Abstract
For many years the growth of solid tumors has been associated with their vascularization. The new vessels are needed to deliver oxygen and nutrients within the tumor mass. At the same time, these poorly stabilized vessels act as “Trojan horses” and open a way out for cancer cells. More recently, tumors have been identified whose growth appears to be independent of endothelial cell activity. Here we describe the ability of cancer cells to differentiate and reorganize themself in channels similar to blood vessels containing blood flow, overcoming the need for the angiogenic process of tumor vascularization. Together with the new vessels arising both from angiogenic and vasculogenic processes, these vessel-like structures can be exploited by tumor cells as a guide for migration and metastatic dissemination. In addition to classical intravascular dissemination, cancer cells can acquire pericytic features, interact with the endothelial basal lamina and migrate toward vessels or outside of the vessels. As expected, these alternative tumor behaviors assume greater importance if we consider that drugs with anti-angiogenic action directed against endothelial cells or their ligands are currently used in cancer therapy.
1. Introduction
Metastasization is a multistep process in which cancer cells detach from the primary tumor (or other metastases) and spread to locoregional or distant lymph nodes, or to non-contiguous secondary sites. Here, if the tissue microenvironment allows them to survive, they generate a new tumor. Metastasis is responsible for about 90% of cancer-associated deaths [1]. The metastatic potential makes cancer highly lethal; indeed, early diagnosis before the metastatic stage forecasts a better prognosis. Despite the significant progress in cancer biology, it is still difficult to predict whether disseminated cancer cells are present at the moment of diagnosis or treatment and, if so, where they reside. About 1700 people die each day of cancer only in United States (GLOBOCAN2020, WHO https://gco.iarc.fr/databases (access date September 2021)), which further attests to the failure in managing the disease once it disseminates into the body. Classically, the metastatic process uses three ways to spread to distant organs: (i) blood circulation (hematogenous), (ii) lymphatic circulation, and (iii) spread through the abdominal and chest cavities (transcoelomic) [2,3]. While the circulatory system appears to be the most common route, the extent of lymphatic versus hematogenous spread depends on the origin and location of the primary tumor [4]. For instance, bone tumors and sarcomas spread primarily through the blood [5,6] while gastrointestinal, lung, and breast tumors spread through the lymphatic system [7,8,9,10]. Finally, transcoelomic spread appears to be restricted to mesotheliomas and ovarian carcinomas [11]. During tumor growth, cancer cells induce the formation and arrangement of a tumoral vascular bed, mainly through angiogenesis. The formation of new vessels contributes to the proliferation and the metastatic spreading of cancer cells [12]. Tumor vasculature is usually disorganized, with thin and leaky walls and lack of complete pericytic coverage [13,14]; thus, cancer cells can easily penetrate the endothelial cell barrier. Cancer cells that enter circulation are called Circulating Tumor Cells (CTCs) [15]. CTCs are frequently found in the circulation of patients with primary tumors; however, the direct histological observation of tumor cells in vascular channels is infrequent. This “technical impasse” might be attributable to the short time the cells spend in the bloodstream, which is estimated to be in the span of minutes. In the bloodstream, CTCs are exposed to blood flow, to shear stress and/or to the action of immune cells. In this regard, it is important to remember that only 0.1% of the intravasate cells remain alive for more than 24 h, and only 0.01% produce metastatic foci [16,17]. Nevertheless, there is increasing evidence that some tumors, primarily melanomas and carcinomas, can adopt different strategies to grow and disseminate. Among these are vascular co-optation [18], vascular mimicry [19], and single or collective extravascular migration, also known as pericytic mimicry or perivascular invasion [19]. With the identification of vascular co-option and vascular mimicry, the paradigm within which tumor growth and dissemination are angiogenesis-dependent has been overcome [20,21], and non-angiogenic tumors have since been described in brain, liver, skin, and lymph nodes [22]. These alternative strategies increase the supply of nutrients into the tumor mass and increase its invasive and migratory capacities without forming new vessels. The lack of new blood vessels may have a bearing on the efficacy of anti-angiogenic therapies; thus, a detailed description of these processes becomes important in understanding cancer biology. On the other hand, extravascular migration still requires the presence of a well-developed vascular network. Here we will review the molecular mechanisms and the morphofunctional alterations described to date which led to these alternative modalities of metastasization.
2. Non-Angiogenic Intratumoral Vascularization
Tumor vascularization resulting from the growth of vessels from pre-existing ones or the recruitment of circulant endothelial cell precursors (EPC) is fundamental for tumor growth [23]. Furthermore, the angiogenic process is an essential component of the metastatic pathway. The vascular bed within the tumor provides the principal route by which malignant cells exit the primary tumor site and enter into circulation. For many tumors, vascular and lymphatic density can provide a prognostic indicator of metastatic potential, where highly vascular primary tumors have a higher incidence of metastasis than poorly vascular tumors [19]. The vascular structure acts as a physical and mechanical impediment to the passage of cancer cells into the bloodstream, affecting their fate and the new destination tissue. Over the years, the process of tumor vascularization has been the target of numerous studies that led to the characterization of the sprouting phenomena of new vessels from pre-existing ones (the angiogenic process), and to the identification of possible longitudinal partition that divided the vessel into, two identified as intussusceptive microvascular growth. These phenomena, well characterized in blood vessels [24], have been recently described into the lymphatic torrent as well [25,26]. In contrast with the angiogenic process, intussusception in blood and lymphatic vessels facilitates tissue vascularization without modifying vascular permeability. In addition, the identification of EPC suggests that vasculogenesis could be an alternative process in tumor vascularization. Indeed, EPCs, attracted into the tumor mass by the growth factors, leave the bloodstream to migrate into the tissues, finding a suitable microenvironment. Here they differentiate into mature endothelial cells and reorganize themselves into vascular structures. These new structures will reconnect to the pre-existing vessels of the tissue itself, allowing active circulation [27]. As already mentioned, the identification of these processes has for years supported the idea of tumor dependence on vessels, as suggested by Folkman in 1971. In the late 1990s, several studies suggested that brain and lung cancer growth proceeds independently from the neovascularization of the tissue, and that the tissue vascular network in fact persists with the structure and distribution of pre-existing vessels. In these tumors, cancer cells can hijack existing blood vessels for tumor growth, survival and metastasis [28,29,30], or exploit their high plasticity to reorganize themself in functional channels. These processes are termed vessel co-option and vascular mimicry (VM), respectively.
