Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology of Neuropathy
4. Risk Factors of Diabetic Neuropathy
4.1. Neuropathy Risk Factors
4.2. Genetic Risk Factors of Neuropathy
4.3. Other Risk Factors
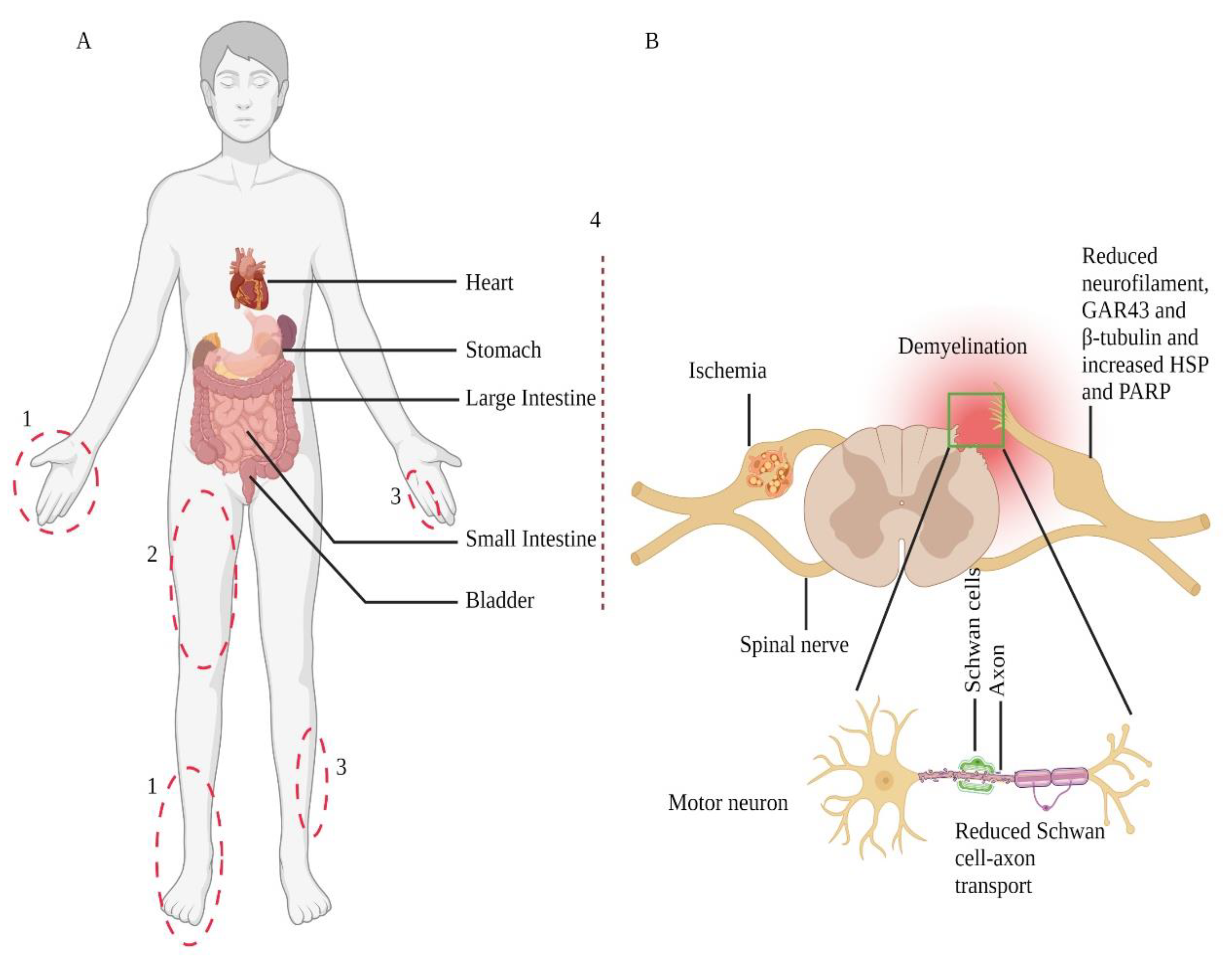
5. Mechanisms of Diabetic Neuropathy and Associated Pain
5.1. Mechanisms of Diabetic Neuropathy
5.2. Hyperglycemia in Neuropathy
5.3. Malfunctioned Insulin Signalling in Neuropathy
6. Diagnosis and Management of Neuropathic Pain
6.1. Assessment of Pain
6.2. Management
7. Pharmacological Targets and Future Perspective
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathy: A position statement by the american diabetes association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Lu, J.; Brooks, M.M.; Albert, S.; Althouse, A.D.; Escobedo, J.; Green, J.; Palumbo, P.; Perkins, B.A.; Whitehouse, F.; et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the bypass angioplasty revascularisation investigation 2 diabetes (bari 2d) cohort. Diabetes Care 2013, 36, 3208–3215. [Google Scholar] [CrossRef]
- Martin, C.L.; Albers, J.W.; Pop-Busui, R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 31–38. [Google Scholar] [CrossRef]
- Baron, R. Mechanisms of disease: Neuropathic pain—A clinical perspective. Nat. Clin. Pract. Neurol. 2006, 2, 95–106. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Barker, L.E.; Williamson, D.F. Projection of the year 2050 burden of diabetes in the us adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 2010, 8, 29. [Google Scholar] [CrossRef]
- Beghi, E.; Monticelli, M.L.; Amoruso, L.; Apollo, F.; Delodovici, M.L.; Grampa, G.; Ciam, M.A.; Cursio, M.; Ferrari, V. Chronic symmetric symptomatic polyneuropathy in the elderly: A field screening investigation in two italian regions. I. Prevalence and general characteristics of the sample. Italian general practitioner study group (igpsg). Neurology 1995, 45, 1832–1836. [Google Scholar]
- Bharucha, N.E.; Bharucha, A.E.; Bharucha, E.P. Prevalence of peripheral neuropathy in the parsi community of bombay. Neurology 1991, 41, 1315–1317. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Kerber, K.A.; Lisabeth, L.L.; Morgenstern, L.B.; Longoria, R.; Rodgers, A.; Longwell, P.; Feldman, E.L. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. 2014, 71, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.A.; Notermans, N.C.; Linssen, R.S.; van den Berg, L.H.; Vrancken, A.F. Incidence of polyneuropathy in utrecht, the netherlands. Neurology 2015, 84, 259–264. [Google Scholar] [CrossRef]
- Ang, L.; Jaiswal, M.; Martin, C.; Pop-Busui, R. Glucose control and diabetic neuropathy: Lessons from recent large clinical trials. Curr. Diabetes Rep. 2014, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.M.; Kahn, L.B.; Baxter, J.; Marshall, J.A.; Hamman, R.F. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The san luis valley diabetes study. Am. J. Epidemiol. 1990, 131, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J., 3rd; Service, F.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The rochester diabetic neuropathy study. Neurology 1993, 43, 817–824. [Google Scholar] [CrossRef]
- Boulton, A.J.; Knight, G.; Drury, J.; Ward, J.D. The prevalence of symptomatic, diabetic neuropathy in an insulin-treated population. Diabetes Care 1985, 8, 125–128. [Google Scholar] [CrossRef]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef]
