Abstract
Diabetic retinopathy (DR) is the most common complication of diabetes and a major cause of vision loss worldwide. The premature death of the microvascular mural cells represents both a pathological hallmark of vasodegeneration in DR and a basis for therapeutic intervention to halt progression to the sight-threatening stages. Recent studies suggest that retinal microvascular mural cells, classed as pericytes in the capillaries and vascular smooth muscle cells in the larger vessels (VSMC), may undergo autophagy-dependent cell death during DR. The present investigation was undertaken to assess electron microscopic evidence for involvement of autophagy in mediation of cell death in the mural cells of the retinal vasculature, in eyes from human diabetic donors and diabetic dogs. All specimens examined showed widespread evidence of autophagosomes in processes of viable pericytes and VSMCs, and the membranous remnants of excessive autophagic activity in their “ghost cell” remnants within the vascular walls. Autophagy was termed “excessive” when it occupied the greater part of the cytoplasm in mural cell processes. This was notable in specimens from short-term diabetic donors with no evidence of basement-membrane thickening or mural cell loss, in which regions of mural cell cytoplasm filled with autophagic bodies appeared to be undergoing cytoplasmic cleavage. No equivalent evidence of autophagy was detected in the adjacent endothelial cells of the retinal vessels. We conclude that increased autophagy in the retinal pericytes and VSMCs is linked to the diabetic milieu, and over time may also act as a trigger for mural cell loss and progressive vasodegeneration.
1. Introduction
Diabetic retinopathy (DR) is the most common complication of diabetes [1], and as the incidence of the disease continues to rise, represents an increasing challenge to healthcare systems around the world [2]. The selective loss of microvascular pericytes was established as a histopathological hallmark of vasodegeneration in diabetic retinopathy over 70 years ago [3] and the concomitant loss of vascular smooth muscle cells (VSMCs) has also been recognised [4]. Such loss of vascular mural cells is described as selective because the associated endothelial cells continue to survive for much longer, and the parent vessels remain perfused [4]. Only when dysfunction and cell injury exceed the reparative capacity of the endothelium do the vessels undergo closure and non-perfusion [5]. The differential vulnerability between endothelial and mural cells in diabetes is likely to be related to their functional and metabolic specialization. For example, pericytes and VSMCs suffer higher levels of pathological polyol pathway activity than their endothelial counterparts [6,7,8], as well as a greater accumulation of advanced glycation end-products (AGEs) [9]. Furthermore, both types of mural cells are vulnerable to toxicity due to oxidised lipid products [10,11] and suffer autophagic cell death as a result [12,13]. In addition, the expression or biological activity of the most important mural cell survival factors, PDGF [14,15,16] and IGF-1 [17], are reduced in the diabetic retina [18,19,20], while VEGF continues to be available to support the survival and proliferation of the endothelial cells. This difference is illustrated by the fact that although apoptotic cell death has been demonstrated in both endothelial cells and pericytes [21], only endothelial cells appear to retain mitotic competency during diabetes, showing a three-fold-increased turnover in rat retinal vasculature after 6 months of diabetes [22]. Unfortunately, the cross-sectional nature of such studies has limited our knowledge of how soon endothelial cell turnover increases and at what point their regenerative capacity becomes exhausted. Loss of mural cells, especially VSMCs, probably exposes the endothelium to increased haemodynamic stress [23]. This, combined with diabetes-induced pro-inflammatory [24] and procoagulant [25] changes in the endothelium, may precipitate vascular closure due to leukocyte damage [26], or thrombotic events due to platelet dysfunction, even before the replicative, or Hayflick limit [27] of ECs has been reached.
In DR, in spite of a combination of metabolic and mechanical stresses that may dispose to cell death, at the molecular level apoptosis represents the outcome of both pro-death and pro-survival factors [28]. In common with many cell types, mural cells may recover from death-inducing insults by intervention of appropriate survival factor signalling [17]. Although vascular cells can express their requisite survival factors in an autonomous fashion during in vitro culture, in both retinal development and the adult retina, the major sources of vascular growth/survival factors are the retinal neurons and glia [19,29,30,31,32]. Therefore, in DR, the loss of neuronal and glial survival signalling renders the vascular cells increasingly vulnerable to acute pro-apoptotic insults. Such insults may be mediated by proinflammatory cytokines, oxidative damage or, in the case of pericytes, exposure to oxidised lipoproteins [10,11,12] that conceivably gain access to the vascular wall and neuropile through breakdown of the blood–retinal barrier. While endothelial cells dying by either apoptosis or necrosis probably detach from their basal lamina and pass into circulation, dead pericytes and vascular smooth muscle cells remain trapped within the ensheathing vascular basement membrane and can be visualised in vascular digests as periodic acid–Schiff-positive “ghosts”, or by electron microscopy [3,4]. How long pericyte and VSMC ghosts persist within the layers of basal lamina in the vascular wall is unknown. We and others have assumed that these pockets of vesicular debris represented either necrosis or post-apoptotic necrosis because of inefficient scavenging of apoptotic bodies enveloped within the thickened vascular basement membrane. However, the recognition of autophagy as either a precursor or effector of cell death in a wide variety of cell types [33], including retinal pericytes and VSMCs [10,11,12,13], calls for a re-evaluation of this interpretation. Autophagy is the process whereby portions of cytoplasm and the contained organelles are engulfed by a crescent-shaped double-membrane structure called the phagophore [34]. Phagophores can be nucleated on various endomembranes, but the endoplasmic reticulum (ER) exit sites (ERES) have been proposed as likely candidates as they are enriched in COP-II-coated membrane, which is known to contribute membrane in the phagophore expansion phase [35,36]. However, strong evidence points to a central role for ER–mitochondrial contact sites in autophagosome initiation [37,38], and mitochondria as an important source of membrane in autophagosome biogenesis [39]. Following enlargement of the vesicle, membrane fusion of the two double-membrane complexes at the ends of the phagophore completes the autophagosome (AP). APs subsequently fuse with lysosomes and the contents are digested, liberating low-molecular-weight energy substrates and amino acids for new protein synthesis, some of which is devoted to autophagy-related proteins such as lysosomal hydrolases [40]. Under normal circumstances autophagy enhances cell survival, but in disease situations may participate in cell death [33,41].
