Microplastic Accumulation in Urban Stream Sediments: Vertical Distribution and Transport Dynamics
Abstract
1. Introduction
2. Materials and Methods
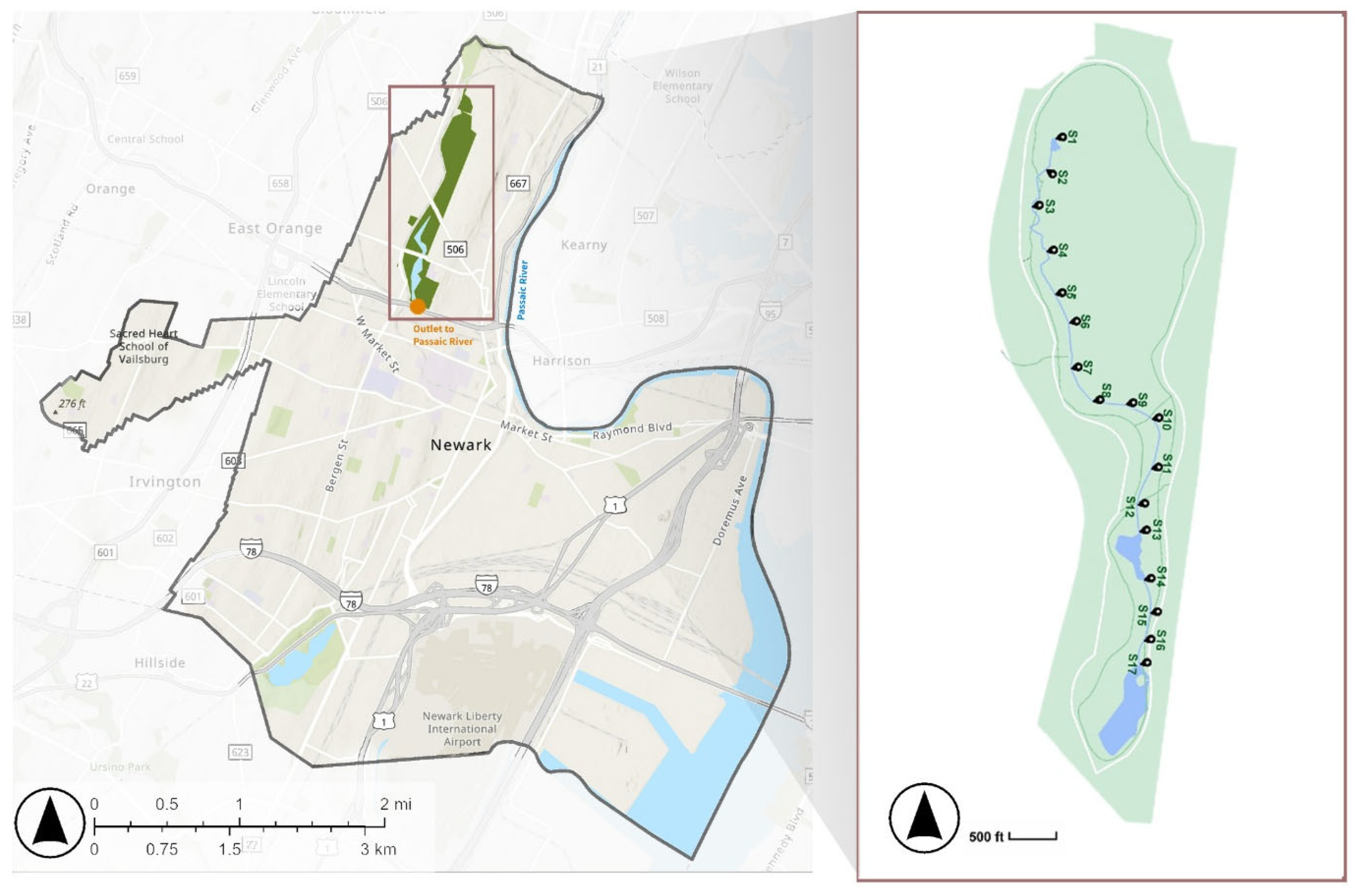
2.1. Site Description
2.2. Sample Collection
2.3. MPs Extraction and Characterization
2.4. Data Analysis
2.5. Quality Assurance and Quality Control (QA/QC)
3. Results & Discussion
3.1. MPs Concentration in Stream Sediments
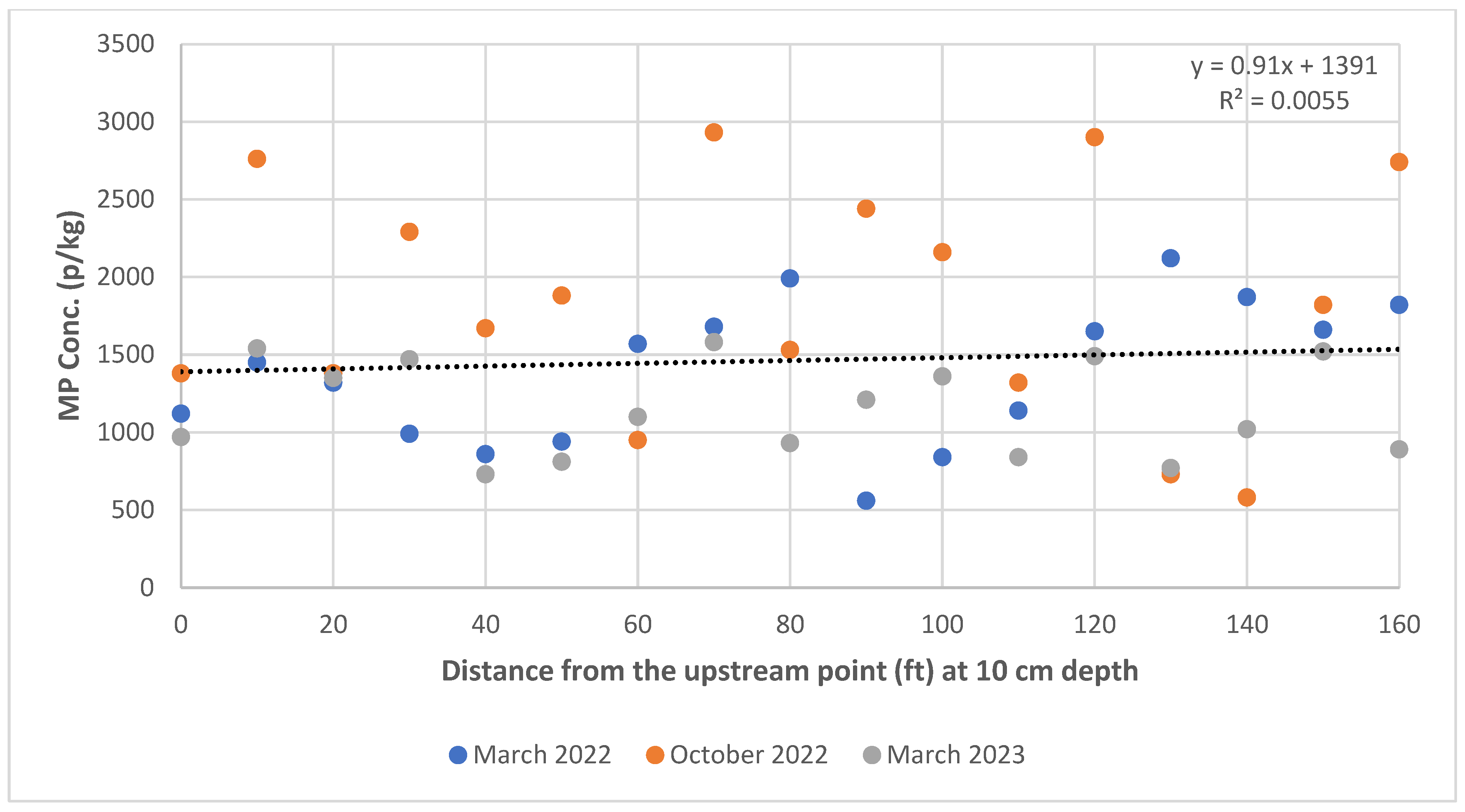
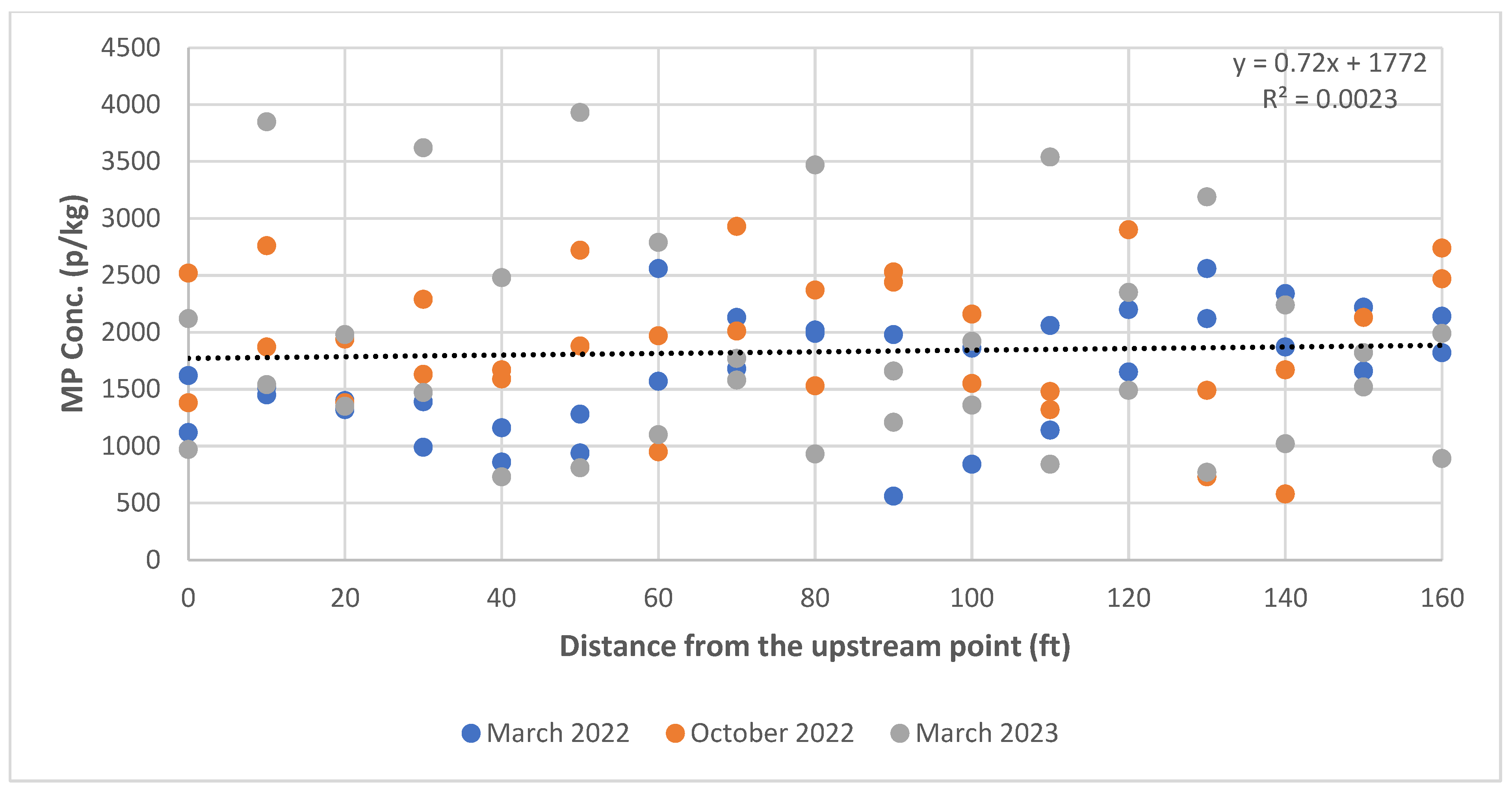
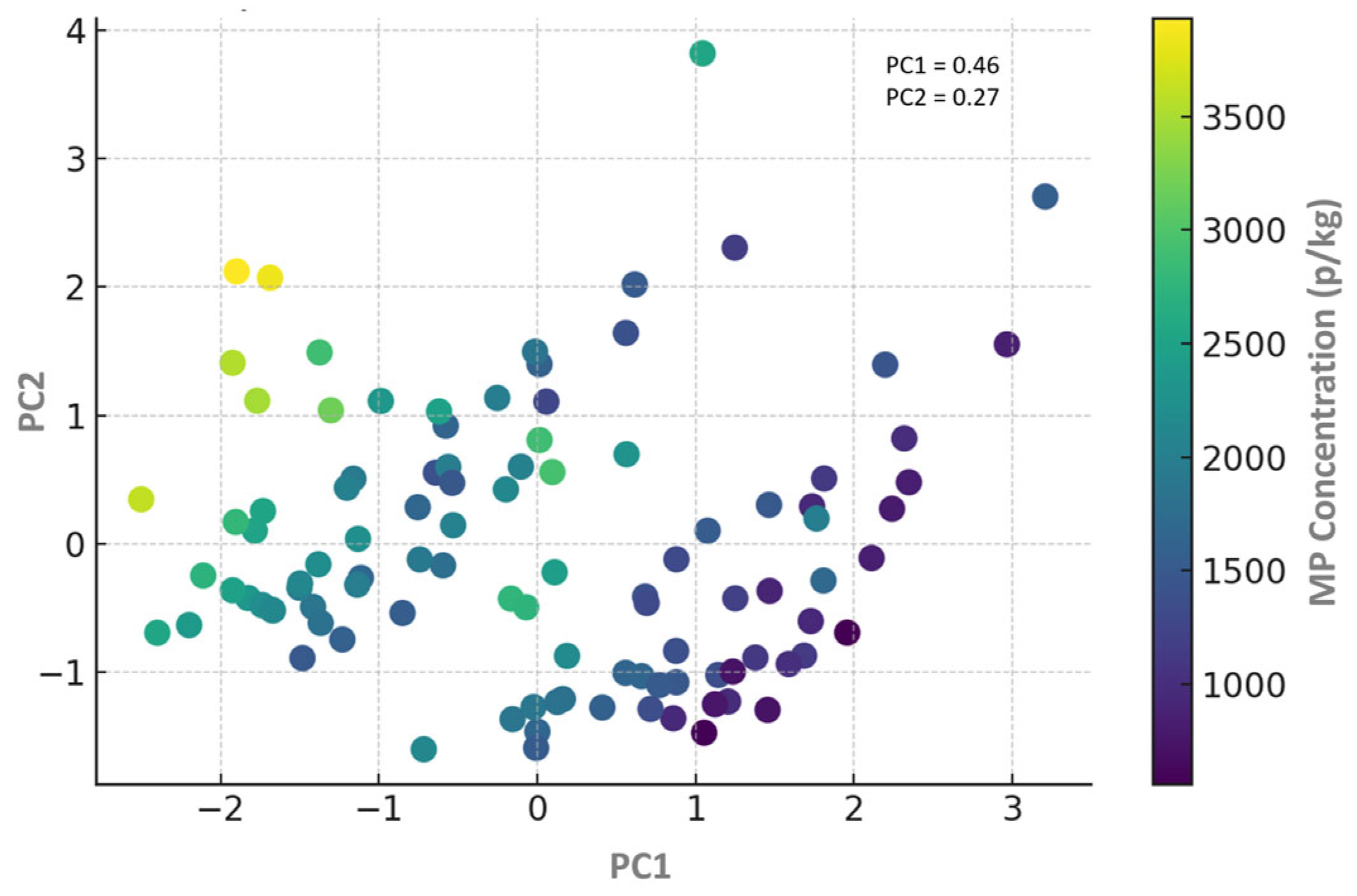
3.2. Spatial Variation in MPs in Stream Sediments
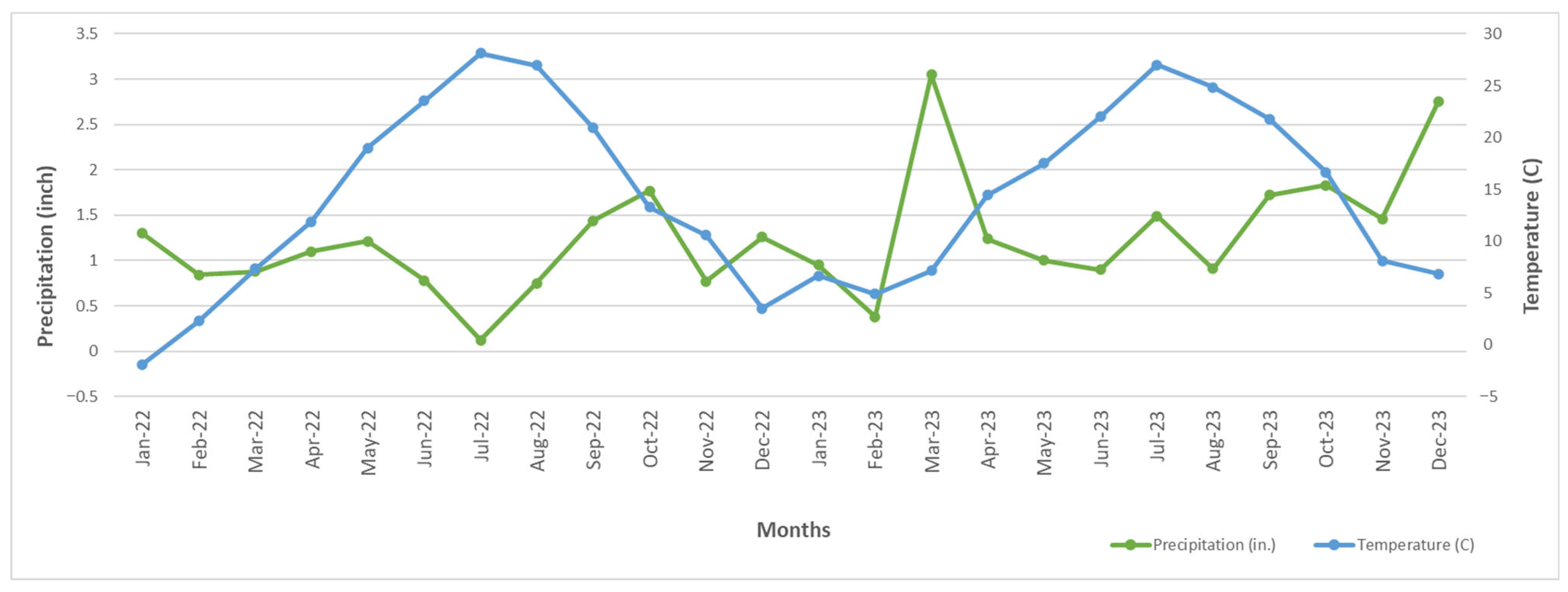
3.3. Seasonal and Temporal Variation in MPs in Stream Sediments
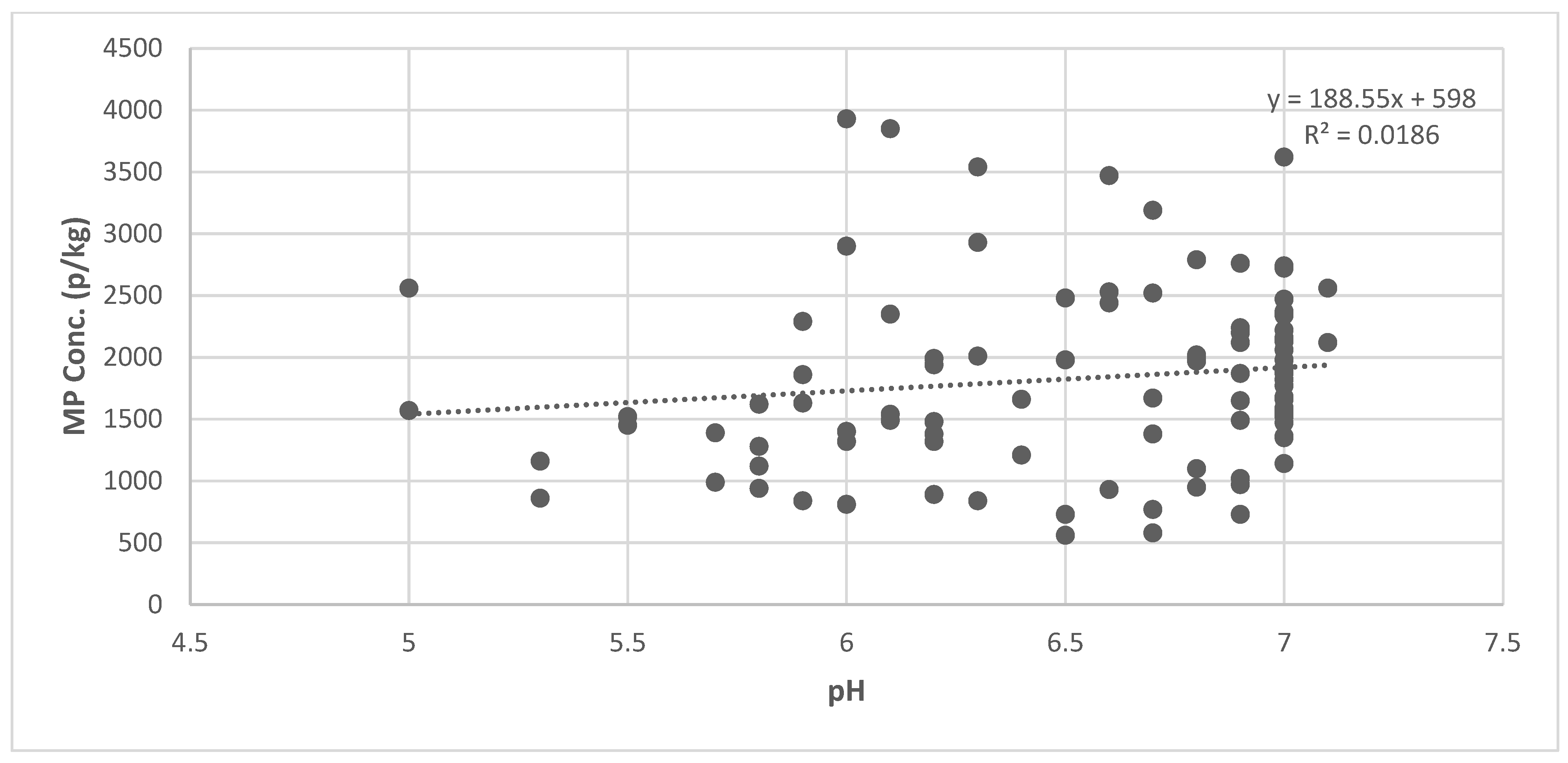
3.4. Influence of pH on MP Concentration
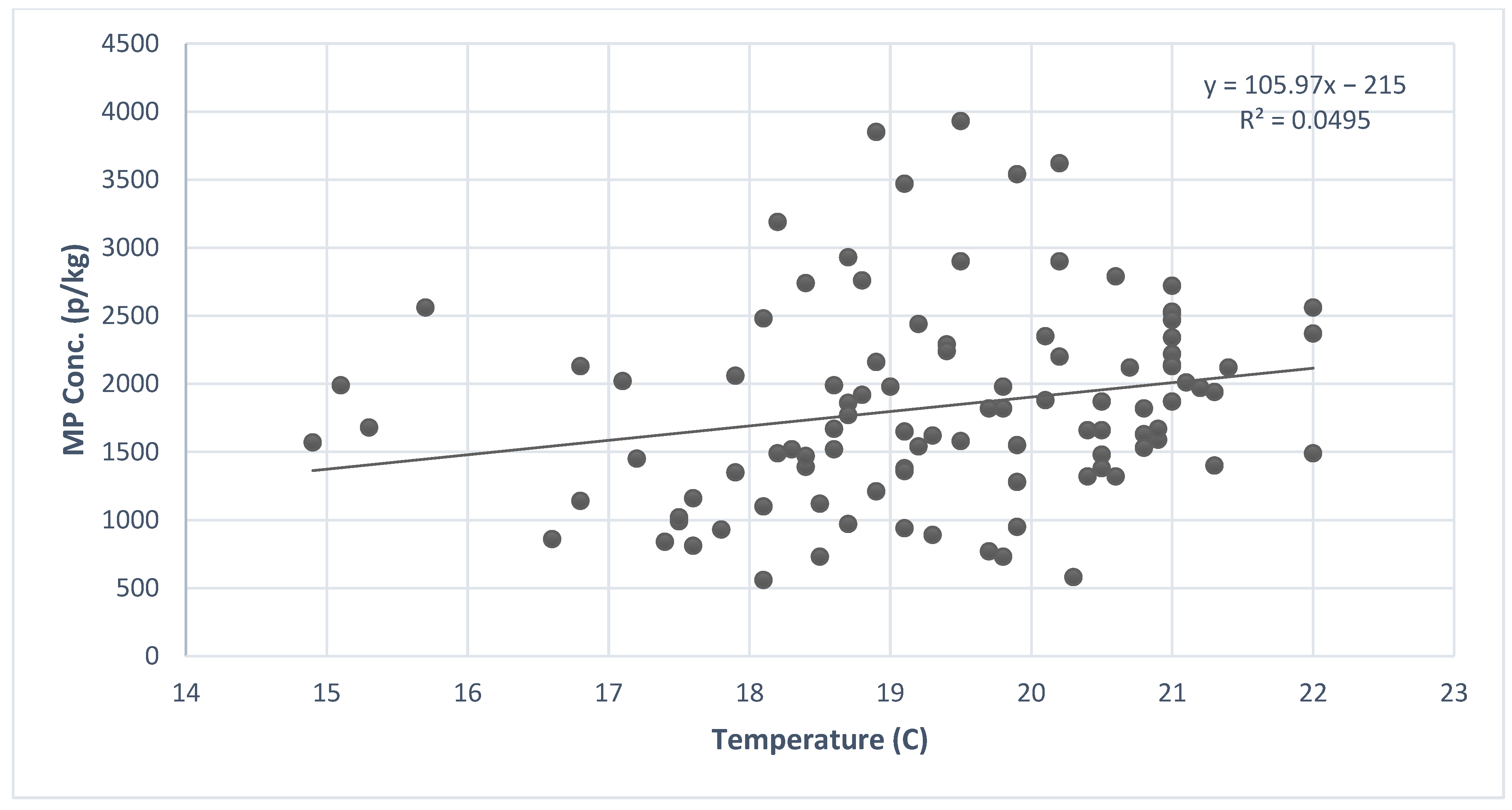
3.5. Influence of Temperature on MP Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.; Charan Rath, C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Pennock, W.H.; Brenckman, C.; Borgaonkar, A.D. Would the Oceans Become Toxic to Humanity Due to Use and Mismanagement of Plastics? Int. J. Environ. Res. Public Health 2024, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.A.; McMahon, D.; Adams, W.A.; Vermaire, J.C. Change in Microplastic Concentration During Various Temporal Events Downstream of a Combined Sewage Overflow and in an Urban Stormwater Creek. Front. Water 2022, 4, 958130. [Google Scholar] [CrossRef]
- Jayalakshmamma, M.P.; Nagara, V.N.; Borgaonkar, A.; Sarkar, D.; Sadik, O.; Boufadel, M. Characterizing microplastics in urban runoff: A multi-land use assessment with a focus on 1–125 μm size particles. Sci. Total Environ. 2023, 904, 166685. [Google Scholar] [CrossRef]