- Partanen, J.; Niskanen, L.; Lehtinen, J.; Mervaala, E.; Siitonen, O.; Uusitupa, M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 89–94. [Google Scholar] [CrossRef]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the who multinational study of vascular disease in diabetes. Diabetologia 2001, 44 (Suppl. 2), S14–S21. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L.; Smith, T.; Havsager, A.M.; Madsen, C.; Kjeldsen, M.J.; Dalsgaard, N.J.; Gaist, D.; Schrøder, H.D.; Sindrup, S.H. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J. Clin. Neuromuscul. Dis. 2001, 3, 47–52. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Most, R.S.; Sinnock, P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care 1983, 6, 87–91. [Google Scholar] [CrossRef]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the united kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The eurodiab iddm complications study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef]
- Sumner, C.J.; Sheth, S.; Griffin, J.W.; Cornblath, D.R.; Polydefkis, M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003, 60, 108–111. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy—A review. Nat. Clin. Pract. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef]
- Leinninger, G.M.; Edwards, J.L.; Lipshaw, M.J.; Feldman, E.L. Mechanisms of disease: Mitochondria as new therapeutic targets in diabetic neuropathy. Nat. Clin. Pract. Neurol. 2006, 2, 620–628. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Ayeleso, A.O.; Mukwevho, E.; Masola, B. Antioxidant strategies in the management of diabetic neuropathy. BioMed Res. Int. 2015, 2015, 515042. [Google Scholar] [CrossRef] [PubMed]
- King, R.H. The role of glycation in the pathogenesis of diabetic polyneuropathy. Mol. Pathol. 2001, 54, 400–408. [Google Scholar]
- Monti, M.C.; Lonsdale, J.T.; Montomoli, C.; Montross, R.; Schlag, E.; Greenberg, D.A. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of american families with type 1 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 4650–4655. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Deshmukh, H.A.; Donnelly, L.A.; Torrance, N.; Colhoun, H.M.; Palmer, C.N.; Smith, B.H. A genome-wide association study provides evidence of sex-specific involvement of chr1p35.1 (zscan20-tlr12p) and chr8p23.1 (hmgb1p46) with diabetic neuropathic pain. EBioMedicine 2015, 2, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Deshmukh, H.A.; van Zuydam, N.R.; Liu, Y.; Donnelly, L.A.; Zhou, K.; Morris, A.D.; Colhoun, H.M.; Palmer, C.N.; Smith, B.H. A genome-wide association study suggests an association of chr8p21.3 (gfra2) with diabetic neuropathic pain. Eur. J. Pain 2015, 19, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Veluchamy, A.; Hébert, H.L.; Campbell, A.; Colhoun, H.M.; Palmer, C.N.A. A genome-wide association study suggests that mapk14 is associated with diabetic foot ulcers. Br. J. Dermatol. 2017, 177, 1664–1670. [Google Scholar] [CrossRef]
- Tang, Y.; Lenzini, P.A.; Pop-Busui, R.; Ray, P.R.; Campbell, H.; Perkins, B.A.; Callaghan, B.; Wagner, M.J.; Motsinger-Reif, A.A.; Buse, J.B.; et al. A genetic locus on chromosome 2q24 predicting peripheral neuropathy risk in type 2 diabetes: Results from the accord and bari 2d studies. Diabetes 2019, 68, 1649–1662. [Google Scholar] [CrossRef]
- Blesneac, I.; Themistocleous, A.C.; Fratter, C.; Conrad, L.J.; Ramirez, J.D.; Cox, J.J.; Tesfaye, S.; Shillo, P.R.; Rice, A.S.C.; Tucker, S.J.; et al. Rare nav1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018, 159, 469–480. [Google Scholar] [CrossRef]