The aim of the current study was to qualitatively assess what, if any, evidence was present in the retinal vasculature of human donor eyes that could support the thesis for involvement of autophagy in the death of pericytes and/or VSCMs in DR. The study also examines evidence from diabetic dogs that represent the only non-insulinopenic long-term animal model of diabetic retinopathy that shows all the vasodegenerative changes of DR as it occurs in human disease [4,42,43]: specifically, basement-membrane thickening, mural cell death, vascular closure, IRMA, and “cotton wool” spots. What constitutes type-2 or “autophagic cell death” was first defined “as a type of cell death that occurs without chromatin condensation, accompanied by massive autophagic vacuolization of the cytoplasm. These vacuoles, by definition, are two-membraned and contain degenerating cytoplasmic organelles or cytosol” [44]. As this definition was based on morphology, with transmission electron microscopy as the desirable modality [44], we considered a review of electron micrographs that focused on retinal vascular pathology but with no particular focus on autophagy, to represent a valid approach that would add to our current knowledge.
2. Methods
Electron micrographs were available for analysis from the central retina of one eye from each of three insulin-dependent spontaneously diabetic dogs that had been obtained from local veterinary practitioners in the 1980s and one eye from each of four experimental alloxan–streptozotocin-induced diabetic dogs. The spontaneous diabetic dogs had been diabetic for between 4 and 6 years, although the exact duration of diabetes was uncertain. The animals were euthanized due to deteriorating health and the eyes fixed in phosphate-buffered 10% formalin; these eyes were refixed in glutaraldehyde before dissection. The experimental diabetic dogs had been induced with a cocktail of alloxan and streptozotocin as previously described [45] and maintained on daily insulin in a state of moderately stable hyperglycaemia (mean blood sugar 20 ± 5 mM/L) for 5 years. The eyes from the experimental diabetic dogs were fixed in 2.5% glutaraldehyde in either 0.1 M phosphate or 0.1 M cacodylate buffer at pH7.4.
Single eyes from six different human donors were obtained from hospitals in the UK or Ireland between 1979 and 1989, in accordance with the Treaty of Helsinki 1964 and with permission of local ethical committees. All the donors had suffered from type-2 diabetes and were aged between 55 and 69 years of age. None had proliferative DR, although the duration of diabetes was not available, except for two males aged 55 and 57 who had been diagnosed diabetic within one year of death. Donor eyes (n = 6) were enucleated withing 6 h of death and fixed, transported, and stored in phosphate-buffered 10% formalin and re-fixed in 2.5% glutaraldehyde prior to dissection. For comparison, tissue blocks of three normal retinas were prepared from superior calottes removed at the dissection of three eyes that had been enucleated due to the presence of malignant melanomas in the anterior uvea. The patients were males aged 34, 53, and 58 years. All tissue blocks for electron microscopy were post-fixed in 1% osmium tetroxide, dehydrated in ascending concentrations of ethanol and embedded in Spurr’s resin. Ultrathin sections of 3 mm strips of retina were prepared from at least six blocks from posterior non-macular retina (the macular region was not available for electron microscopic analysis) and stained with 2% uranyl acetate in 50% ethanol followed by Reynold’s lead citrate.
Although 291 electron micrographs of human retina and 116 from the retina of diabetic dogs were recorded in the original studies, no meaningful statistical analysis was possible in the present study as the electron micrographs of retinal blood vessels were not randomly selected. The vascular images in particular tended to exclude oblique profiles where basement-membrane thickness was ambiguous and focused on vessels showing overt pathology, such as pericyte degeneration or loss. Again, in the context of autophagy as the focus of the current analysis, it should be appreciated that occasional autophagosomes in either endothelial or mural cells would have been interpreted as non-pathological features and not specifically recorded. Only when autophagic bodies were prominent in cells showing overt evidence of cell death, or when occupying a significant volume of the cytoplasm of otherwise normal cells, would they have been recorded as features of interest. As a consequence of such selectivity, no gradation in autophagic activity could be detected.
3. Results
3.1. Differentiation of Post-Mortem (PM) Change from Diabetes-Related Mural Cell Death
Although the overwhelming majority of the retinal vessel profiles examined in this investigation showed typical diabetes-related basement-membrane thickening, the images recorded tended to represent vascular profiles that also featured mural or endothelial cell pathology or vascular occlusion. As the retinas of the experimental diabetic dogs received optimal fixation and preservation, they were used as the gold standard in assessing the possible impact of delayed fixation on the retinas from the spontaneous diabetic dogs and human donors. Invariably, fixation delay in human donor retinas was significantly better tolerated by endothelial cells than by pericytes. Endothelial cells showed normal cell organelles, nuclear chromatin, and the predominant basal-plasma-membrane disposition of caveolae that is typical of functioning retinal vessels (Figure 1A,B). There was also evidence of continued plasma-membrane dynamics, such as the presence of coated pits at the luminal plasma membranes (Figure 1F). However, in contrast to optimally fixed eyes, the endothelial cells of the retinal vessels in donor eyes showed many filopodia-like luminal-plasma-membrane protrusions (Figure 1C,D). These were interpreted as a PM phenomenon reflecting hypoxia-linked cell activation. PM oxygen deprivation was also reflected by the ultrastructure of the pericytes in donor retina, although this was more profound with increased granularity of the nuclear chromatin (Figure 1E,F), and a swollen cytoplasm containing similarly distended organelles with the vacant, electron-lucent appearance typical of early PM necrosis (Figure 1A,B,E,F). The Muller cell and astrocyte processes comprising the glia limitans, the glial foot-processes responsible for the formation, and maintenance of the glial component of the vascular basement membranes, showed greater resistance to PM hypoxia than the adjacent pericytes and often contained a high concentration of particulate glycogen (Figure 1A–C,E).