- Kay, P.; Hiscoe, R.; Moberley, I.; Bajic, L.; McKenna, N. Wastewater treatment plants as a source of microplastics in river catchments. Environ. Sci. Pollut. Res. 2018, 25, 20264–20267. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Brenckman, C.M.; Parameswarappa Jayalakshmamma, M.; Pennock, W.H.; Ashraf, F.; Borgaonkar, A.D. A Review of Harmful Algal Blooms: Causes, Effects, Monitoring, and Prevention Methods. Water 2025, 17, 1980. [Google Scholar] [CrossRef]
- Ren, X.; Mao, M.; Feng, M.; Peng, T.; Long, X.; Yang, F. Fate, abundance and ecological risks of microcystins in aquatic environment: The implication of microplastics. Water Res. 2024, 251, 121121. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, M.; Stoewer, S.-C.; Fischer, E.K. On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ. Pollut. 2019, 254, 112970. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, M.; Chen, X.; Hu, L.; Xu, Y.; Fu, W.; Li, C. A comparative review of microplastics in lake systems from different countries and regions. Chemosphere 2022, 286, 131806. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in Surface Waters of Urban Rivers: Concentration, Sources, and Associated Bacterial Assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Montecinos, S.; Gil, M.; Tognana, S.; Salgueiro, W.; Amalvy, J. Distribution of microplastics present in a stream that receives discharge from wastewater treatment plants. Environ. Pollut. 2022, 314, 120299. [Google Scholar] [CrossRef]
- Dikareva, N.; Simon, K.S. Microplastic pollution in streams spanning an urbanisation gradient. Environ. Pollut. 2019, 250, 292–299. [Google Scholar] [CrossRef]
- Gonzalez-Saldias, F.; Sabater, F.; Gomà, J. Microplastic distribution and their abundance along rivers are determined by land uses and sediment granulometry. Sci. Total Environ. 2024, 933, 173165. [Google Scholar] [CrossRef]