Vessel co-opting tumors differ from angiogenic ones in their ability to preserve the vascular scaffold of the surrounding normal tissue instead of inducing a destructive wound healing-like reaction along with angiogenesis, fibrosis and inflammation [31,32]. Usually, vascular co-option occurs in solid tumors, such as in the brain, breast, kidney, and lung [30], with a well-organized vascular bed. In this process, pericytes play an important role in supporting and stabilizing ECs [33]. Even if the molecular mechanisms driving vessel co-option are poorly understood, pericytes physically interacting with cancer cells support cancer invasion [31,34]. The identification of vascular channels lacking ECs was introduced by Maniotis et al., who reported the plasticity of aggressive cancer cells forming de novo vascular networks in highly aggressive uveal melanomas [35]. VM has been described in a plethora of tumors, including carcinomas of the breast [36], ovary [37], bladder [38], lung [39] and prostate [40], as well as sarcomas [41], glioblastomas [42], astrocytomas [43] and melanomas [44]. The functional channels (that are not vessels) are composed of tumor cells with stem cell features, and present characteristic patterns in addition to the erythrocytes inside them. The presence of VM is associated with a high tumor grade, short survival, invasion, and metastasis. Although the molecular mechanisms of VM are not entirely clear, the hypoxia-inducible factor 2α (HIF2α)/vascular endothelial (VE)-cadherin axes are a key pathway [19]. Microarray analyses highlighted in melanoma cell formation channel the downregulation of several melanoma-specific genes, such as melanoma-cell adhesion molecule (MCAM), melan-A (MLANA), and microphthalmia-associated transcription factor (MITF), suggesting a regression in the differentiation state of the tumors. On the contrary, EC-related genes including the tyrosine kinase receptor 1 (TIE1), epithelial-cell kinase (EPHA2), VE-cadherin (CDH5), neuropilin 1 (NPR1) and hypoxia-inducible factor 1α (HIF1α) are up-regulated [45].
An exciting hypothesis associates the vascularization of non-angiogenic tumors with anti-angiogenic therapies (AATs). VEGF inhibitors such as bevacizumab and VEGFR tyrosine kinase inhibitors (RTK-Is) have been widely used in clinics. However, the benefits of AATs are only partial and do not last a long time. It has been demonstrated that the prolonged use of AATs can aggravate hypoxia, which in turn promotes revascularization. In experimental in vitro models of melanoma, the ability of vemurafenib-resistant cancer cells to organize themselves in perfused vascular-like channels has been described [46]. The RTK-I sunitinib increased VM under hypoxic conditions in renal carcinoma models [47] and triple-negative breast cancer cells through the overexpression of HIF-1, VE-cadherin, and Twist1 [48]. Again, in orthotopic glioblastoma models, bevacizumab or vatalanib administration upregulates the IL8/CXCR2 pathway and VM [49,50]. Thus, the non-angiogenic tumors show an altered drug response to AATs, bypassing the endothelial cell-dependent angiogenesis to supply themselves with oxygen and nutrients.
3. Epithelial to Mesenchymal Transition
During the metastatic process cells acquire distinctive mesenchymal properties, with a cellular transdifferentiation process defined as the epithelial to mesenchymal transition (EMT) [51]. EMT plays a central role, especially in carcinoma metastasization. Although during EMT cancer cells acquire their invasive capacity, the EMT switch is not enough to describe all migratory phenotypes of cancer cells. During EMT, cancer cells lose several epithelial markers, reduce adhesion molecules (i.e., the so-called “cadherin switch” from Epithelial to Neural cadherin), and up-regulate mesenchymal-related genes required to acquire invasive behavior. In this process, cells lose polarity and the expression of E-cadherin, and gain the expression of Smooth Muscle Actin and Fibroblast Specific Protein-1 [52], among others. The loss of E-cadherin expression is correlated with an invasive and undifferentiated phenotype in many epithelium-derived cancer cells. Meanwhile, the acquisition of N-cadherin is associated with heightened invasive potential [53,54]. The intermediate filaments also switch; vimentin replaces keratin, which regulates multiple cellular processes in epithelial cells, for example during the slug dependent EMT [55]. This new cell arrangement induces a change in cell motility, causing the so-called “amoeboid” movement typical of mesenchymal cells. Carcinoma cells use transition mechanisms to detach from primary tumors and migrate to distal sites where they can form metastases. The amoeboid movement is characterized by a dominant cell side that interacts with the EMC and releases proteolytic enzymes, while the cytoskeleton contracts and the tail of the cell detaches. It is increasingly clear that the EMT program is a continuum of transitional stages between the epithelial and mesenchymal phenotypes, and does not involve a binary choice between full-epithelial and full-mesenchymal phenotype [56]. Furthermore, cancer cells expressing a mix of epithelial and mesenchymal phenotypes are more effective in circulation, colonization at the secondary site, and the development of metastasis. It is also important to point out that although EMT facilitates the dissemination of cells, the formation of metastases also requires the loss of the mesenchymal phenotype through the inverse Mesenchymal to Epithelial Transition process (MET) [57].