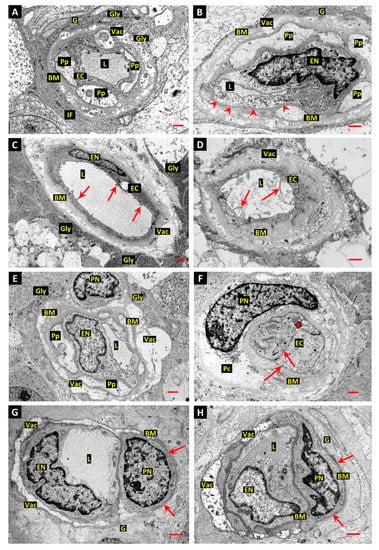
Figure 1.
Assessment of post-mortem change in human retinal capillaries. (A,B): Retinal capillary profiles from diabetic donor eye retina show intact endothelial cells (EC) with a normal distribution of well-preserved mitochondria, endoplasmic reticulum, and free polyribosomes. In contrast, the adjacent pericyte processes (Pp) are pale and swollen with sparse, swollen mitochondria and endoplasmic reticulum. However, the plasma membranes appear intact and still show dense matrix-attachment sites. The glial processes forming the glia limitans are well-preserved and show darkly stained aggregates of glycogen particles (Gly) and dense bundles of intermediate filaments (IF) interspersed with microtubules. The distinctive basal polarization of the plasma-membrane caveolae was preserved and caveolae fully occupied large stretches of the basal plasma membrane of the endothelial cells (arrow heads in panel B). Age-related basement membrane (BM) vacuolation (Vac). Panel A, bar 1.0 µm; panel B, bar 1.0 µm. (C): Electron micrograph of small retinal vessel from diabetic donor. The vessel is larger than a capillary but because the mural cell covering has been lost, it is unclear whether it was a small arteriole or venule. The basement membrane is thickened and laminated, and the endothelial cell shows numerous luminal microvilli (arrows). The glial cells forming the glia limitans contain dense masses of glycogen particles. Age-related BM vacuolation (Vac). Bar 1.0 µm. (D): Retinal capillary from diabetic donor retina shows complete pericyte loss and the basement membrane is grossly thickened and laminated. The endothelial cell appears viable and numerous luminal microvilli are present, suggestive of early post-mortem (PM) migratory activity. Bar 1.0 µm. (E): Retinal capillary from diabetic donor shows an intact endothelial cell and pericyte with swollen cell processes and increased granularity of the chromatin in the cell nucleus (PN) compared to that of the endothelial nucleus (EN). The perinuclear cytoplasm of the pericyte retains some mitochondria and cytoplasmic polysomes. Glycogen deposits are present in the processes of the glia limitans (Gly). Bar 1.0 µm. (F): Retinal capillary showing a swollen pericyte with more advanced PM necrosis than that depicted in panel E. The nuclear chromatin is more granular, and the nuclear envelope is disintegrating. Much of the content of the perinuclear cytoplasm (Pc) has been completely lost and only small remnants of the plasma membrane can be detected. The extreme swelling of the pericyte has closed the vascular lumen (thick arrow), although the endothelia cell cytoplasm and organelles appear intact. Clathrin-coated pits are present at both the luminal and basal plasma membranes (arrows). Bar 1.0 µm. (G,H): Retinal capillaries from promptly fixed retinas of 58-year-old (panel G) and 55-year-old (panel H) normal human surgical specimens. Both vessels show the same “Swiss cheese” BM vacuolation that is typical of adult human retinal capillaries (Vac). However, in contrast to the thickened and laminated BMs in retinal capillaries of diabetic donors, the segments of BM unaffected by vacuolation at the glial interface (arrows), and at the pericyte–endothelial interfaces, are relatively thin and regular. Furthermore, the nuclear chromatin, cytoplasmic electron density, and cytoplasmic organelles are similar in pericytes and ECs. Endothelial nucleus (EN); pericyte nucleus (PN); vessel lumen (L); glial processes (G). Bars 1.0 µm.
Normal human retinal capillaries (Figure 1G,H) from promptly fixed surgical specimens show the same “Swiss cheese” BM vacuolation observed at the outer aspect of the retinal vessels in diabetic subjects and which are typically present in adult human retinal capillaries (Vac). However, in contrast to the thickened and laminated BMs in retinal capillaries of diabetic donors, the segments of BM unaffected by vacuolation at the glial interface (arrows), and at the pericyte–endothelial interfaces, are relatively thin and regular. Furthermore, the nuclear chromatin, cytoplasmic electron density, and cytoplasmic organelles are similar in pericytes and ECs.
3.2. Evidence of Mural Cell Autophagy in Diabetic Retinas
In comparison to the pale empty cytoplasm of mural cells undergoing PM necrosis, the pericyte and VSMC “ghosts” that had undergone diabetes-associated cell death were filled with vesicular and granular debris (Figure 2A–F and Figure 3A,B), that on closer inspection was often enclosed in the remnants of endomembranes (Figure 2E,F). The ghost cells remained enclosed in the pocket of basement membrane that they had occupied in life and only small remnants of their plasma membrane remained around intact cell processes (Figure 3A,B). Interestingly, other pockets of better-preserved membrane-enclosed structures were found to reside in intact mural cell processes in both diabetic dogs (Figure 2G,H) and donor retinas (Figure 3C–F). Such bodies were extremely heterogeneous in their form and contents, and some examples clearly showed enclosure by the double membrane that typifies autophagosomes, with membrane-bound segments of cytoplasm and organelles within (Figure 3C–F). The autophagosomes featured in Figure 3D,F appear to have been fixed at a relatively early stage following generation of the initial phagophore.
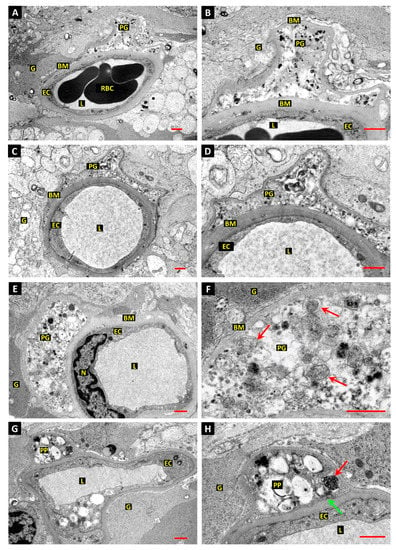
Figure 2.