- Berg, E.M.; Speir, S.L.; Shogren, A.J.; Dee, M.M.; Vincent, A.E.S.; Tank, J.L.; Kelly, J.J.; Hoellein, T.J. Transport and Retention of Microplastic Fibers in Streams Are Impacted by Benthic Algae, Discharge, and Substrate. Limnol. Oceanogr. 2025, 70, 1093–1107. [Google Scholar] [CrossRef]
- Vincent, A.E.S.; Hoellein, T.J. Distribution and Transport of Microplastic and Fine Particulate Organic Matter in Urban Streams. Ecol. Appl. 2021, 31, e02429. [Google Scholar] [CrossRef]
- Drummond, J.D.; Schneidewind, U.; Li, A.; Hoellein, T.J.; Krause, S.; Packman, A.I. Microplastic accumulation in riverbed sediment via hyporheic exchange from headwaters to mainstems. Sci. Adv. 2022, 8, eabi9305. [Google Scholar] [CrossRef]
- Jayalakshmamma, M.P.; Nagara, V.N.; Borgaonkar, A.; Sarkar, D.; Obropta, C.; Boufadel, M. Temporal and spatial distribution of microplastics in green infrastructures: Rain gardens. Chemosphere 2024, 362, 142543. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, J.; Liu, H.; Lu, X. Vertical distribution of microplastics in the sediment profiles of the Lake Taihu, eastern China. Sustain. Environ. Res. 2022, 32, 44. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. CH4 and CO2 emissions from the decomposition of microplastics in the bottom sediment—Preliminary studies. Environments 2022, 9, 91. [Google Scholar] [CrossRef]
- Tristanova, T.; Ismanto, A.; Widiaratih, R.; Zainuri, M.; Sugianto, D.N.; Rochaddi, B.; Ismuniarti, D.H.; Wulandari, S.Y.; Hernawan, U.; Hadibarata, T. Modeling the fate of microplastics in the Sengkarang Estuary, Pekalongan City, Central Java, Indonesia. Environ. Qual. Manag. 2024, 34, e22239. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Spanjer, A.R.; Rosen, M.R.; Thom, T. Microplastics in Lake Mead national recreation area, USA: Occurrence and biological uptake. PLoS ONE 2020, 15, e0228896. [Google Scholar] [CrossRef]