4. The Angiotropic Process: Walking on the Abluminal Side of Vessels
Recently, pathologists described, in melanoma samples, the presence of single cancer cells or cell aggregates near vessels on their outer side. Based on these histological observations, Lugassy and Barnhill have hypothesized, for the first time, the phenomenon of angiotropism. Cancer cells, acquiring the typical forms and positions of pericytes, can migrate extravascularly, returning to a neural crest cell migratory phenotype [58]. According to this concept, melanoma cells closely associated with the endothelium in a pericytic location, which are generally detected at the advancing front of the tumor and without evidence of intravasation, are defined as angiotropic melanoma cells [59,60]. Histological analysis has revealed that both micro and large vessels can be affected by the angiotropic phenomena, while data about neovessels or stabilized vessels are not conclusive yet. In this complex, the ECs show no signs of physiological damage or intravasation. Thus, in parallel to the classical metastatic dissemination, the migration of cancer cells outside the vessels has been referred to as extra vascular migratory metastasis (EVMM) (Figure 1, Table 1).
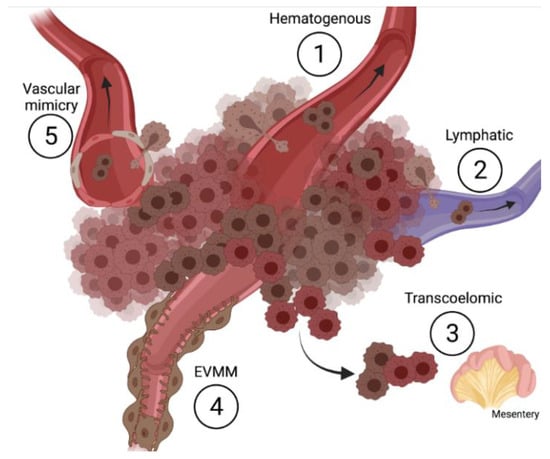
Figure 1.
Schematic representation of metastatic routes (created with BioRender.com).

Table 1.
Short description of the different routes of cancer dissemination.
In this view, EVMM can be considered as an important alternative to tumor dissemination. EVMM and pericyte mimicry, mainly described in melanoma [59], have been reported in various tumors including cutaneous squamous cell carcinoma, prostatic adenocarcinoma, carcinosarcomas of the ovaries and endometrium, glioma, liposarcoma and glioblastoma [61,62,63]. During EVMM, the perivascular localization of melanoma cells has been associated with the acquisition of stem-like plasticity and the expression of specific pericytic markers, including PDGFRβ, CD146, CD44, CD73, CD105, and CD144 [64]. All these observations resume the glioblastoma behavior where EVMM occurs, and cancer stem cells give rise to up to 80% of the pericytic compartment. Despite these similarities, further effort is required to understand whether all the pericytes in both glioblastoma and melanoma exert similar migratory and metastatic abilities as well as play a role in vessel stabilization. In vitro, in vivo, and clinical evidence of EVMM is summarized in Table 2. A recent study suggested that up to 37% of cases of melanoma exhibit EVMM [65]. Therefore, EVMM is probably a common phenomenon underestimated until now, likely due to technical limitations. Moreover, it has been observed that patients with progressive disease of melanoma that were sentinel lymph node-negative had progressive disease both in sentinel-basin and at distant sites. Therefore, it has also been hypothesized that EVMM, rather than hematogenous spread, might be responsible for the observed progressive disease with single organ involvement. For this, the understanding of the mechanisms of action of EVMM is of outstanding importance. For instance, differential analysis between angiotropic melanomas and non-angiotropic melanomas highlighted 15 critical genes involved in the modulation of EVMM [66]. Among these, KIF14 (kinesin family member 14), ECT2 (epithelial cell transforming sequence 2 oncogenes), and HMMR (hyaluronan-mediated motility receptor) seem to be implicated in cytokinesis. In addition, co-culture experiments demonstrated that the interaction of angiotropic melanoma cells with the abluminal vascular surface promotes the differential expression of genes related to cell migration (CCL2, ICAM1 and IL6), cancer progression (CCL2, ICAM1, SELE, TRAF1, IL6, SERPINB2 and CXCL6), EMT (CCL2 and IL6), stemness (CCL2, PDGFB, EVX1 and CFDP1), and pericytic recruitment (PDGFB) [67].

Table 2.
Cancer types displaying perivascular migration.
5. Role of the Extracellular Matrix
Angiotropic tumors have been described as acquiring pericytic localization and being directly associated with the basal lamina of ECs. Ultrastructurally, the basal lamina is an amorphous matrix. Among the extracellular matrix (ECM) proteins, laminins are the principal non-collagenous components involved in cell migration, adhesion, and differentiation. Laminins are heterotrimeric glycoproteins composed of different combinations of alpha (five genetic variants), beta (four genetic variants), and gamma (three genetic variants) chains. In angiotropic melanomas, tumor microvessels showed minimal focal vascular positivity for α3, β3, and γ2 laminin, while β2 laminin positivity characterizes the vessels of the tumors. In this context, tumor cells spread on the abluminal surface of small vessels that are enriched in β2 laminin [83]. Alteration in the basal lamina is induced by, i.e., neutrophil elastase, which cleaves laminin-332 (α3, β3, γ2) and enhances the metastatic potential of melanoma cells [58]. Accordingly, in vitro and in vivo experiments clearly showed the ability of the C16 peptide (KAFDITYVRLKF), derived from the laminin γ1 chain, to enhance the angiotropic extravascular migration and pulmonary metastasization of melanoma cells [84]. The expression of LAMC2, LAMA4, and ITGB3 is increased in vascularized angiotropic melanoma areas with respect to the avascular ones in the same tumor [85]. Furthermore, the expression of laminin receptors represents an unfavorable prognostic factor in melanoma. CD36, a membrane glycoprotein involved in angiogenesis, supports vascular mimicry formation in human melanoma cancer cells, acting through α3 integrin-laminin interaction; in silico analysis of CD36 expression within the melanoma cohort of The Cancer Genome Atlas suggests that melanoma patients with high expression of CD36 have a poorer clinical outcome [86].