Evidence of autophagy associated with cell death of retinal pericytes in diabetic dogs. (A–D): Electron micrographs from retina of 5-year experimental diabetic dog shows a dilated capillary blood vessel with an intact endothelial cell (EC) but no viable pericyte covering. The basement membrane (BM) is grossly thickened and a pericyte “ghost” (PG) filled with vesicular and granular electron-dense material is present within the BM. Red blood cells (RBC), vessel lumen (L), glial processes (G). Bars 1.0 µm. (E,F): Retinal capillary as those depicted in panels A–D, shows a pericyte “ghost” filled with granular and vesicular material; endothelial cell nucleus (N). Higher magnification reveals that the vesicles are enclosed by secondary layers of endomembranes, but no intact plasma membrane is discernible. Bar 1.0 µm. (G,H): Retinal capillary as those depicted in panels A–D, but with intact pericyte processes. One pericyte process (PP) shows cytoplasmic vacuolation and heterogeneous membrane-bound material suggestive of autophagy. A double-membrane-enclosed dense body (red arrow) may represent a late autophagosome. A clathrin-coated pit (green arrow) is present at the inner aspect of the plasma membrane, indicating continued membrane dynamics. Bar 1.0 µm.
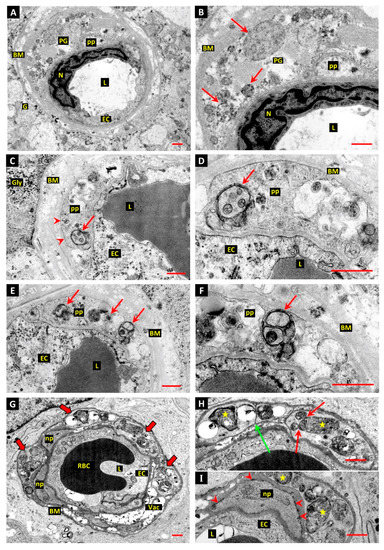
Figure 3.
Evidence of autophagy associated with death of retinal pericytes in diabetic humans. (A,B): Electron micrographs of diabetic human donor retina shows a capillary blood vessel with an intact endothelial cell (EC) but no viable pericyte covering; endothelial cell nucleus (N), vessel lumen (L), glial cell (G). The basement membrane (BM) is grossly thickened and laminated, and a pericyte “ghost” (PG) filled with vesicular and granular material is present within the BM. The plasma membrane appears to have disintegrated, apart from that surrounding a small pericyte process (pp). Bar 1.0 µm. (C–F): Retinal capillary from diabetic human donor shows pericyte processes (pp) with intact plasma membranes (arrow heads in panel C) containing autophagosomes. The autophagosomes contain enclosed cytoplasm and organelles (arrows). Panels E and F feature another profile of the pericyte depicted in panels C and D, with more autophagosomes containing sequestered cytoplasm and organelles (arrows). The well-preserved autophagosomes depicted in panels D and F are thought to represent early stages in the development of the compartments. Bars 1.0 µm. (G–I): Capillary-sized retinal vessel from a human donor diagnosed diabetic within 1 year of enucleation shows several mural cell processes filled with autophagosomes (panel G—thick arrows). Panel G: Red blood cell (RBC); basement membrane vacuolisation (Vac). The plasma membranes are intact and double membranes can be defined around several of the autophagic bodies (stars in panels H and I). Fusion between autophagic bodies, or secondary lysosomes, and autophagosomes is evident (red arrows in panel H). One process is divided by tightly apposed plasma membranes with no associated basal lamina, suggesting cytoplasmic cleavage (green arrow in panel H). Two apparently normal pericyte processes (np) are also present (panels G and I). Bar 1.0 µm.
In one eye from a donor who had been diagnosed diabetic within 1 year of enucleation, several processes, presumed to belong to the same pericyte, were almost totally filled by autophagic vacuoles (Figure 3G–I). These vacuoles showed evidence of fusion with other membrane-bound bodies, presumed to represent secondary lysosomes (Figure 3H), and several showed unambiguous sequestration by double membranes (Figure 3H,I). An unusual feature, observed in two cell processes involved in such excessive autophagy, was tight apposition of their plasma membranes without intervening basal lamina, suggesting that they were engaged in cytoplasmic cleavage (Figure 3H). Processes of an apparently normal pericyte were present in the same vascular profile (Figure 3G,I) and the basement membrane (BM) was not grossly thickened, although it had the typical age-related “Swiss cheese” vacuolation that is common in adult human retinal capillaries [46]. The spontaneous diabetic dog retinas in the current study showed similar BM vacuolation (Figure 1G,H), although it was not observed in the retinal vessels of the experimental diabetic dogs, presumably because they were over 2 years younger than the spontaneous diabetic animals.
Vascular smooth muscle cell (VSMC) “ghosts” show similar membranous and granular deposits as those of pericytes, likewise confined to well circumscribed pockets within the basement membranes of the host vessels (Figure 4A–H). Ghost cells in the larger retinal arteries which possessed more than one layer of VSMCs, tended to be located in the outer layer of myocytes at the glial interface. At certain stages, VSMC ghosts were filled with dense concentrations of vesicular bodies, showing membranous whorls (Figure 4D,F) with evidence of fusion (Figure 4D). Furthermore, VSMC ghost cells filled with autophagic remnants could be found in close proximity to normal VSMCs with which they had apparently shared a junction (Figure 4E,F). Interestingly, excessive autophagy was present in a mural cell of a retinal venule from a short-term type-2 diabetic donor, and, in common with the pericytes engaged in such activity, showed similar evidence of cytoplasmic cleavage (Figure 4G,H). Significantly, the region of cytoplasm involved in extreme autophagy and cleavage was confined to one pole of the cell, while the cell nucleus and perinuclear cytoplasm appeared normal (Figure 4G,H).