- Lozeron, P.; Nahum, L.; Lacroix, C.; Ropert, A.; Guglielmi, J.M.; Said, G. Symptomatic diabetic and non-diabetic neuropathies in a series of 100 diabetic patients. J. Neurol. 2002, 249, 569–575. [Google Scholar] [CrossRef]
- Gorson, K.C.; Ropper, A.H. Additional causes for distal sensory polyneuropathy in diabetic patients. J. Neurol. Neurosurg. Psychiatry 2006, 77, 354–358. [Google Scholar] [CrossRef]
- Dunnigan, S.K.; Ebadi, H.; Breiner, A.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 2013, 36, 3684–3690. [Google Scholar] [CrossRef]
- Mizisin, A.P.; Shelton, G.D.; Wagner, S.; Rusbridge, C.; Powell, H.C. Myelin splitting, schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998, 95, 171–174. [Google Scholar] [CrossRef]
- Pan, S.; Chan, J.R. Regulation and dysregulation of axon infrastructure by myelinating glia. J. Cell Biol. 2017, 216, 3903–3916. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L.H. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.N.; Clark, A.W.; Zochodne, D.W. Neurofilament and tubulin gene expression in progressive experimental diabetes: Failure of synthesis and export by sensory neurons. Brain J. Neurol. 1999, 122 Pt 11, 2109–2118. [Google Scholar] [CrossRef]
- Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Shevalye, H.; Obrosova, I.G. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 2013, 62, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pan, P.; Anyika, M.; Blagg, B.S.; Dobrowsky, R.T. Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chem. Neurosci. 2015, 6, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.J.; Pan, P.; Farmer, K.L.; Zhao, H.; Blagg, B.S.; Dobrowsky, R.T. Modulating molecular chaperones improves sensory fiber recovery and mitochondrial function in diabetic peripheral neuropathy. Exp. Neurol. 2012, 235, 388–396. [Google Scholar] [CrossRef]
- Ilnytska, O.; Lyzogubov, V.V.; Stevens, M.J.; Drel, V.R.; Mashtalir, N.; Pacher, P.; Yorek, M.A.; Obrosova, I.G. Poly(adp-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 2006, 55, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Shevalye, H.; Maksimchyk, Y.; Drel, V.R.; Obrosova, I.G. Parp inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: Correlation with peripheral nerve function. Free. Radic. Biol. Med. 2011, 50, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.M.; Kato, K.; McLean, L.L.; Soules, M.E.; Feldman, E.L. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid. Redox Signal. 2009, 11, 425–438. [Google Scholar] [CrossRef]
- Fernyhough, P. Mitochondrial dysfunction in diabetic neuropathy: A series of unfortunate metabolic events. Curr. Diabetes Rep. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, P.; McGavock, J. Mechanisms of disease: Mitochondrial dysfunction in sensory neuropathy and other complications in diabetes. Handb. Clin. Neurol. 2014, 126, 353–377. [Google Scholar]
- Rumora, A.E.; Lentz, S.I.; Hinder, L.M.; Jackson, S.W.; Valesano, A.; Levinson, G.E.; Feldman, E.L. Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.A.; Hogue-Angeletti, R.A.; Frazier, W.A. Nerve growth factor and insulin: Evidence of similarities in structure, function, and mechanism of action. Recent Prog. Horm. Res. 1974, 30, 575–596. [Google Scholar] [PubMed]
- Fernyhough, P.; Willars, G.B.; Lindsay, R.M.; Tomlinson, D.R. Insulin and insulin-like growth factor i enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993, 607, 117–124. [Google Scholar] [CrossRef]
- Brussee, V.; Cunningham, F.A.; Zochodne, D.W. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 2004, 53, 1824–1830. [Google Scholar] [CrossRef]