6. Approaches to Studying Angiotropism and EVMM
The emergence of new strategies through which cancer cells can metastasize is always accompanied by the need for new experimental plans to describe them. To this end, different in vitro, ex vivo, and in vivo assays have been developed (Table 2). Here, we summarize the available models for the evaluation of angiotropism or EVMM of melanoma cell lines.
6.1. In Vitro 3D Models
In 2002 Lugassy and colleagues described the ability of primary human melanoma cells to migrate along the capillary-like structures of EC. Indeed, ECs plated onto a 3D Matrigel self-organize tube-like structures on which melanoma cells migrate with a velocity of 0.01 μm/min to 2 μm/min. Interestingly, this rate corresponds to a distance of 0.5 cm to 105 cm/year, agreeable with the diagnosis of metastasis [87]. Recently, we implemented this protocol and set up a long co-culture method. To this purpose, spheroids of human melanoma cells and human endothelial cells were embedded into 3D Matrigel scaffolds for ten days. As expected, ECs formed 3D tubular structures that came out of the spheroid. Melanoma cells used these structures to leave the spheroid, migrating in close contact with the outside of the sprouts over the next 4/6 days (Figure 2A).
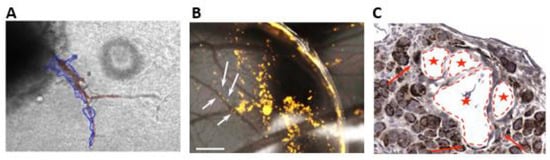
Figure 2.
Experimental approaches for studying EVMM. (A) 3D matrigel-embedded spheroids of endothelial (highlighted in brown) and melanoma (highlighted in blue) cells; (B) Chick embryo chorioallantoic membrane (CAM). White arrows highlight melanoma cells (yellow) in close contact with the abluminal side of blood vessels. Scale bar 2 mm; (C) Histological image of metastatic melanoma cells in CAM section. Melanoma cells are stained with anti-human mitochondria (brown dots, DAB), and the lumen of the vessels are highlighted with red stars.
Although in vitro cell cultures hitherto represented an indispensable and reliable tool for biomedical research, 3D cell culture models are now closer to in vivo conditions. 3D cell culture favors the maintenance of cell polarity and more physiological cell–cell and cell–ECM complex interactions. Moreover, by using specific extracellular matrices and organic and/or inorganic scaffolds [88], researchers can modulate the mechanical supports and the availability of soluble factors potentially adsorbed on scaffolds. Setting up suitable 3D culture conditions usually remains time-consuming. Nevertheless, the long lasting culture of tumor cell spheroids or organoids allows the observation of tumor behavior and phenotypes that cannot be reached with 2D cultures [89,90]. For this, and even if 3D culture still lacks the microenvironmental complexity in which cancer cells reside in animal models (e.g., interaction with the immune system), the long term culture condition has proven to be widely useful for cancer research and drug screening [91].
6.2. In Vivo Models
The optical transparency of the zebrafish embryo makes it an excellent in vivo model to study tumor progression, tumor angiogenesis, and tumor metastasis, allowing high-resolution live imaging [92]. Briefly, labeled cancer cells are injected into the yolk of the zebrafish embryo two days post-fertilization. The migration of cancer cells outside the bloodstream can be followed in live time-lapse imaging for 2 to 3 days post-injection. Of course, the zebrafish embryo is a suitable model to evaluate the classic intravascular metastasization and the EVMM simultaneously. Alternatively, EVMM can be analyzed in the chick embryo chorioallantoic membrane model (CAM). The CAM is a highly vascularized membrane that wraps the embryo and transports oxygen, calcium, and metabolites. In this context, tumor cells can be seeded on the CAM and followed over time in ovo [27]. The migration of cancer cells and their association with blood vessels can be monitored by using a stereomicroscope over 72 h (Figure 2B). Moreover, the samples can be fixed and analyzed by histology to visualize the angio-complex (Figure 2C). Specific approaches have been developed to study the angiotropic and EVMM processes. For this purpose, GFP-tagged human metastatic melanoma cells were stereotactically injected into the brain, and a vascular tracer was administered in the jugular vein of immunodeficient mice allowing the visualization of the interaction of melanoma cells with the brain vasculature [93]. The constant development of optically transparent chambers for live-imaging techniques may lead to new studies and new knowledge in the field of EVMM [94].
The in vivo models of zebrafish and chick embryo CAM have added complexity to the previously described in vitro models. Both these models share the advantages of being easy to handle, relatively cheap, highly suitable for imaging and, as substitutes for mouse models, perfectly reflective of the “Replacement” purpose of the 3R law. The zebrafish switches from embryo to larvae in a few days, while the chick embryo CAM hatches in 21 days. These relatively short time windows can influence the timing of the experiments and limit the drug screening strategy. Of note, the embryonic environments of CAM and zebrafish may inhibit tumor growth, reducing the expression of pro-oncogenes [95,96]. On the other hand, the same embryonic features can support the growth, differentiation, and migration of germline tumors, including teratomas [27].