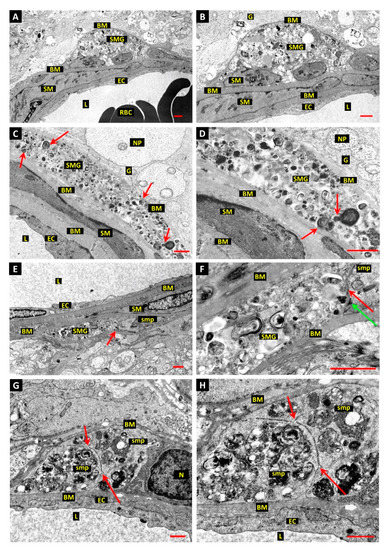
Figure 4.
Evidence of autophagy associated with mural cell death in arterioles and venules of diabetic dog and human retinas. (A,B): Electron micrograph of vascular smooth muscle “ghost” (SMG) in the wall of a retinal arteriole from 5-year experimental diabetic dog is represented as a pocket of heterogeneous cell debris, consisting of electron-dense granular material and vesicular structures enclosed by the basement membrane. The “ghost” cell is located at the glial interface on the outer aspect of the vascular wall. Basement membrane (BM); endothelial cell (EC); red blood cells (RBC); smooth muscle cell (SM); glial cells (G); vessel lumen (L); neural cell process (NP). Bar 1.0 µm. (C,D): Longitudinal section of vascular smooth muscle cell “ghost” (SMG) in retinal arteriole of 5-year experimental diabetic dog shows the fusiform profile of the original cell filled with vesicular material (examples arrowed). Endomembrane preservation is poor, but fusion of autophagic bodies can be inferred (arrows in panel D). Bar 1.0 µm. (E,F): Presumptive junction between VSMC “ghost” and normal smooth muscle cell process (smp). The zone of contact is indicated by red arrows in panel E and panel F. The dense plasma-membrane attachment site (green arrow, panel F) adjacent to the zone of contact (red arrow, panel F) suggests that the site represents pre-existing contact between neighbouring VSMCs. Bar 1.0 µm. (G,H): Electron micrograph of a short-term type-2 diabetic human donor shows one pole of a mural cell from a large retinal vein engaged in intensive autophagy. The autophagic zone is clearly demarcated from the adjacent normal nucleus and cytoplasm, possibly by an invagination of the plasma membrane sectioned en face. Furthermore, the autophagic pole of the cell appears to be engaged in cytoplasmic cleavage, with tightly apposed invaginations of the plasma membrane showing no associated basal lamina. Two tripartite junctions of the cleavage segments are indicated by red arrows. Bar 1.0 µm.
In comparison to the pericytes and VSMCs observed in the present study, the adjacent endothelial cells showed little evidence of autophagy.
4. Discussion
Autophagy, also known as macroautophagy to distinguish it from the many substrate-specific variants of the process [47], is an essential player in the nutrient and energy economy of normal cells [48]. It is also an essential mechanism of last resort for the elimination of misfolded proteins when the more specific mechanisms of proteostasis fail in neurodegenerative disease [49]. Although an established cell-survival mechanism, autophagy as a mode of cell death has been controversial since it was first accepted as type-2 cell death [44]. This ambiguity centres on the fact that severely stressed cells may induce autophagy as a survival mechanism, but if the cell cannot be saved then apoptosis may ensue in a scenario called death with autophagy [50]. By contrast, deregulation of the autophagic process can lead to cell death when a critical percentage of the cell’s resources have been consumed [41,50], leading to death by autophagy. Of course, the only way to prove autophagy as the final death mechanism is to show that it is independent of apoptosis and that inhibition of autophagy can rescue the cells from death [51]. In light of these issues, a recent convention has recommended the use of the term autophagy-dependent cell death [52,53] in place of autophagic cell death. In the context of the present study, the term autophagic cell death cannot be applied as we were presented with snap-shots of autophagic activity in mural cells without a temporal sequence. The best-preserved profiles of typical autophagy were located in cells that were obviously viable at the time of fixation, while the ghost cells that were clearly dead, at best, showed only the packed vesicular remnants of the death process. Therefore, the most useful conclusions that can be drawn from the evidence provided are circumstantial and subject to interpretation.
Nevertheless, the current study has shown excessive autophagy in viable pericytes and VSMCs, and gross accumulations of vesicular remnants in their dead counterparts during DR. These findings support a role for autophagy, in either effecting cell death, or triggering death by other means. No other process related to cell death can explain the composition of the mural cell ghosts observed in this study and previously [4,23]. The residual mass of vesicular bodies in ghost cells, and the necessary expansion of the endomembrane system to account for these, is not a feature of either necrosis or apoptosis, but is consistent with the agreed ultrastructural classification of autophagy-dependent cell death [44]. Morphologically, necrosis is characterised by swelling of the cytoplasm and its organelles, and progressive solubilisation of the structural elements, leading to eventual loss of plasma-membrane integrity and fragmentation [44]. Indeed, the PM autolysis of pericytes in donor eyes in the present study shows all the morphological features of necrotic cell death consistently observed in diverse cell types [44,54]. Again, ghost cells show little resemblance to any of the features of apoptosis. Maintenance of the original cell outlines by ghost cells is inconsistent with the morphology of apoptotic death, which is characterised by extreme shrinkage accompanied by nuclear and cytoplasmic cleavage to yield apoptotic bodies. Cells dying through apoptosis have hyper-condensed chromatin bound by segments of the nuclear envelope [55] and the final cytoplasmic cleavage utilises the plasma membrane with no requirement for the gross expansion of endomembranes required to generate the dense mass of vesicular contents observed in mural cell ghosts in the current investigation. Even if apoptotic body phagocytosis is delayed, as is likely for cells undergoing apoptosis within the confines of the vascular basement membrane, post-apoptotic necrosis is a passive process with no need or possibility of new membrane genesis. In contrast, autophagy, while it generates energy-producing substrates during starvation [48], is itself an energy-dependent process [56] requiring new membrane and phospholipid synthesis [57,58].