- Sugimoto, K.; Murakawa, Y.; Zhang, W.; Xu, G.; Sima, A.A. Insulin receptor in rat peripheral nerve: Its localisation and alternatively spliced isoforms. Diabetes/Metab. Res. Rev. 2000, 16, 354–363. [Google Scholar] [CrossRef]
- Toth, C.; Brussee, V.; Zochodne, D.W. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia 2006, 49, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Kan, M.; Martinez, J.A.; Zochodne, D.W. Local insulin and the rapid regrowth of diabetic epidermal axons. Neurobiol. Dis. 2011, 43, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cheng, C.; Sun, H.; Zochodne, D.W. Near nerve local insulin prevents conduction slowing in experimental diabetes. Brain Res. 1997, 763, 209–214. [Google Scholar] [CrossRef]
- Grote, C.W.; Morris, J.K.; Ryals, J.M.; Geiger, P.C.; Wright, D.E. Insulin receptor substrate 2 expression and involvement in neuronal insulin resistance in diabetic neuropathy. Exp. Diabetes Res. 2011, 2011, 212571. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Truini, A. A review of neuropathic pain: From guidelines to clinical practice. Pain Ther. 2017, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luo, L.; Hu, Y.; Fang, K.; Liu, J. Clinical practice guidelines for the management of neuropathic pain: A systematic review. BMC Anesthesiol. 2016, 16, 12. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N. Diagnosis and assessment of neuropathic pain: The saga of clinical tools. Pain 2011, 152, S74–s83. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The iasp classification of chronic pain for icd-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Brown, J.J.; Pribesh, S.L.; Baskette, K.G.; Vinik, A.I.; Colberg, S.R. A comparison of screening tools for the early detection of peripheral neuropathy in adults with and without type 2 diabetes. J. Diabetes Res. 2017, 2017, 1467213. [Google Scholar] [CrossRef]
- Olaleye, D.; Perkins, B.A.; Bril, V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res. Clin. Pract. 2001, 54, 115–128. [Google Scholar] [CrossRef]
- Perkins, B.A.; Olaleye, D.; Zinman, B.; Bril, V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001, 24, 250–256. [Google Scholar] [CrossRef]
- England, J.D.; Gronseth, G.S.; Franklin, G.; Miller, R.G.; Asbury, A.K.; Carter, G.T.; Cohen, J.A.; Fisher, M.A.; Howard, J.F.; Kinsella, L.J.; et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the american academy of neurology, the american association of electrodiagnostic medicine, and the american academy of physical medicine and rehabilitation. Neurology 2005, 64, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Keller, J.; Maier, C.; Pannek, J. Diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2014, 122, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; Kansagara, D.; Horwitch, C.; Barry, M.J.; Forciea, M.A.; Fitterman, N.; Balzer, K.; Boyd, C.; Humphrey, L.L.; et al. Hemoglobin a1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: A guidance statement update from the american college of physicians. Ann. Intern. Med. 2018, 168, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gutierrez, R.; Lipska, K.J.; McCoy, R.G. Intensive glycemic control in type 2 diabetes mellitus—A balancing act of latent benefit and avoidable harm: A teachable moment. JAMA Intern. Med. 2016, 176, 300–301. [Google Scholar] [CrossRef][Green Version]
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef]
