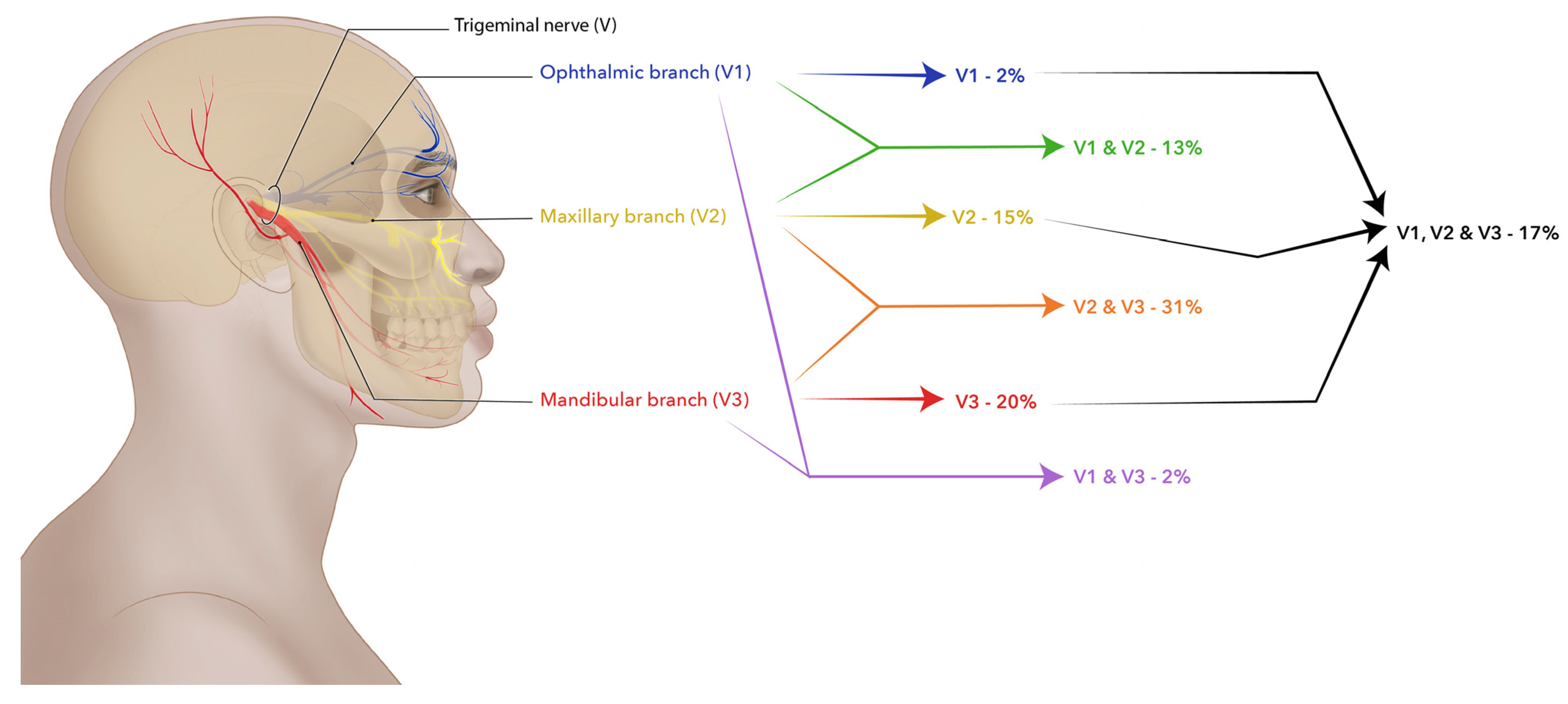
| Pain division | V1 | 3 1.9 | 0 0.0 | 2 66.7 | 0 0.0 | 1 33.3 | 0 0.0 | 0.751 | 0 0.0 | 0 0.0 | 0 0.0 | 2 100 | 0 0.0 | 0.161 |
| V2 | 24 15.2 | 7 29.2 | 6 25.0 | 9 37.5 | 2 8.3 | 0 0.0 | 3 30.0 | 3 30.0 | 4 40.0 | 0 0.0 | 0 0.0 | |||
| V3 | 32 20.3 | 11 34.4 | 9 28.1 | 8 25.0 | 4 12.5 | 0 0.0 | 9 69.2 | 1 7.7 | 2 15.4 | 1 7.7 | 0 0.0 | |||
| V1, V2 | 21 13.3 | 9 42.9 | 2 9.5 | 4 19.0 | 5 23.8 | 1 4.8 | 6 54.5 | 1 9.1 | 2 18.2 | 1 9.1 | 1 9.1 | |||
| V1, V3 | 3 1.9 | 2 66.7 | 0 0.0 | 1 33.3 | 0 0.0 | 0 0.0 | 2 66.7 | 0 0.0 | 1 33.3 | 0 0.0 | 0 0.0 | |||
| V2, V3 | 49 31.0 | 18 36.7 | 14 28.6 | 12 24.5 | 3 6.1 | 2 4.1 | 8 36.4 | 6 27.3 | 4 18.2 | 4 18.2 | 0 0.0 | |||
| V1, V2, V3 | 26 16.5 | 8 30.8 | 7 26.9 | 6 23.1 | 4 15.4 | 1 3.8 | 3 37.5 | 2 25.0 | 1 12.5 | 1 12.5 | 1 12.5 | |||
| Atypical pain | Yes | 7 4.4 | 0 0.0 | 1 14.3 | 3 42.9 | 3 42.9 | 0 0.0 | 0.052 | 0 0.0 | 0 0.0 | 0 0.0 | 2 100 | 0 0.0 | 0.008 |
| No | 153 95.6 | 56 36.6 | 39 25.5 | 37 24.2 | 17 11.1 | 4 2.6 | 31 46.3 | 13 19.4 | 14 20.9 | 7 10.4 | 2 3.0 | |||
| Category | Variable | Number (Percentage %) | Category | Variable | Number (Percentage %) |
|---|---|---|---|---|---|
| Pain character | Aggravating factors | ||||
| Stabbing pain | 98 (61.3) | Activity | 52 (32.5) | ||
| Electrical shock pain | 134 (83.8) | Eating | 135 (84.4) | ||
| Sharp pain | 136 (85.0) | Heat | 38 (23.8) | ||
| Dull pain | 49 (30.6) | Positioning | 85 (53.1) | ||
| Aching pain | 53 (33.1) | Talking | 128 (80.0) | ||
| Tender pain | 79 (49.4) | Cold | 96 (60.0) | ||
| Pressure pain | 48 (30.0) | Coughing/Deep breaths | 53 (33.1) | ||
| Throbbing pain | 88 (55.0) | Touch | 119 (74.4) | ||
| Cramping pain | 18 (11.3) | Brushing teeth | 116 (72.5) | ||