An intriguing finding of the present study was evidence of cytoplasmic cleavage in mural cells engaged in extreme autophagy, firstly, in a pericyte process in the wall of a capillary sized vessel, and secondly, in a mural cell of a retinal venule (Figure 3H and Figure 4G,H). Significantly, the extreme autophagy in the venous mural cell was confined to one pole of the cell and the portion of cytoplasm involved was sharply demarcated from the adjacent nucleus and perinuclear cytoplasm, which appeared normal at this stage. The lack of intervening basal lamina between the tightly apposed invaginations of the plasma membrane precluded the possibility that the segments of cytoplasm involved could have been independent cell processes, as mural cell processes are completely encased in basal lamina, except where they make direct contact with the endothelial cells. The only cell death-related phenomenon that produces cytoplasmic cleavage is plasma membrane “blebbing” during apoptosis [59]. Interestingly, the death-associated protein kinase (DAPk) has been shown to be involved in both apoptotic membrane blebbing [60] and endoplasmic reticulum (ER) stress-induced caspase activation and autophagic cell death [61,62]. As ER-stress has been implicated in the autophagic cell death of pericytes exposed to oxidised lipids in the study by Fu et al. [12], it is possible that DAPk, although not assayed in that work, could have been involved. Such a mechanism could account for cleavage of the autophagosome-filled cytoplasm of pericytes described in the present study. To date, membrane blebbing in apoptosis has only been described during in vitro culture and it can reasonably be assumed that the same process occurring within the confines of the retinal vascular basement membrane would be unable to form the spherical blebs that typify blebbing in the open fluid environment of a culture dish [63]. Rather, it is likely that the blebs would be crowded tightly together, as shown by the closely apposed plasma-membrane segments within the vascular wall in the present study.
Notably, two of the best-preserved examples of mural cells engaged in excessive autophagy and cytoplasmic cleavage were present in eyes from donors that were reported to have a relatively short duration of diabetes. As the donors had type-2 diabetes, the exact time of onset is uncertain, although the relatively normal BM thickness in the vessels concerned suggests that the duration of diabetes was short. This raises the intriguing possibility that diabetes-related pericyte stress, sufficient to induce extreme autophagy, such as when exposed to oxidatively modified lipids [10,12], can occur early in the disease process. This may precipitate acute pericyte death in cell-specific events that do not require the slow cumulative toxicity of the diabetic environment to become manifest. Within this paradigm, pericyte death in DR may be reconsidered as episodic, rather than linear, with autophagy-dependent pericyte death occurring as discrete events related to the energy status of individual cells [64,65], and available survival signalling within the local neuro-vascular unit.
Interestingly, as evidence of even maintenance levels of autophagy was difficult to find in the adjacent endothelium, excessive mural cell autophagy in early DR may reflect a particular vulnerability of these cells to the high level of oxidative and metabolic stress in the diabetic retina [66,67,68]. Autophagy is a powerful mediator of survival in stressed cells and mitophagy in particular may be triggered by reactive oxygen (ROS) species as a mechanism to reduce mitochondrial volume and associated ROS generation [69]. Indeed, a recent study has shown a general increase in mitophagy during early diabetic retinopathy in Ins2Akita/+ mice, but which declined later in the disease process to allow the accumulation of dysfunctional mitochondria and increased ROS production [70]. However, it is unknown whether insulin treatment could have reversed or deferred the long-term failure of mitophagy, established once the hyperglycaemic state had become chronic. Nevertheless, aberrant ROS generation by mitochondria [66], and other sources, such as NADPH-oxidase [71], is an established feature of vascular cells in DR and therefore related triggers of autophagy need to be considered in the possible autophagy-dependent death of mural cells. Specifically, the high level of oxidant-induced DNA damage in diabetes [72,73], particularly the preponderance of single-strand breaks (SSBs) [73], has been implicated as an activator of poly-ADP-ribose polymerase-1 (PARP-1). PARP plays a vital role in the DNA-damage response [74] and induction of autophagy [75]. Overactivity of PARP in DR leads to pathological consumption of its substrate NAD+ that in turn rapidly depletes cellular ATP and may induce cell death [76]. However, such ATP loss will be rapidly detected by the AMP-dependent protein kinase (AMPK), the central energy sensor and activator of autophagy [77], so that if energy homeostasis may be restored through autophagy, death may be deferred.
In addition to oxidative stress, autophagy in retinal mural cells in DR may be mediated by the instabilities in blood glucose and survival signalling that accompany treatment with exogenous insulin. Metabolic stress induced by rapid fluctuations in blood sugar can trigger apoptotic cell death in cultured pericytes [78], possibly due to the downregulation of glucose transporters that occurs during hyperglycaemia in pericytes but not in endothelial cells [79]. In explanation, endothelial cells may be less vulnerable to metabolic stress due to their predominantly glycolytic metabolism [80]. Indeed, in the current study it was obvious that both endothelial cells and the Muller cells adjacent to the vessels were able to withstand PM hypoxia more successfully than the pericytes. On this issue, it is relevant that Muller cells also have a predominantly glycolytic metabolism [81] and store glycogen so that glucose availability may not be limiting during hypoxia. Indeed, the glial glycogen stores were obvious in the diabetic donor retinas in the current study. However, precisely why pericytes are differentially vulnerable to hypoxia is currently unknown. Considering the central role of retinal hypoxia, both functional and ischaemic during DR [23], endothelial versus pericyte survival and resilience to hypoxia represent an important differential that remains to be exploited therapeutically.
This study has provided evidence of excessive activation of autophagy in the diabetic retina but has also exposed serious gaps in our current knowledge of mural cell energy metabolism. These deficiencies in our understanding of pericyte biology suggest an ongoing need for research on basic mural cell metabolism, both in vivo and in vitro, and within a physiologically relevant oxygen environment. Such studies will be able to correctly place autophagy within the framework of the normal mural cell energy economy and define its role as a pivotal process determining either life or death [41] in diabetic retinopathy.