- Albers, J.W.; Herman, W.H.; Pop-Busui, R.; Feldman, E.L.; Martin, C.L.; Cleary, P.A.; Waberski, B.H.; Lachin, J.M. Effect of prior intensive insulin treatment during the diabetes control and complications trial (dcct) on peripheral neuropathy in type 1 diabetes during the epidemiology of diabetes interventions and complications (edic) study. Diabetes Care 2010, 33, 1090–1096. [Google Scholar] [CrossRef]
- Fullerton, B.; Jeitler, K.; Seitz, M.; Horvath, K.; Berghold, A.; Siebenhofer, A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2014, 2014, Cd009122. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 2012, 6, Cd007543. [Google Scholar] [CrossRef]
- Group, U.S. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33). Uk prospective diabetes study (ukpds) group. Lancet 1998, 352, 837–853. [Google Scholar]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H., Jr.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the accord randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Pantalone, K.M.; Misra-Hebert, A.D.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Chagin, K.; Dey, T.; Milinovich, A.; Weng, W.; Bauman, J.M.; et al. Effect of glycemic control on the diabetes complications severity index score and development of complications in people with newly diagnosed type 2 diabetes. J. Diabetes 2018, 10, 192–199. [Google Scholar] [CrossRef]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The legacy effect in type 2 diabetes: Impact of early glycemic control on future complications (the diabetes & aging study). Diabetes Care 2019, 42, 416–426. [Google Scholar] [PubMed]
- Gibbons, C.H. Treatment-induced neuropathy of diabetes. Curr. Diabetes Rep. 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H. Treatment induced neuropathy of diabetes-long term implications in type 1 diabetes. J. Diabetes Its Complicat. 2017, 31, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.V.; Tiwari, S.; Purandare, V.B.; Khedkar, S.; Bhosale, S.S.; Unnikrishnan, A.G. Choice of wound care in diabetic foot ulcer: A practical approach. World J. Diabetes 2014, 5, 546–556. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An scn9a channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Rauck, R.L.; Shaibani, A.; Biton, V.; Simpson, J.; Koch, B. Lacosamide in painful diabetic peripheral neuropathy: A phase 2 double-blind placebo-controlled study. Clin. J. Pain 2007, 23, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wymer, J.P.; Simpson, J.; Sen, D.; Bongardt, S. Efficacy and safety of lacosamide in diabetic neuropathic pain: An 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clin. J. Pain 2009, 25, 376–385. [Google Scholar] [CrossRef]
- Curtin, C.M.; Kenney, D.; Suarez, P.; Hentz, V.R.; Hernandez-Boussard, T.; Mackey, S.; Carroll, I.R. A double-blind placebo randomised controlled trial of minocycline to reduce pain after carpal tunnel and trigger finger release. J. Hand Surg. 2017, 42, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Szekely, B.; Lemarié, J.; Martin, F.; Gentili, M.; Ben Ammar, S.; Lepeintre, J.F.; Garreau de Loubresse, C.; Chauvin, M.; Bouhassira, D.; et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: A randomised, double-blind, controlled study. Pain 2013, 154, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, M.; Ueda, H.; Hozumi, J.; Inoue, R.; Kogure, T.; Yamada, Y.; Kogure, T. Minocycline does not decrease intensity of neuropathic pain intensity, but does improve its affective dimension. J. Pain Palliat. Care Pharmacother. 2016, 30, 31–35. [Google Scholar] [CrossRef]