The mouse model represents the “top-level model” in preclinical cancer research. Indeed, mice present several advantages, including their high conservation with humans in term of anatomy, physiology and genetics, which help to better model the metastatic process [97,98]. Moreover, they provide a good tool for drug discovery and verification. The variety of mouse strains, including humanized mice, are open to many cancer cell lines and patient derived xenograft (PDX) research [99,100]. However, obtaining and maintaining the animals can be very expensive, and requires highly specialized personnel.
7. Final Remarks
Solid tumors are characterized by the ability to support themselves in their growth and in the colonization of new body districts. However, the fastest route (hematogenous) may not be the best choice. Per this view, extravascular migration, although less characterized, assumes vital importance for cancer progression. In conclusion, more significant efforts should be devoted to elucidating these processes, as they could be potential and exciting therapeutic targets.
Author Contributions
Conceptualization, M.C. and C.R.; methodology, M.C., C.R. and E.G.; writing—original draft preparation, M.C. and C.R.; writing—review and editing, M.C., C.R. and S.M.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) to S.M. (IG17276). E.G. was supported by Fondazione Umberto Veronesi (FUV) Fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Barbolina, M.V. Molecular Mechanisms Regulating Organ-Specific Metastases in Epithelial Ovarian Carcinoma. Cancers 2018, 10, 444. [Google Scholar] [CrossRef]
- Wong, S.Y.; Hynes, R.O. Lymphatic or hematogenous dissemination: How does a metastatic tumor cell decide? Cell Cycle 2006, 5, 812–817. [Google Scholar] [CrossRef]
- Pennacchioli, E.; Tosti, G.; Barberis, M.; De Pas, T.M.; Verrecchia, F.; Menicanti, C.; Testori, A.; Mazzarol, G. Sarcoma spreads primarily through the vascular system: Are there biomarkers associated with vascular spread? Clin. Exp. Metastasis 2012, 29, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Perissinotto, E.; Cavalloni, G.; Leone, F.; Fonsato, V.; Mitola, S.; Grignani, G.; Surrenti, N.; Sangiolo, D.; Bussolino, F.; Piacibello, W.; et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 490–497. [Google Scholar]
- Rahman, M.; Mohammed, S. Breast cancer metastasis and the lymphatic system. Oncol. Lett. 2015, 10, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Riquet, M.; Rivera, C.; Gibault, L.; Pricopi, C.; Mordant, P.; Badia, A.; Arame, A.; Le Pimpec Barthes, F. Lymphatic spread of lung cancer: Anatomical lymph node chains unchained in zones. Rev. De Pneumol. Clin. 2014, 70, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, C.S.; Foroulis, C.N.; Kostikas, K.; Konstantinou, M.; Kalkandi, P.; Dimadi, M.; Bouros, D.; Lioulias, A. Involvement of lymphatic metastatic spread in non-small cell lung cancer accordingly to the primary cancer location. Lung Cancer 2004, 44, 183–191. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xu, T.; Xue, R.; Yu, L.; Zhu, Y.; Wu, Y.; Zhang, Q.; Li, D.; Shen, S.; et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 2020, 11, 1993. [Google Scholar] [CrossRef]
- Romani, C.; Zizioli, V.; Silvestri, M.; Ardighieri, L.; Bugatti, M.; Corsini, M.; Todeschini, P.; Marchini, S.; D’Incalci, M.; Zanotti, L.; et al. Low Expression of Claudin-7 as Potential Predictor of Distant Metastases in High-Grade Serous Ovarian Carcinoma Patients. Front. Oncol. 2020, 10, 1287. [Google Scholar] [CrossRef]
- Nyangoga, H.; Mercier, P.; Libouban, H.; Basle, M.F.; Chappard, D. Three-dimensional characterization of the vascular bed in bone metastasis of the rat by microcomputed tomography (MicroCT). PLoS ONE 2011, 6, e17336. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Hynes, R.O. The initial hours of metastasis: The importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Seano, G.; Jain, R.K. Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis 2020, 23, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cortes, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dudley, A.C. Models and molecular mechanisms of blood vessel co-option by cancer cells. Angiogenesis 2020, 23, 17–25. [Google Scholar] [CrossRef]
- Frentzas, S.; Simoneau, E.; Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Kostaras, E.; Nathan, M.; Wotherspoon, A.; Gao, Z.H.; Shi, Y.; et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016, 22, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, F.; Gatter, K. Non-angiogenic tumours unveil a new chapter in cancer biology. J. Pathol. 2015, 235, 381–383. [Google Scholar] [CrossRef]
- Ronca, R.; Benkheil, M.; Mitola, S.; Struyf, S.; Liekens, S. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev. 2017, 37, 1231–1274. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Djonov, V. Intussusceptive microvascular growth in tumors. Cancer Lett. 2012, 316, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Gayoso, S.; Garcia, M.P.; Gonzalez-Gomez, M.; Diaz-Flores, L., Jr.; Sanchez, R.; Carrasco, J.L.; Madrid, J.F. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol. Histopathol. 2020, 35, 1083–1103. [Google Scholar] [CrossRef]