Author Contributions
Formal analysis, T.A.G.; writing—original draft preparation, T.A.G.; writing—review and editing, A.W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was performed on anonymous archival data, so no institutional ethical approval was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study involved qualitative evaluation of electron micrographs. No numerical data was produced.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Cogan, D.G.; Toussaint, D.; Kuwabara, T. Retinal Vascular Patterns. IV. Diabetic Retinopathy. Arch. Ophthalmol. 1961, 66, 366–378. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Stitt, A.W.; Anderson, H.R.; Archer, D.B. Selective Loss of Vascular Smooth Muscle Cells in the Retinal Microcirculation of Diabetic Dogs. Br. J. Ophthalmol. 1994, 78, 54–60. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Archer, D.B.; Curtis, T.M.; Stitt, A.W. Arteriolar Involvement in the Microvascular Lesions of Diabetic Retinopathy: Implications for Pathogenesis. Microcirculation 2007, 14, 25–38. [Google Scholar] [CrossRef]
- Tawata, M.; Ohtaka, M.; Hosaka, Y.; Onaya, T. Aldose Reductase mRNA Expression and Its Activity Are Induced by Glucose in Fetal Rat Aortic Smooth Muscle (A10) Cells. Life Sci. 1992, 51, 719–726. [Google Scholar] [CrossRef]
- Morrison, A.D. Effect of inhibition of polyol pathway activity on aortic smooth muscle metabolism. Clin. Investig. Med. 1990, 13, 119–122. [Google Scholar]
- Hohman, T.C.; Nishimura, C.; Robison, W.G., Jr. Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp. Eye Res. 1989, 48, 55–60. [Google Scholar] [CrossRef]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 1997, 150, 523–531. [Google Scholar]
- Fu, D.; Wu, M.; Zhang, J.; Du, M.; Yang, S.; Hammad, S.M.; Wilson, K.; Chen, J.; Lyons, T.J. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia 2012, 55, 3128–3140. [Google Scholar] [CrossRef]
- Martinet, W.; De Bie, M.; Schrijvers, D.M.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Yu, J.Y.; Yang, S.; Wu, M.; Hammad, S.M.; Connell, A.R.; Du, M.; Chen, J.; Lyons, T.J. Survival or death: A dual role for autophagy in stress-induced pericyte loss in diabetic retinopathy. Diabetologia 2016, 59, 2251–2261. [Google Scholar] [CrossRef]
- Martinet, W.; De Meyer, G.R. Autophagy in atherosclerosis. Curr. Atheroscler. Rep. 2008, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Babic, S.; De Gooyer, T.; Stitt, A.W.; Jaworski, K.; Ong, L.G.; Kelly, D.J.; Gilbert, R.E. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am. J. Pathol. 2004, 164, 1263–1273. [Google Scholar] [CrossRef]
- Stitt, A.W.; Hughes, S.J.; Canning, P.; Lynch, O.; Cox, O.; Frizzell, N.; Thorpe, S.R.; Cotter, T.G.; Curtis, T.M.; Gardiner, T.A. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia 2004, 47, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef]
- Bennett, M.R.; Evan, G.I.; Schwartz, S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Investig. 1995, 95, 2266–2274. [Google Scholar] [CrossRef]
- Geraldes, P.; Hiraoka-Yamamoto, J.; Matsumoto, M.; Clermont, A.; Leitges, M.; Marette, A.; Aiello, L.P.; Kern, T.S.; King, G.L. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009, 15, 1298–1306. [Google Scholar] [CrossRef]
- Cox, O.T.; Simpson, D.A.; Stitt, A.W.; Gardiner, T.A. Sources of PDGF expression in murine retina and the effect of short-term diabetes. Mol. Vis. 2003, 9, 665–672. [Google Scholar]
- Gerhardinger, C.; McClure, K.D.; Romeo, G.; Podesta, F.; Lorenzi, M. IGF-I mRNA and signaling in the diabetic retina. Diabetes 2001, 50, 175–183. [Google Scholar] [CrossRef][Green Version]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Gardiner, T.A.; Archer, D.B. A morphologic and autoradiographic study of cell death and regeneration in the retinal microvasculature of normal and diabetic rats. Am. J. Ophthalmol. 1985, 100, 51–60. [Google Scholar] [CrossRef]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Li, X.; Weber, N.C.; Cohn, D.M.; Hollmann, M.W.; DeVries, J.H.; Hermanides, J.; Preckel, B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Flusberg, D.A.; Sorger, P.K. Surviving apoptosis: Life-death signaling in single cells. Trends Cell Biol. 2015, 25, 446–458. [Google Scholar] [CrossRef]
- Simpson, D.A.; Murphy, G.M.; Bhaduri, T.; Gardiner, T.A.; Archer, D.B.; Stitt, A.W. Expression of the VEGF gene family during retinal vaso-obliteration and hypoxia. Biochem. Biophys. Res. Commun. 1999, 262, 333–340. [Google Scholar] [CrossRef]
- Stitt, A.W.; Simpson, D.A.; Boocock, C.; Gardiner, T.A.; Murphy, G.M.; Archer, D.B. Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours. J. Pathol. 1998, 186, 306–312. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Brown, L.F.; Roy, S.; Mizutani, M.; Zucker, C.L.; Lorenzi, M. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am. J. Pathol. 1998, 152, 1453–1462. [Google Scholar] [PubMed]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15, 4738–4747. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Carlsson, S.R.; Simonsen, A. Membrane dynamics in autophagosome biogenesis. J. Cell Sci. 2015, 128, 193–205. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Sanchez-Wandelmer, J.; Ktistakis, N.T.; Reggiori, F. ERES: Sites for autophagosome biogenesis and maturation? J. Cell Sci. 2015, 128, 185–192. [Google Scholar] [CrossRef]
- Bockler, S.; Westermann, B. Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev. Cell. 2014, 28, 450–458. [Google Scholar] [CrossRef]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L.; Kern, T.S. Retinopathy in animal models of diabetes. Diabetes Metab. Rev. 1995, 11, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L.; Bloodworth, J.M., Jr. Experimental Diabetic Retinopathy in Dogs. Arch. Ophthalmol. 1965, 73, 205–210. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12 (Suppl. 2), 1463–1467. [Google Scholar] [CrossRef]