- Vanelderen, P.; Van Zundert, J.; Kozicz, T.; Puylaert, M.; De Vooght, P.; Mestrum, R.; Heylen, R.; Roubos, E.; Vissers, K. Effect of minocycline on lumbar radicular neuropathic pain: A randomised, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015, 122, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Vinik, A.; Rosenstock, J.; Sharma, U.; Feins, K.; Hsu, C.; Merante, D. Efficacy and safety of mirogabalin (ds-5565) for the treatment of diabetic peripheral neuropathic pain: A randomised, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care 2014, 37, 3253–3261. [Google Scholar] [CrossRef]
- Elzinga, S.; Murdock, B.J.; Guo, K.; Hayes, J.M.; Tabbey, M.A.; Hur, J.; Feldman, E.L. Toll-like receptors and inflammation in metabolic neuropathy; a role in early versus late disease? Exp. Neurol. 2019, 320, 112967. [Google Scholar] [CrossRef]
- Zhu, T.; Meng, Q.; Ji, J.; Lou, X.; Zhang, L. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci. Lett. 2015, 585, 28–32. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Nunes Vdos, S.; Mastronardi, C.A.; Neeman, T.; Paz-Filho, G.J. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of controlled cross-sectional studies. J. Diabetes Its Complicat. 2016, 30, 357–367. [Google Scholar] [CrossRef]
- Pradeepa, R.; Surendar, J.; Indulekha, K.; Chella, S.; Anjana, R.M.; Mohan, V. Association of serum adiponectin with diabetic microvascular complications among south indian type 2 diabetic subjects—(cures-133). Clin. Biochem. 2015, 48, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Kannenberg, J.M.; Huth, C.; Carstensen-Kirberg, M.; Rathmann, W.; Koenig, W.; Heier, M.; Püttgen, S.; Thorand, B.; Peters, A.; et al. Proinflammatory cytokines predict the incidence and progression of distal sensorimotor polyneuropathy: Kora f4/ff4 study. Diabetes Care 2017, 40, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tang, D.D.; Yin, E.G.; Wei, L.L.; Chen, P.; Deng, S.P.; Tu, L.L. Diagnostic significance of serum levels of nerve growth factor and brain derived neurotrophic factor in diabetic peripheral neuropathy. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2018, 24, 5943–5950. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, Y.J.; Ahn, C.W.; Park, K.S.; Kim, J.C.; Nam, J.S.; Im, Y.S.; Lee, J.E.; Lee, S.C.; Lee, H.K. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet. Med. J. Br. Diabet. Assoc. 2009, 26, 1228–1234. [Google Scholar] [CrossRef]
- Pourhamidi, K.; Skärstrand, H.; Dahlin, L.B.; Rolandsson, O. Hsp27 concentrations are lower in patients with type 1 diabetes and correlate with large nerve fiber dysfunction. Diabetes Care 2014, 37, e49–e50. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Hannan, J.M.A.; Azam, S.; Jakaria, M. Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy. Int. J. Transl. Med. 2021, 1, 175-186. https://doi.org/10.3390/ijtm1030013
Ansari P, Hannan JMA, Azam S, Jakaria M. Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy. International Journal of Translational Medicine. 2021; 1(3):175-186. https://doi.org/10.3390/ijtm1030013
Chicago/Turabian StyleAnsari, Prawej, J.M.A. Hannan, Shofiul Azam, and Md. Jakaria. 2021. "Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy" International Journal of Translational Medicine 1, no. 3: 175-186. https://doi.org/10.3390/ijtm1030013
APA StyleAnsari, P., Hannan, J. M. A., Azam, S., & Jakaria, M. (2021). Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy. International Journal of Translational Medicine, 1(3), 175-186. https://doi.org/10.3390/ijtm1030013