- Diaz-Flores, L.; Gutierrez, R.; Pino Garcia, M.; Gonzalez-Gomez, M.; Diaz-Flores, L., Jr.; Carrasco, J.L. Intussusceptive lymphangiogenesis in the sinuses of developing human foetal lymph nodes. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2019, 226, 73–83. [Google Scholar] [CrossRef]
- Corsini, M.; Ravelli, C.; Grillo, E.; Dell’Era, P.; Presta, M.; Mitola, S. Simultaneously characterization of tumoral angiogenesis and vasculogenesis in stem cell-derived teratomas. Exp. Cell Res. 2021, 400, 112490. [Google Scholar] [CrossRef]
- Baker, G.J.; Yadav, V.N.; Motsch, S.; Koschmann, C.; Calinescu, A.A.; Mineharu, Y.; Camelo-Piragua, S.I.; Orringer, D.; Bannykh, S.; Nichols, W.S.; et al. Mechanisms of glioma formation: Iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia 2014, 16, 543–561. [Google Scholar] [CrossRef]
- Yagi, Y.; Aly, R.G.; Tabata, K.; Barlas, A.; Rekhtman, N.; Eguchi, T.; Montecalvo, J.; Hameed, M.; Manova-Todorova, K.; Adusumilli, P.S.; et al. Three-Dimensional Histologic, Immunohistochemical, and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 589–600. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Latacz, E.; Caspani, E.; Barnhill, R.; Lugassy, C.; Verhoef, C.; Grunhagen, D.; Van Laere, S.; Fernandez Moro, C.; Gerling, M.; Dirix, M.; et al. Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis 2020, 23, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Reynolds, A.R.; Kuczynski, E.A.; Gatter, K.; Vermeulen, P.B.; Kerbel, R.S.; Harris, A.L.; Pezzella, F. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer 2018, 18, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Caspani, E.M.; Crossley, P.H.; Redondo-Garcia, C.; Martinez, S. Glioblastoma: A pathogenic crosstalk between tumor cells and pericytes. PLoS ONE 2014, 9, e101402. [Google Scholar] [CrossRef] [PubMed]
- Folberg, R.; Hendrix, M.J.; Maniotis, A.J. Vasculogenic mimicry and tumor angiogenesis. Am. J. Pathol. 2000, 156, 361–381. [Google Scholar] [CrossRef]
- Shirakawa, K.; Wakasugi, H.; Heike, Y.; Watanabe, I.; Yamada, S.; Saito, K.; Konishi, F. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int. J. Cancer 2002, 99, 821–828. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Liu, W.X.; Wang, K. VEGF and SEMA4D have synergistic effects on the promotion of angiogenesis in epithelial ovarian cancer. Cell. Mol. Biol. Lett. 2018, 23, 2. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Xie, H.; Liu, T.; Chen, Y.; Ma, Z.; Pei, X.; Yang, W.; Li, L. Androgen receptor suppresses prostate cancer metastasis but promotes bladder cancer metastasis via differentially altering miRNA525-5p/SLPI-mediated vasculogenic mimicry formation. Cancer Lett. 2020, 473, 118–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Nong, L.; Chen, M.; Gu, X.; Zhao, W.; Liu, M.; Cheng, W. Baicalein suppresses vasculogenic mimicry through inhibiting RhoA/ROCK expression in lung cancer A549 cell line. Acta Biochim. Biophys. Sin. 2020, 52, 1007–1015. [Google Scholar] [CrossRef]
- Yeo, C.; Han, D.S.; Lee, H.J.; Lee, E.O. Epigallocatechin-3-Gallate Suppresses Vasculogenic Mimicry through Inhibiting the Twist/VE-Cadherin/AKT Pathway in Human Prostate Cancer PC-3 Cells. Int. J. Mol. Sci. 2020, 21, 439. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Vottis, C.T.; Megaloikonomos, P.D.; Agrogiannis, G.D.; Theocharis, S. Neovascularization in Ewing’s sarcoma. Neoplasma 2018, 65, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Zhao, P.; Zhao, H.; Gao, W.; Wang, L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and Angiogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Cao, W.; Xu, C.; Li, X.; Yang, X. Twist1 promotes astrocytoma development by stimulating vasculogenic mimicry. Oncol. Lett. 2019, 18, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bellido, D.; Fernandez-Cortes, M.; Rodriguez, M.I.; Serrano-Saenz, S.; Carracedo, A.; Garcia-Diaz, A.; Oliver, F.J. VE-cadherin promotes vasculogenic mimicry by modulating kaiso-dependent gene expression. Cell Death Differ. 2019, 26, 348–361. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Hess, A.R.; Seftor, R.E. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, E.; Laurenzana, A.; Peppicelli, S.; Biagioni, A.; Margheri, F.; Ruzzolini, J.; Bianchini, F.; Fibbi, G.; Rosso, M.D.; Nediani, C.; et al. uPAR controls vasculogenic mimicry ability expressed by drug-resistant melanoma cells. Oncol. Res. 2021. (Online ahead of print). [Google Scholar] [CrossRef]
- Serova, M.; Tijeras-Raballand, A.; Dos Santos, C.; Martinet, M.; Neuzillet, C.; Lopez, A.; Mitchell, D.C.; Bryan, B.A.; Gapihan, G.; Janin, A.; et al. Everolimus affects vasculogenic mimicry in renal carcinoma resistant to sunitinib. Oncotarget 2016, 7, 38467–38486. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Zhao, X.; Ma, Y.; Ji, R.; Gu, Q.; Dong, X.; Li, J.; Liu, F.; Jia, X.; et al. Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer. Mol. Cancer 2014, 13, 207. [Google Scholar] [CrossRef]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928. [Google Scholar] [CrossRef]
