Micro- and Nanoplastics on Human Health and Diseases: Perspectives and Recent Advances
Abstract
1. Introduction
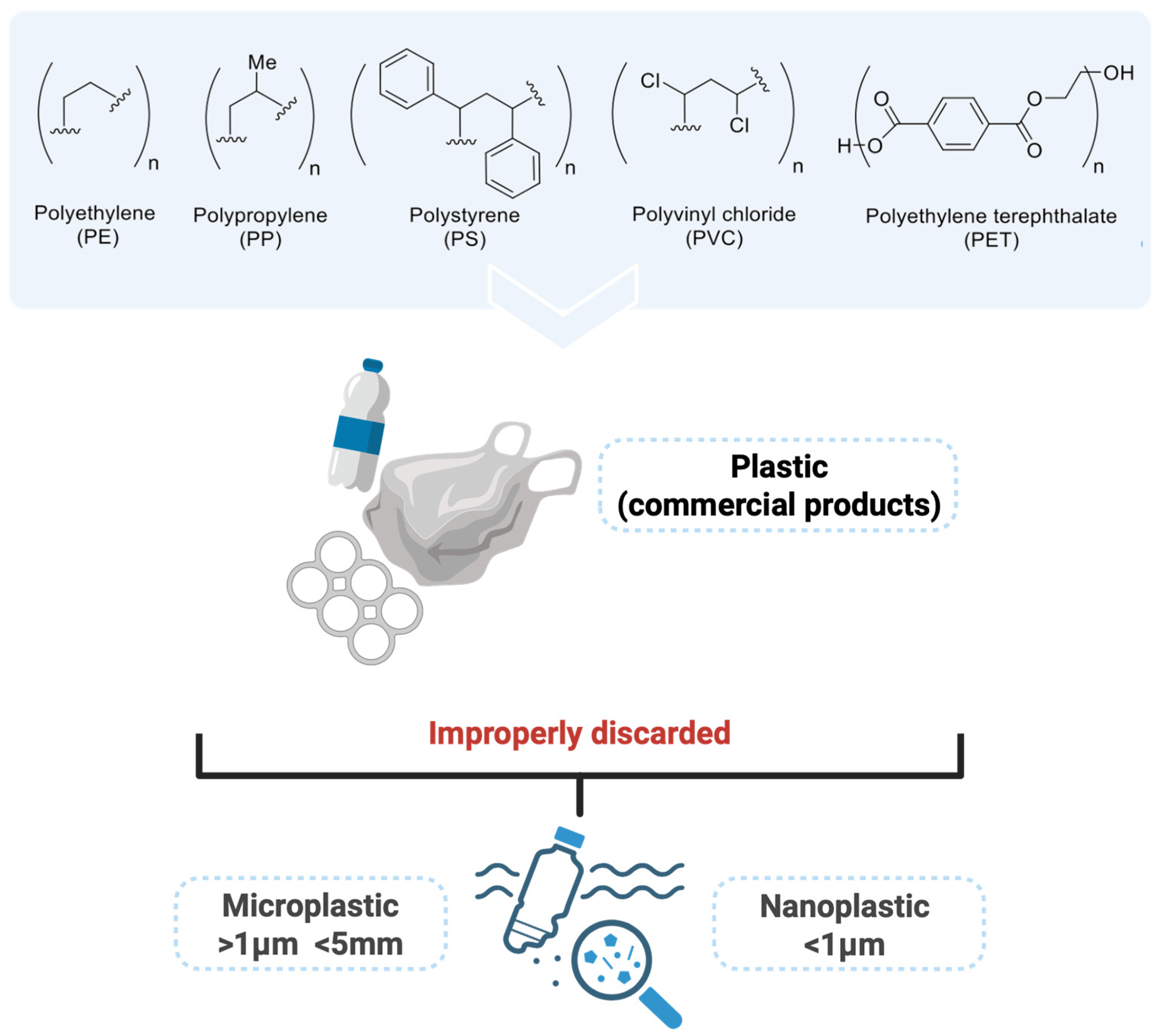
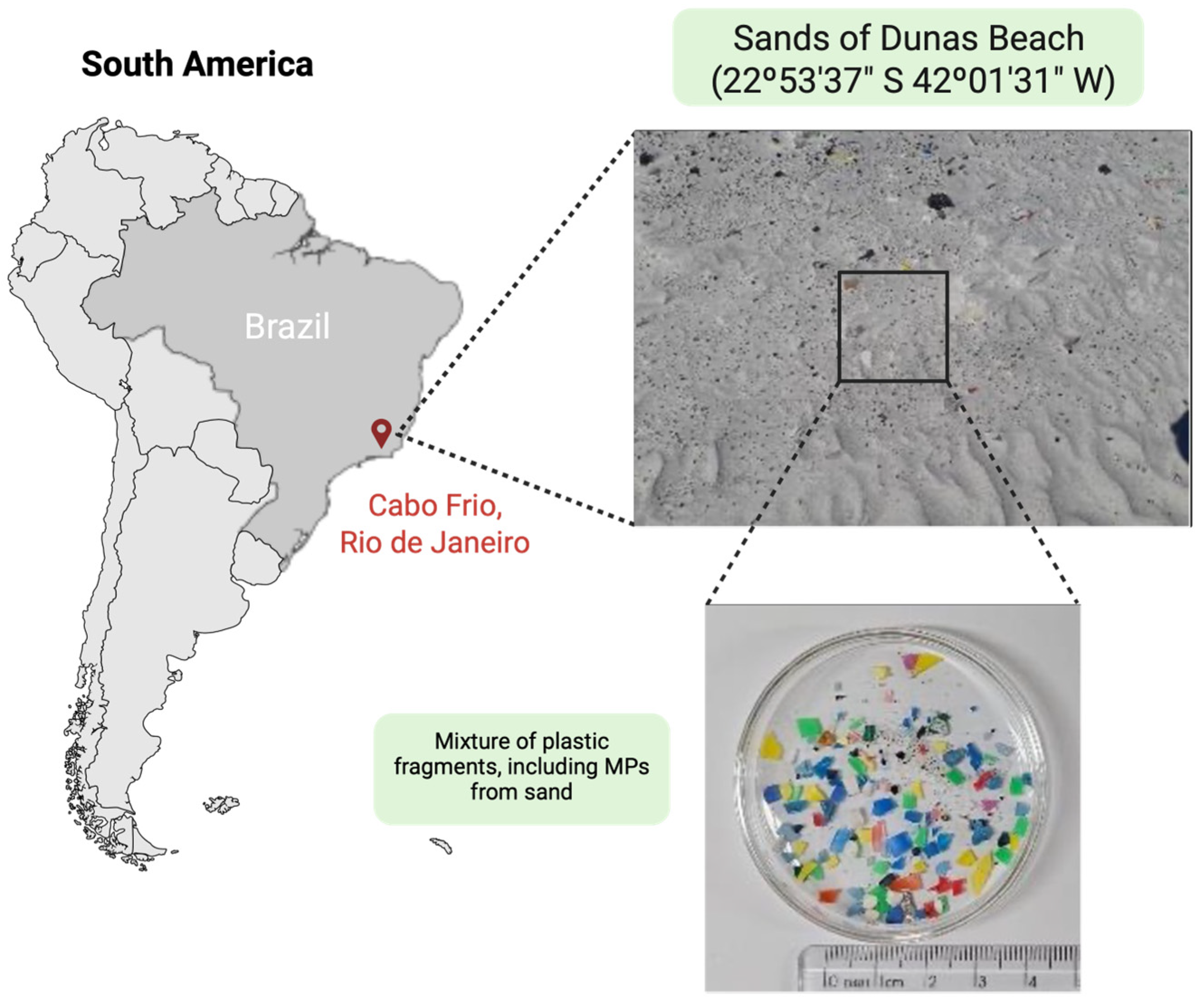
2. MPs in the Environment
3. Biological Effects of Polystyrene Particles (PS-MPs and PS-NPs)