| Burning pain | 69 (43.1) | Brushing hair | 50 (31.3) | ||
| Pulling pain | 22 (13.8) | Shaving | 37 (23.1) | ||
| Changes in quality of life | Putting makeup | 54 (33.8) | |||
| None | 10 (6.3) | Alleviating factors | |||
| Sleep changes | 70 (43.8) | Rest | 40 (25.0) | ||
| Reduced appetite | 49 (30.6) | Medication | 129 (80.6) | ||
| Reduced physical activity | 78 (48.8) | Heat | 37 (23.1) | ||
| Emotional | 31 (19.4) | Cold | 8 (5.0) | ||
| Altered relationships | 43 (26.9) | ||||
| Variable | Number Percentage % | TN GKOS at 1 Year | p-Value | TN GKOS at 2 Years | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 1C | 2 | 3 | 1A | 1B | 1C | 2 | 3 | |||||
| Diabetes mellitus | Yes | 25 14.0 | 9 36.0 | 5 20.0 | 8 32.0 | 3 12.0 | 0 0.0 | 0.765 | 5 38.5 | 3 23.1 | 3 23.1 | 2 15.4 | 0 0.0 | 0.933 |
| No | 153 86 | 54 35.3 | 39 25.5 | 35 22.9 | 20 13.1 | 5 3.3 | 28 43.1 | 13 20.0 | 12 18.5 | 9 13.8 | 3 4.6 | |||
| Hypertension | Yes | 85 47.8 | 27 31.8 | 23 27.1 | 22 25.9 | 12 14.1 | 1 1.2 | 0.576 | 15 41.7 | 9 25.0 | 7 19.4 | 4 11.1 | 1 2.8 | 0.864 |
| No | 93 52.2 | 36 38.7 | 21 22.6 | 21 22.6 | 11 11.8 | 4 4.3 | 18 42.9 | 7 16.7 | 8 19.0 | 7 16.7 | 2 4.8 | |||
| Hyperlipidemia | Yes | 32 18.0 | 13 40.6 | 9 28.1 | 7 21.9 | 2 6.3 | 1 3.1 | 0.749 | 8 50.0 | 3 18.8 | 3 18.8 | 2 12.5 | 0 0.0 | 0.890 |
| No | 146 82.0 | 50 34.2 | 35 24.0 | 36 24.7 | 21 14.4 | 4 2.7 | 25 40.3 | 13 21.0 | 12 19.4 | 9 14.5 | 3 4.8 | |||
| Obesity | Yes | 5 2.8 | 0 0.0 | 1 20.0 | 3 60.0 | 0 0.0 | 1 20.0 | 0.030 | 0 0.0 | 0 0.0 | 0 0.0 | 1 50.0 | 1 50.0 | 0.005 |
| No | 173 97.2 | 63 36.4 | 43 24.9 | 40 23.1 | 23 13.3 | 4 2.3 | 33 43.4 | 16 21.1 | 15 19.7 | 10 13.2 | 2 2.6 | |||