- Angara, K.; Borin, T.F.; Rashid, M.H.; Lebedyeva, I.; Ara, R.; Lin, P.C.; Iskander, A.; Bollag, R.J.; Achyut, B.R.; Arbab, A.S. CXCR2-Expressing Tumor Cells Drive Vascular Mimicry in Antiangiogenic Therapy-Resistant Glioblastoma. Neoplasia 2018, 20, 1070–1082. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361. [Google Scholar] [CrossRef]
- Araki, K.; Shimura, T.; Suzuki, H.; Tsutsumi, S.; Wada, W.; Yajima, T.; Kobayahi, T.; Kubo, N.; Kuwano, H. E/N-cadherin switch mediates cancer progression via TGF-beta-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br. J. Cancer 2011, 105, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011, 30, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Landsberg, J.; Tuting, T.; Barnhill, R.L.; Lugassy, C. The Role of Neutrophilic Inflammation, Angiotropism, and Pericytic Mimicry in Melanoma Progression and Metastasis. J. Investig. Dermatol. 2016, 136, 372–377. [Google Scholar] [CrossRef]
- Barnhill, R.L.; Lugassy, C. Angiotropic malignant melanoma and extravascular migratory metastasis: Description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 2004, 36, 485–490. [Google Scholar] [CrossRef]
- Lugassy, C.; Kleinman, H.K.; Vermeulen, P.B.; Barnhill, R.L. Angiotropism, pericytic mimicry and extravascular migratory metastasis: An embryogenesis-derived program of tumor spread. Angiogenesis 2020, 23, 27–41. [Google Scholar] [CrossRef]
- Lugassy, C.; Vernon, S.E.; Warner, J.W.; Le, C.Q.; Manyak, M.; Patierno, S.R.; Barnhill, R.L. Angiotropism of human prostate cancer cells: Implications for extravascular migratory metastasis. BJU Int. 2005, 95, 1099–1103. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; Sloan, A.E.; Mao, Y.; Lathia, J.D.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Rustagi, T.; Gleeson, F.C.; Chari, S.T.; Lehrke, H.D.; Takahashi, N.; Malikowski, T.M.; Abu Dayyeh, B.K.; Chandrasekhara, V.; Iyer, P.G.; Kendrick, M.L.; et al. Safety, Diagnostic Accuracy, and Effects of Endoscopic Ultrasound Fine-Needle Aspiration on Detection of Extravascular Migratory Metastases. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 2533–2540.e1. [Google Scholar] [CrossRef] [PubMed]
- Lugassy, C.; Zadran, S.; Bentolila, L.A.; Wadehra, M.; Prakash, R.; Carmichael, S.T.; Kleinman, H.K.; Peault, B.; Larue, L.; Barnhill, R.L. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: An alternative to intravascular cancer dissemination. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2014, 7, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.P.; Duncan, L.M.; Muzikansky, A.; Kraft, S. Angiotropism in primary cutaneous melanoma is associated with disease progression and distant metastases: A retrospective study of 179 cases. J. Cutan. Pathol. 2019, 46, 498–507. [Google Scholar] [CrossRef]
- Lugassy, C.; Lazar, V.; Dessen, P.; van den Oord, J.J.; Winnepenninckx, V.; Spatz, A.; Bagot, M.; Bensussan, A.; Janin, A.; Eggermont, A.M.; et al. Gene expression profiling of human angiotropic primary melanoma: Selection of 15 differentially expressed genes potentially involved in extravascular migratory metastasis. Eur J Cancer 2011, 47, 1267–1275. [Google Scholar] [CrossRef]
- Lugassy, C.; Wadehra, M.; Li, X.; Corselli, M.; Akhavan, D.; Binder, S.W.; Peault, B.; Cochran, A.J.; Mischel, P.S.; Kleinman, H.K.; et al. Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2013, 6, 19–29. [Google Scholar] [CrossRef][Green Version]
- Fornabaio, G.; Barnhill, R.L.; Lugassy, C.; Bentolila, L.A.; Cassoux, N.; Roman-Roman, S.; Alsafadi, S.; Del Bene, F. Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci. Rep. 2018, 8, 10448. [Google Scholar] [CrossRef]
- Lugassy, C.; Kleinman, H.K.; Vernon, S.E.; Welch, D.R.; Barnhill, R.L. C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br. J. Dermatol. 2007, 157, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, L.A.; Prakash, R.; Mihic-Probst, D.; Wadehra, M.; Kleinman, H.K.; Carmichael, T.S.; Peault, B.; Barnhill, R.L.; Lugassy, C. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci. Rep. 2016, 6, 23834. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, A.K.; Rushing, E.J.; Kirschenbaum, D.; Mangana, J.; Mittmann, C.; Moch, H.; Lugassy, C.; Barnhill, R.L.; Mihic-Probst, D. Eight autopsy cases of melanoma brain metastases showing angiotropism and pericytic mimicry. Implications for extravascular migratory metastasis. J. Cutan. Pathol. 2019, 46, 570–578. [Google Scholar] [CrossRef]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Kienast, Y.; Fuhrmann, M.; Von Baumgarten, L.; Burgold, S.; Mitteregger, G.; Kretzschmar, H.; Herms, J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 2009, 57, 1306–1315. [Google Scholar] [CrossRef]
- Alieva, M.; Leidgens, V.; Riemenschneider, M.J.; Klein, C.A.; Hau, P.; van Rheenen, J. Intravital imaging of glioma border morphology reveals distinctive cellular dynamics and contribution to tumor cell invasion. Sci. Rep. 2019, 9, 2054. [Google Scholar] [CrossRef] [PubMed]