- Anderson, H.R.; Stitt, A.W.; Gardiner, T.A.; Lloyd, S.J.; Archer, D.B. Induction of alloxan/streptozotocin diabetes in dogs: A revised experimental technique. Lab. Anim. 1993, 27, 281–285. [Google Scholar] [CrossRef]
- Ishikawa, T. Fine structure of retinal vessels in man and the macaque monkey. Investig. Ophthalmol. 1963, 2, 1–15. [Google Scholar]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef]
- Singh, R.; Cuervo, A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011, 13, 495–504. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsuda, N. Proteostasis and neurodegeneration: The roles of proteasomal degradation and autophagy. Biochim. Biophys. Acta 2014, 1843, 197–204. [Google Scholar] [CrossRef]
- Nishida, K.; Yamaguchi, O.; Otsu, K. Crosstalk between autophagy and apoptosis in heart disease. Circ. Res. 2008, 103, 343–351. [Google Scholar] [CrossRef]
- Denton, D.; Xu, T.; Kumar, S. Autophagy as a pro-death pathway. Immunol. Cell Biol. 2015, 93, 35–42. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Bialik, S.; Dasari, S.K.; Kimchi, A. Autophagy-dependent cell death—Where, how and why a cell eats itself to death. J. Cell Sci. 2018, 131, jcs215152. [Google Scholar] [CrossRef] [PubMed]
- Tinari, A.; Giammarioli, A.M.; Manganelli, V.; Ciarlo, L.; Malorni, W. Analyzing morphological and ultrastructural features in cell death. Methods Enzymol. 2008, 442, 1–26. [Google Scholar] [PubMed]
- Lane, J.D.; Allan, V.J.; Woodman, P.G. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J. Cell Sci. 2005, 118 Pt 17, 4059–4071. [Google Scholar] [CrossRef] [PubMed]
- Plomp, P.J.; Gordon, P.B.; Meijer, A.J.; Hoyvik, H.; Seglen, P.O. Energy dependence of different steps in the autophagic-lysosomal pathway. J. Biol. Chem. 1989, 264, 6699–6704. [Google Scholar] [CrossRef]
- Schutter, M.; Giavalisco, P.; Brodesser, S.; Graef, M. Local Fatty Acid Channeling into Phospholipid Synthesis Drives Phagophore Expansion during Autophagy. Cell 2020, 180, 135–149.e14. [Google Scholar] [CrossRef] [PubMed]
- Andrejeva, G.; Gowan, S.; Lin, G.; Wong Te Fong, A.C.L.; Shamsaei, E.; Parkes, H.G.; Mui, J.; Raynaud, F.; Asad, Y.; Vizcay-Barrena, G.; et al. De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy 2020, 16, 1044–1060. [Google Scholar] [CrossRef]
- Mills, J.C.; Stone, N.L.; Erhardt, J.; Pittman, R.N. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 1998, 140, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Inbal, B.; Bialik, S.; Sabanay, I.; Shani, G.; Kimchi, A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell Biol. 2002, 157, 455–468. [Google Scholar] [CrossRef]
- Levin-Salomon, V.; Bialik, S.; Kimchi, A. DAP-kinase and autophagy. Apoptosis 2014, 19, 346–356. [Google Scholar] [CrossRef]
- Gozuacik, D.; Bialik, S.; Raveh, T.; Mitou, G.; Shohat, G.; Sabanay, H.; Mizushima, N.; Yoshimori, T.; Kimchi, A. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008, 15, 1875–1886. [Google Scholar] [CrossRef]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.E.; Munro, J.; Rath, N.; Al Zander, S.; Mleczak, A.; Sumpton, D.; Morrice, N.; et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, W.; Menza, S.A.; Levine, J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. 1998, 274, F315–F327. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Single, B.; Castoldi, A.F.; Kuhnle, S.; Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997, 185, 1481–1486. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Hombrebueno, J.R.; Cairns, L.; Dutton, L.R.; Lyons, T.J.; Brazil, D.P.; Moynagh, P.; Curtis, T.M.; Xu, H. Uncoupled turnover disrupts mitochondrial quality control in diabetic retinopathy. JCI Insightig. 2019, 4, e129760. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH oxidase in retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Szabo, C.; Kern, T.S. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 2004, 53, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G.; Li, F.; Abatan, O.I.; Forsell, M.A.; Komjati, K.; Pacher, P.; Szabo, C.; Stevens, M.J. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes 2004, 53, 711–720. [Google Scholar] [CrossRef]
- Burkart, V.; Wang, Z.Q.; Radons, J.; Heller, B.; Herceg, Z.; Stingl, L.; Wagner, E.F.; Kolb, H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat. Med. 1999, 5, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gamez, J.A.; Rodriguez-Vargas, J.M.; Quiles-Perez, R.; Aguilar-Quesada, R.; Martin-Oliva, D.; de Murcia, G.; Menissier de Murcia, J.; Almendros, A.; Ruiz de Almodovar, M.; Oliver, F.J. PARP-1 is involved in autophagy induced by DNA damage. Autophagy 2009, 5, 61–74. [Google Scholar] [CrossRef]
- Del Nagro, C.; Xiao, Y.; Rangell, L.; Reichelt, M.; O’Brien, T. Depletion of the central metabolite NAD leads to oncosis-mediated cell death. J. Biol. Chem. 2014, 289, 35182–35192. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yanoff, M.; Cohen, S.; Ye, X. Cultured retinal capillary pericytes die by apoptosis after an abrupt fluctuation from high to low glucose levels: A comparative study with retinal capillary endothelial cells. Diabetologia 1996, 39, 537–547. [Google Scholar] [CrossRef]
- Mandarino, L.J.; Finlayson, J.; Hassell, J.R. High glucose downregulates glucose transport activity in retinal capillary pericytes but not endothelial cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 964–972. [Google Scholar]
- Mertens, S.; Noll, T.; Spahr, R.; Krutzfeldt, A.; Piper, H.M. Energetic response of coronary endothelial cells to hypoxia. Am. J. Physiol. 1990, 258, H689–H694. [Google Scholar] [CrossRef]
- Winkler, B.S.; Arnold, M.J.; Brassell, M.A.; Puro, D.G. Energy metabolism in human retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3183–3190. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).