| Hypothyroidism | Yes | 24 13.5 | 7 29.2 | 5 20.8 | 7 29.2 | 5 20.8 | 0 0.0 | 0.577 | 6 75.0 | 1 12.5 | 0 0.0 | 1 12.5 | 0 0.0 | 0.330 |
| No | 154 86.5 | 56 36.4 | 39 25.3 | 36 23.4 | 18 11.7 | 5 3.2 | 27 38.6 | 15 21.4 | 15 21.4 | 10 14.3 | 3 4.3 | |||
| Multiple sclerosis | Yes | 5 2.8 | 1 20.0 | 2 40.0 | 1 20.0 | 1 20.0 | 0 0.0 | 0.876 | 1 100 | 0 0.0 | 0 0.0 | 0 0.0 | 0 0.0 | 0.847 |
| No | 173 97.2 | 62 35.8 | 42 24.3 | 42 24.3 | 22 12.7 | 5 2.9 | 32 41.6 | 16 20.8 | 15 19.5 | 11 14.3 | 3 3.9 | |||
| Meningioma | Yes | 2 1.1 | 1 50.0 | 0 0.0 | 0 0.0 | 1 50.0 | 0 0.0 | 0.506 | 1 100 | 0 0.0 | 0 0.0 | 0 0.0 | 0 0.0 | 0.847 |
| No | 176 98.9 | 62 35.2 | 44 25.0 | 43 24.4 | 22 12.5 | 5 2.8 | 32 41.6 | 16 20.8 | 15 19.5 | 11 14.3 | 3 3.9 | |||
| Neurological dysfunction | Yes | 2 1.1 | 0 0.0 | 2 100 | 0 0.0 | 0 0.0 | 0 0.0 | 0.188 | 1 100 | 0 0.0 | 0 0.0 | 0 0.0 | 0 0.0 | 0.847 |
| No | 176 98.9 | 63 35.8 | 42 23.9 | 43 24.4 | 23 13.1 | 5 2.8 | 32 41.6 | 16 20.8 | 15 19.5 | 11 14.3 | 3 3.9 | |||
| Stroke | Yes | 12 6.7 | 6 50.0 | 4 33.3 | 1 8.3 | 1 8.3 | 0 0.0 | 0.547 | 4 66.7 | 0 0.0 | 1 16.7 | 1 16.7 | 0 0.0 | 0.636 |
| No | 166 93.3 | 57 34.3 | 40 24.1 | 42 25.3 | 22 13.3 | 5 3.0 | 29 40.3 | 16 22.2 | 14 19.4 | 10 13.9 | 3 4.2 | |||
| Dementia | Yes | 2 1.1 | 0 0.0 | 1 50.0 | 1 50.0 | 0 0.0 | 0 0.0 | 0.714 | ||||||
| No | 176 98.9 | 63 35.8 | 43 24.4 | 42 23.9 | 23 13.1 | 5 2.8 | ||||||||
| Seizures | Yes | 1 0.6 | 0 0.0 | 0 0.0 | 0 0.0 | 1 100 | 0 0.0 | 0.148 | ||||||
| No | 177 99.4 | 63 35.6 | 44 24.9 | 43 24.3 | 22 12.4 | 5 2.8 | ||||||||
| Familial tremor | Yes | 1 0.6 | 0 0.0 | 0 0.0 | 0 0.0 | 1 100 | 0 0.0 | 0.148 | 0 0.0 | 0 0.0 | 1 100 | 0 0.0 | 0 0.0 | 0.373 |
| No | 177 99.4 | 63 35.6 | 44 24.9 | 43 24.3 | 22 12.4 | 5 2.8 | 33 42.9 | 16 20.8 | 14 18.2 | 11 14.3 | 3 3.9 | |||