- Pittroff, M.; Loui, C.; Oswald, S.E.; Bochow, M.; Kamp, J.; Dierkes, G.; Lensing, H.-J.; Munz, M. Riverbed depth-specific microplastics distribution and potential use as process marker. Environ. Sci. Pollut. Res. 2024, 31, 45326–45340. [Google Scholar] [CrossRef]
- Li, C.; Tang, K.H.D. Effects of pH and temperature on the leaching of di (2-ethylhexyl) phthalate and di-n-butyl phthalate from microplastics in simulated marine environment. Biointerface Res. Appl. Chem. 2023, 13, 269. [Google Scholar]
- Feng, S.; Lu, H.; Xue, Y.; Liu, Y.; Li, H.; Zhou, C.; Zhang, X.; Yan, P. Occurrence of microplastics in the headwaters of Yellow River on the Tibetan Plateau: Source analysis and ecological risk assessment. J. Hazard. Mater. 2024, 477, 135327. [Google Scholar] [CrossRef] [PubMed]
- Mutshekwa, T.; Munyai, L.F.; Mugwedi, L.; Cuthbert, R.N.; Dondofema, F.; Dalu, T. Seasonal occurrence of microplastics in sediment of two South African recreational reservoirs. Water Biol. Secur. 2023, 2, 100185. [Google Scholar] [CrossRef]
- Aralappanavar, V.K.; Mukhopadhyay, R.; Yu, Y.; Liu, J.; Bhatnagar, A.; Praveena, S.M.; Li, Y.; Paller, M.; Adyel, T.M.; Rinklebe, J. Effects of microplastics on soil microorganisms and microbial functions in nutrients and carbon cycling–A review. Sci. Total Environ. 2024, 924, 171435. [Google Scholar] [CrossRef]
- Fox, J.M.; Schwoerer, G.D.; Schreiner, K.M.; Minor, E.C.; Maurer-Jones, M.A. Microplastics in the Water Column of Western Lake Superior. ACS ES&T Water 2022, 2, 1659–1666. [Google Scholar] [CrossRef]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Distribution of microplastic in relation to water quality parameters in the Brantas River, East Java, Indonesia. Environ. Technol. Innov. 2021, 24, 101915. [Google Scholar] [CrossRef]