- Zagzag, D.; Esencay, M.; Mendez, O.; Yee, H.; Smirnova, I.; Huang, Y.; Chiriboga, L.; Lukyanov, E.; Liu, M.; Newcomb, E.W. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: One plausible explanation of Scherer’s structures. Am. J. Pathol. 2008, 173, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Koay, M.H.E.; Stewart, C.J.R. Extravascular Migratory Metastasis (Pericytic Mimicry) in Sarcomatoid Squamous Cell Carcinoma of the Vulva: A Report of 2 Cases. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2019, 38, 27–31. [Google Scholar] [CrossRef]
- Fedda, F.; Migden, M.R.; Curry, J.L.; Torres-Cabala, C.A.; Tetzlaff, M.T.; Aung, P.P.; Prieto, V.G.; Ivan, D.; Myers, J.N.; Nagarajan, P. Angiotropism in recurrent cutaneous squamous cell carcinoma: Implications for regional tumor recurrence and extravascular migratory spread. J. Cutan. Pathol. 2019, 46, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Katayama, E.; Saruta, H.; Nanri, A.; Nakama, T.; Ohata, C. Angiotropic syringomatous carcinoma. J. Cutan. Pathol. 2017, 44, 397–400. [Google Scholar] [CrossRef]
- Dyke, J.M.; Crook, M.L.; Platten, M.; Stewart, C.J. Extravascular migratory metastasis in gynaecological carcinosarcoma. Histopathology 2014, 65, 363–370. [Google Scholar] [CrossRef]
- Shen, J.; Shrestha, S.; Rao, P.N.; Asatrian, G.; Scott, M.A.; Nguyen, V.; Giacomelli, P.; Soo, C.; Ting, K.; Eilber, F.C.; et al. Pericytic mimicry in well-differentiated liposarcoma/atypical lipomatous tumor. Hum. Pathol. 2016, 54, 92–99. [Google Scholar] [CrossRef]
- Levy, M.J.; Gleeson, F.C.; Zhang, L. Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2009, 7, 246–248. [Google Scholar] [CrossRef]
- Lugassy, C.; Shahsafaei, A.; Bonitz, P.; Busam, K.J.; Barnhill, R.L. Tumor microvessels in melanoma express the beta-2 chain of laminin. Implications for melanoma metastasis. J. Cutan. Pathol. 1999, 26, 222–226. [Google Scholar] [CrossRef]
- Kuratomi, Y.; Nomizu, M.; Tanaka, K.; Ponce, M.L.; Komiyama, S.; Kleinman, H.K.; Yamada, Y. Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br. J. Cancer 2002, 86, 1169–1173. [Google Scholar] [CrossRef]
- Lugassy, C.; Torres-Munoz, J.E.; Kleinman, H.K.; Ghanem, G.; Vernon, S.; Barnhill, R.L. Overexpression of malignancy-associated laminins and laminin receptors by angiotropic human melanoma cells in a chick chorioallantoic membrane model. J. Cutan. Pathol. 2009, 36, 1237–1243. [Google Scholar] [CrossRef]
- Martini, C.; DeNichilo, M.; King, D.P.; Cockshell, M.P.; Ebert, B.; Dale, B.; Ebert, L.M.; Woods, A.; Bonder, C.S. CD36 promotes vasculogenic mimicry in melanoma by mediating adhesion to the extracellular matrix. BMC Cancer 2021, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.; Dy, K.; Lugassy, C. Angiotropism in cutaneous melanoma: A prognostic factor strongly predicting risk for metastasis. J. Investig. Dermatol. 2002, 119, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Cen, J.; Ma, X.; Cui, L.; Qiu, Z.; Zhang, Z.; Li, H.; Yang, R.Z.; Wang, C.; et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat. Cell Biol. 2019, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Kopper, O.; de Witte, C.J.; Lohmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Liu, C.; Qin, T.; Huang, Y.; Li, Y.; Chen, G.; Sun, C. Drug screening model meets cancer organoid technology. Transl. Oncol. 2020, 13, 100840. [Google Scholar] [CrossRef] [PubMed]
- Belleri, M.; Paganini, G.; Coltrini, D.; Ronca, R.; Zizioli, D.; Corsini, M.; Barbieri, A.; Grillo, E.; Calza, S.; Bresciani, R.; et al. beta-Galactosylceramidase Promotes Melanoma Growth via Modulation of Ceramide Metabolism. Cancer Res. 2020, 80, 5011–5023. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Thareja, N.S.; Carmichael, T.S.; Barnhill, R.L.; Lugassy, C.; Bentolila, L.A. Visualizing Pericyte Mimicry of Angiotropic Melanoma by Direct Labeling of the Angioarchitecture. Methods Mol. Biol. 2021, 2235, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Abdouh, M.; Arena, V.; Arena, M.; Arena, G.O. Reprogramming Malignant Cancer Cells toward a Benign Phenotype following Exposure to Human Embryonic Stem Cell Microenvironment. PLoS ONE 2017, 12, e0169899. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Kasemeier-Kulesa, J.; Kulesa, P.M.; Postovit, L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 2007, 7, 246–255. [Google Scholar] [CrossRef]
- Bailey, C.M.; Kulesa, P.M. Dynamic interactions between cancer cells and the embryonic microenvironment regulate cell invasion and reveal EphB6 as a metastasis suppressor. Mol. Cancer Res. MCR 2014, 12, 1303–1313. [Google Scholar] [CrossRef]
- Giacobbe, A.; Abate-Shen, C. Modeling metastasis in mice: A closer look. Trends Cancer 2021, 7, 916–929. [Google Scholar] [CrossRef]
- Lee, M.W.; Miljanic, M.; Triplett, T.; Ramirez, C.; Aung, K.L.; Eckhardt, S.G.; Capasso, A. Current methods in translational cancer research. Cancer Metastasis Rev. 2021, 40, 7–30. [Google Scholar] [CrossRef]
- Cassidy, J.W.; Caldas, C.; Bruna, A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 2015, 75, 2963–2968. [Google Scholar] [CrossRef]
- Garman, B.; Anastopoulos, I.N.; Krepler, C.; Brafford, P.; Sproesser, K.; Jiang, Y.; Wubbenhorst, B.; Amaravadi, R.; Bennett, J.; Beqiri, M.; et al. Genetic and Genomic Characterization of 462 Melanoma Patient-Derived Xenografts, Tumor Biopsies, and Cell Lines. Cell Rep. 2017, 21, 1936–1952. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).