| Psychiatric disorders (depression, bipolar, anxiety, sleep disorders) | Yes | 18 10.1 | 6 33.3 | 4 22.2 | 5 27.8 | 1 5.6 | 2 11.1 | 0.204 | 3 50.0 | 1 16.7 | 1 16.7 | 1 16.7 | 0 0.0 | 0.979 |
| No | 160 89.9 | 57 35.6 | 40 25.0 | 38 23.8 | 22 13.8 | 3 1.9 | 30 41.7 | 15 20.8 | 14 19.4 | 10 13.9 | 3 4.2 | |||
| Temporomandibular joint dysfunction | Yes | 2 1.1 | 1 50.0 | 0 0.0 | 1 50.0 | 0 0.0 | 0 0.0 | 0.827 | 0 0.0 | 1 50.0 | 1 50.0 | 0 0.0 | 0 0.0 | 0.538 |
| No | 176 98.9 | 62 35.2 | 44 25.0 | 42 23.9 | 23 13.1 | 5 2.8 | 33 43.4 | 15 19.7 | 14 18.4 | 11 14.5 | 3 3.9 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrahi, A.; Cantrell, R.; Norris, C.; Dhandapani, K.; Barrett, J.; Vender, J. Trigeminal Neuralgia Treatment Outcomes Following Gamma Knife Stereotactic Radiosurgery. Int. J. Transl. Med. 2022, 2, 543-554. https://doi.org/10.3390/ijtm2040041
Jarrahi A, Cantrell R, Norris C, Dhandapani K, Barrett J, Vender J. Trigeminal Neuralgia Treatment Outcomes Following Gamma Knife Stereotactic Radiosurgery. International Journal of Translational Medicine. 2022; 2(4):543-554. https://doi.org/10.3390/ijtm2040041
Chicago/Turabian StyleJarrahi, Abbas, Rebecca Cantrell, Cynthia Norris, Krishnan Dhandapani, John Barrett, and John Vender. 2022. "Trigeminal Neuralgia Treatment Outcomes Following Gamma Knife Stereotactic Radiosurgery" International Journal of Translational Medicine 2, no. 4: 543-554. https://doi.org/10.3390/ijtm2040041
APA StyleJarrahi, A., Cantrell, R., Norris, C., Dhandapani, K., Barrett, J., & Vender, J. (2022). Trigeminal Neuralgia Treatment Outcomes Following Gamma Knife Stereotactic Radiosurgery. International Journal of Translational Medicine, 2(4), 543-554. https://doi.org/10.3390/ijtm2040041