- Essex County Parks. Branch Brook Park Overview. 2023. Available online: https://essexcountyparks.org/parks/branch-brook-park (accessed on 15 August 2025).
- US Census Bureau. Population Estimates. 2021. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045221 (accessed on 15 August 2025).
- EPA. Passaic River Basin Restoration Efforts. Available online: https://www.epa.gov/urbanwaterspartners/passaic-river-partnership-history-and-accomplishments (accessed on 15 September 2025).
- Mishra, S.; Dash, D.; Das, A.P. Detection, characterization and possible biofragmentation of synthetic microfibers released from domestic laundering wastewater as an emerging source of marine pollution. Mar. Pollut. Bull. 2022, 185, 114254. [Google Scholar] [CrossRef] [PubMed]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum; NOS-OR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015; Volume 48, p. 10296. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]
- Jayalakshmamma, M.P.; Ji, W.; Abou Khalil, C.; Marhaba, T.F.; Abrams, S.; Lee, K.; Zhang, H.; Boufadel, M. Removal of hydrocarbons from heterogenous soil using electrokinetics and surfactants. Environ. Chall. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Ji, W.; Jayalakshmamma, M.P.; Abou Khalil, C.; Zhao, L.; Boufadel, M. Removal of hydrocarbon from soils possessing macro-heterogeneities using electrokinetics and surfactants. Chem. Eng. J. Adv. 2020, 4, 100030. [Google Scholar] [CrossRef]
- Rimondi, V.; Monnanni, A.; De Beni, E.; Bicocchi, G.; Chelazzi, D.; Cincinelli, A.; Fratini, S.; Martellini, T.; Morelli, G.; Venturi, S. Occurrence and quantification of natural and microplastic items in urban streams: The case of Mugnone Creek (Florence, Italy). Toxics 2022, 10, 159. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK–Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; Guardia, M.J.L.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Henny, C.; Suryono, T.; Rohaningsih, D.; Yoga, G.P.; Sudarso, J.; Waluyo, A. The Occurrence of Microplastics in the Surface Water of Several Urban Lakes in the Megacity of Jakarta. IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012023. [Google Scholar] [CrossRef]
- Clayer, F.; Jartun, M.; Buenaventura, N.; Guerrero, J.-L.; Lusher, A. Bypass of Booming Inputs of Urban and Sludge-Derived Microplastics in a Large Nordic Lake. Environ. Sci. Technol. 2021, 55, 7949–7958. [Google Scholar] [CrossRef]
- Felismino, M.E.L.; Helm, P.A.; Rochman, C.M. Microplastic and other anthropogenic microparticles in water and sediments of Lake Simcoe. J. Great Lakes Res. 2021, 47, 180–189. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Basooma, R.; Nabwire, R. Occurrence, distribution and size relationships of plastic debris along shores and sediment of northern Lake Victoria. Environ. Pollut. 2020, 257, 113442. [Google Scholar] [CrossRef]
- Oni, B.A.; Ayeni, A.O.; Agboola, O.; Oguntade, T.; Obanla, O. Comparing microplastics contaminants in (dry and raining) seasons for Ox-Bow Lake in Yenagoa, Nigeria. Ecotoxicol. Environ. Saf. 2020, 198, 110656. [Google Scholar] [CrossRef]
- Bao, K.; Jiang, H.; Su, P.; Lü, P.; Yan, Z. Vertical Profiles of Microplastics in the Hyporheic Zone Sediment: A Case Study in the Yangtze River, Nanjing Section. Sustainability 2023, 15, 7895. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef]
- Chen, P.; Kane, I.; Clare, M.; Soutter, E.; Mienis, F.; Wogelius, R.A.; Keavney, E. Direct Evidence That Microplastics Are Transported to the Deep Sea by Turbidity Currents. Environ. Sci. Technol. 2025, 59, 7278–7287. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.; Christiansen, K.S.; Bode, E.T.; Hoellein, T.J. A Watershed-Scale, Citizen Science Approach to Quantifying Microplastic Concentration in a Mixed Land-Use River. Water Res. 2018, 147, 382–392. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A. Microplastic pollution in St. Lawrence river sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Radford, F.; Horton, A.; Hudson, M.; Shaw, P.; Williams, I. Agricultural soils and microplastics: Are biosolids the problem? Front. Soil. Sci. 2023, 2, 941837. [Google Scholar] [CrossRef]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef] [PubMed]
- Ockelford, A.; Cundy, A.; Ebdon, J.E. Storm response of fluvial sedimentary microplastics. Sci. Rep. 2020, 10, 1865. [Google Scholar] [CrossRef]
- Liu, P.; Liao, H.; Deng, Y.; Zhang, W.; Zhou, Z.; Sun, D.; Ke, Z.; Zhou, A.; Tang, H. Microplastic Pollution and Its Potential Correlation with Environmental Factors in Daya Bay, South China Sea. J. Mar. Sci. Eng. 2023, 11, 1465. [Google Scholar] [CrossRef]
- Nkosi, M.S.; Cuthbert, R.N.; Wu, N.; Shikwambana, P.; Dalu, T. Microplastic abundance, distribution, and diversity in water and sediments along a subtropical river system. Environ. Sci. Pollut. Res. Int. 2023, 30, 91440–91452. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Ramavandi, B.; Ghasemi, A.; Sorial, G.A. Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 2021, 264, 128543. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms in mangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; De la Rosa, J.R.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Guo, F.; Liu, B.; Zhao, J.; Hou, Y.; Wu, J.; Hu, H.; Zhou, C.; Hu, H.; Zhang, T.; Yang, Z. A Song of Ice and Fire: Temperature-Dependent Effects of Microplastics on Sediment Bacteriome and Metabolome. Chemosphere 2024, 350, 141190. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Li, Q.; Xia, X.; Zhang, H. The effects of land use types on microplastics in river water: A case study on the mainstream of the Wei River, China. Environ. Monit. Assess. 2024, 196, 349. [Google Scholar] [CrossRef]
- Themba, N.N.; Dondofema, F.; Cuthbert, R.N.; Munyai, L.F.; Dalu, T. Abundance and distribution of microplastics in benthic sediments and Cladocera taxa in a subtropical Austral reservoir. Integr. Environ. Assess. Manag. 2024, 20, 2256–2270. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Fate of microplastics under the influence of climate change. iScience 2023, 26, 107649. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parameswarappa Jayalakshmamma, M.; Borgaonkar, A.D.; Sarkar, D.; Obropta, C.; Boufadel, M. Microplastic Accumulation in Urban Stream Sediments: Vertical Distribution and Transport Dynamics. Microplastics 2025, 4, 65. https://doi.org/10.3390/microplastics4030065
Parameswarappa Jayalakshmamma M, Borgaonkar AD, Sarkar D, Obropta C, Boufadel M. Microplastic Accumulation in Urban Stream Sediments: Vertical Distribution and Transport Dynamics. Microplastics. 2025; 4(3):65. https://doi.org/10.3390/microplastics4030065
Chicago/Turabian StyleParameswarappa Jayalakshmamma, Meghana, Ashish D. Borgaonkar, Dibyendu Sarkar, Christopher Obropta, and Michel Boufadel. 2025. "Microplastic Accumulation in Urban Stream Sediments: Vertical Distribution and Transport Dynamics" Microplastics 4, no. 3: 65. https://doi.org/10.3390/microplastics4030065
APA StyleParameswarappa Jayalakshmamma, M., Borgaonkar, A. D., Sarkar, D., Obropta, C., & Boufadel, M. (2025). Microplastic Accumulation in Urban Stream Sediments: Vertical Distribution and Transport Dynamics. Microplastics, 4(3), 65. https://doi.org/10.3390/microplastics4030065