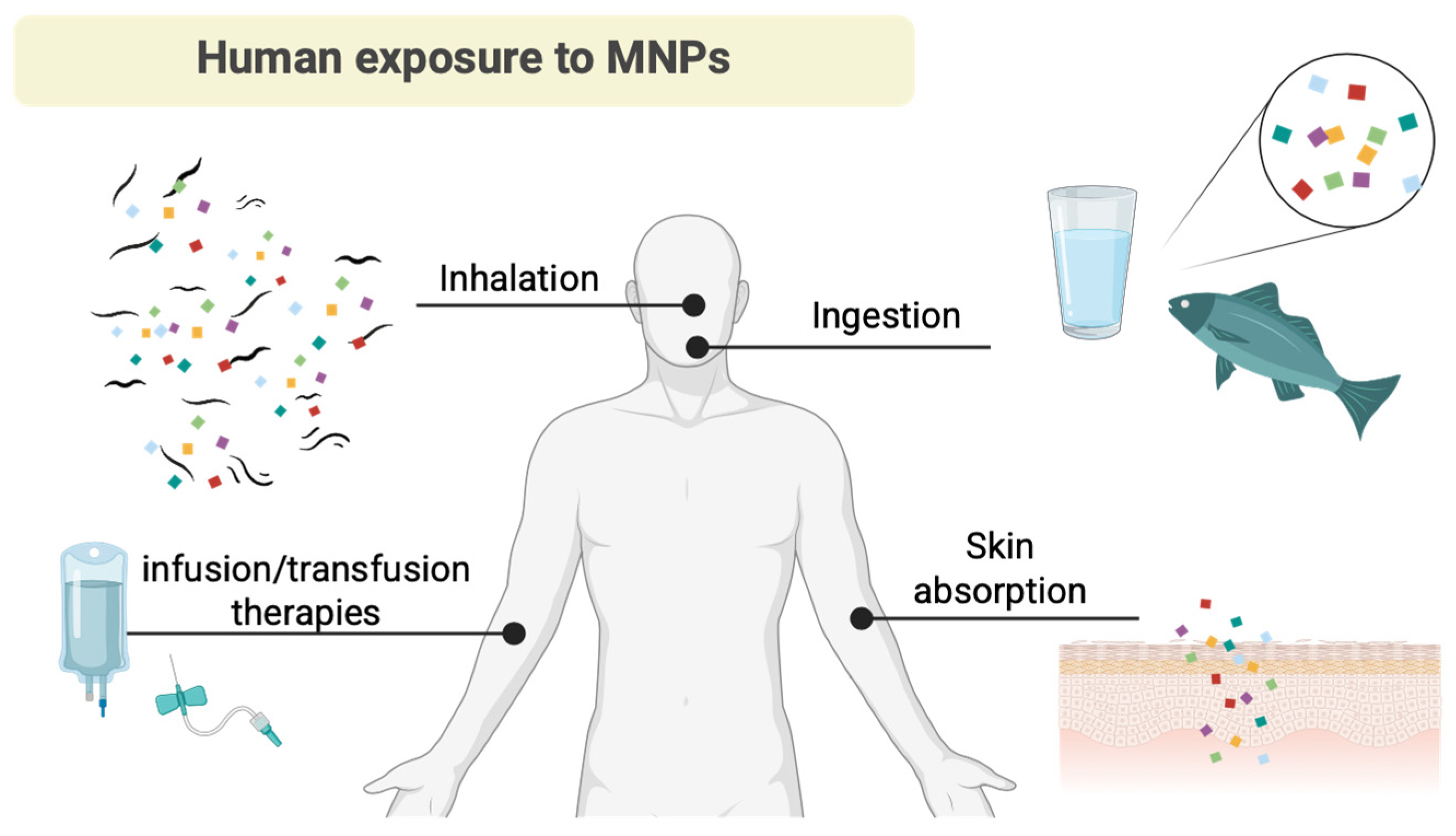
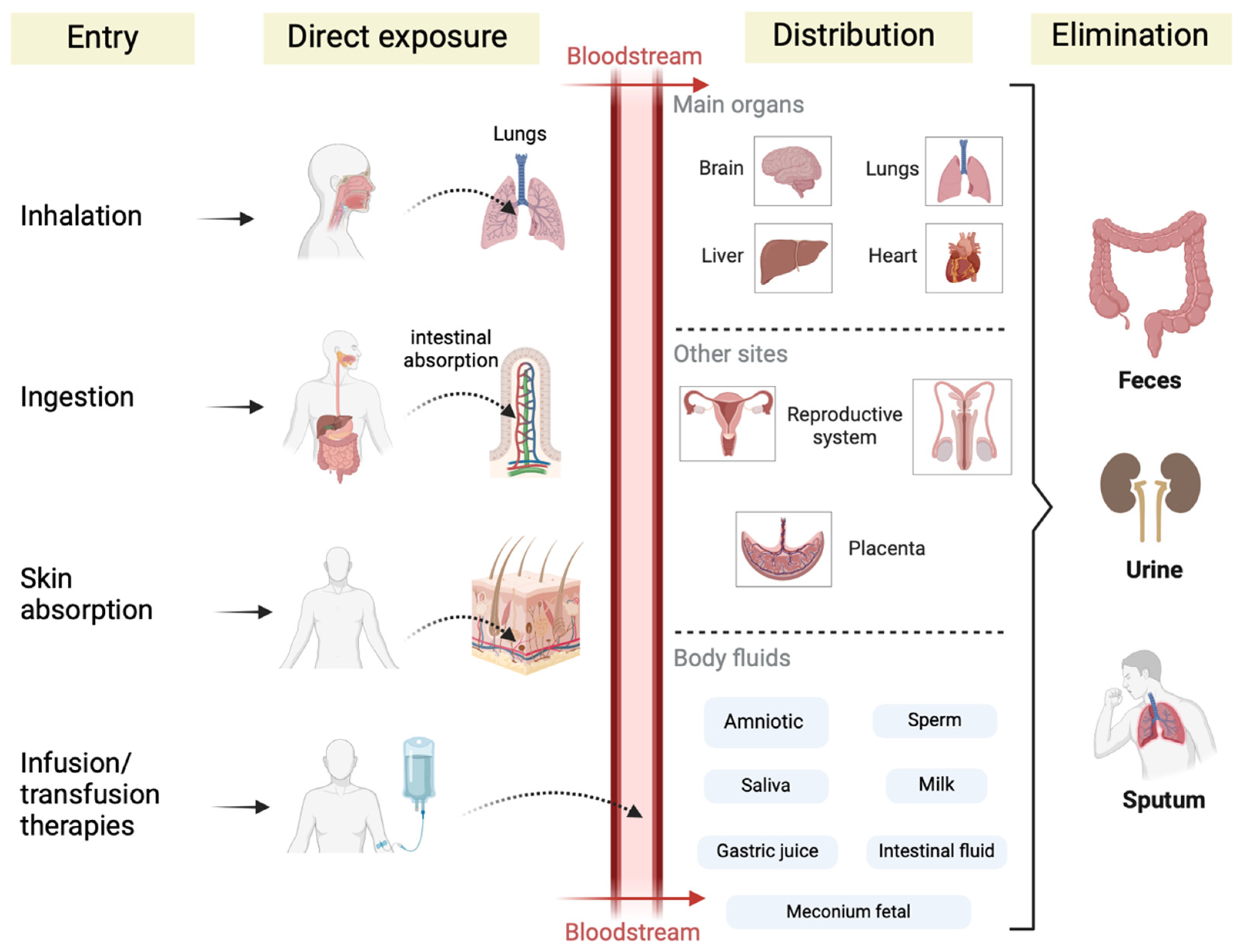
4. Occurrence of MNPs in the Bloodstream, Reproductive System, and Gastrointestinal Tract
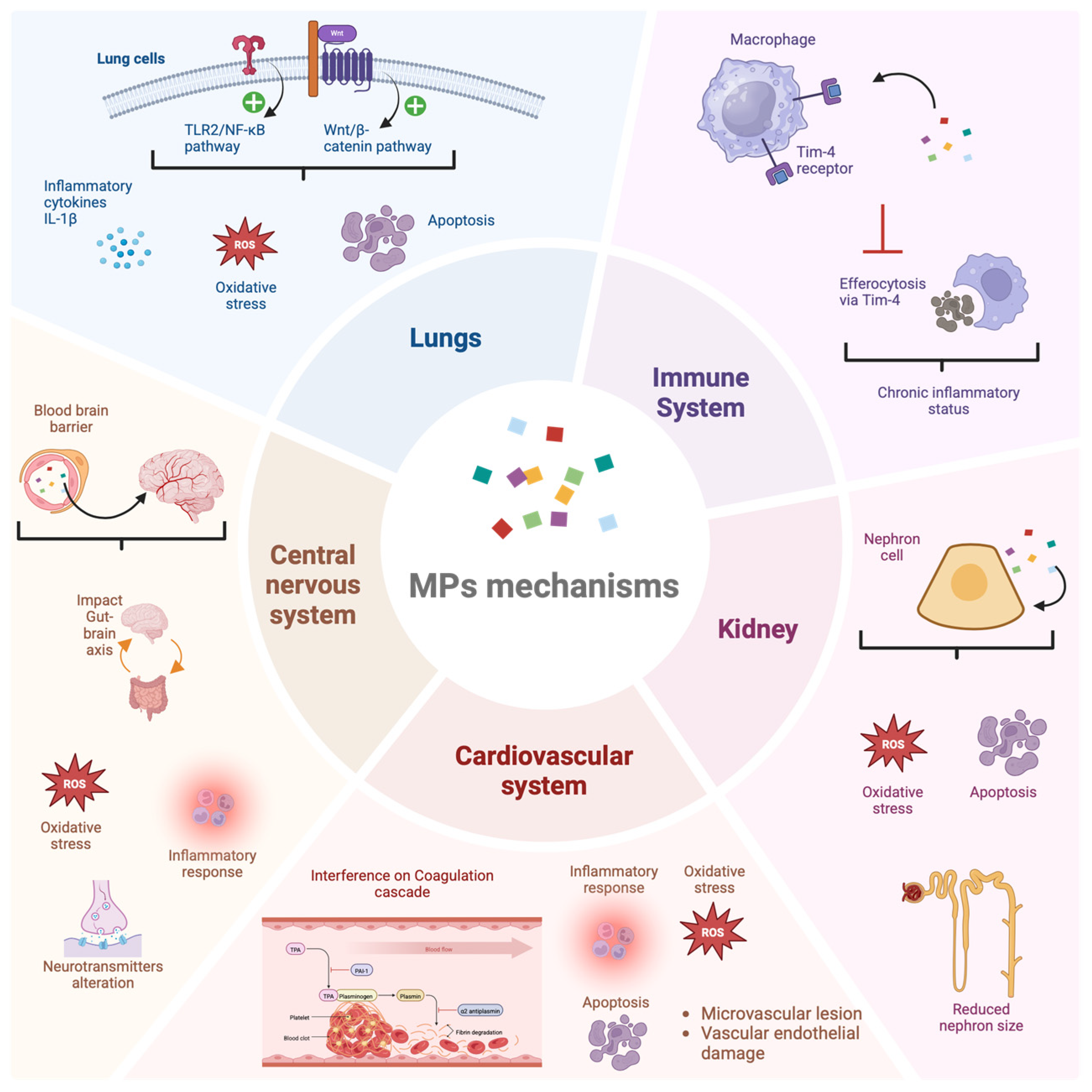
5. Occurrence of MPs in the Respiratory Tract via Inhalation
6. MP Occurrence and the Cardiovascular System Treats
7. MPs and the Development of Cancer
8. Elimination and Clearance of MNPs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeb, A.; Liu, W.; Ali, N.; Shi, R.; Wang, J.; Li, J.; Yin, C.; Liu, J.; Yu, M.; Liu, J. Microplastic pollution in terrestrial ecosystems: Global implications and sustainable solutions. J. Hazard. Mater. 2024, 461, 132636. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2020, 757, 143872. [Google Scholar] [CrossRef]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 101–120. [Google Scholar]
- Proki, M.D.; Radovanovi, T.B.; Gavri, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Ferreira, P.G.; da Silva, F.C.; Ferreira, V.F. The Importance of Chemistry for the Circular Economy. Rev. Virtual Quim. 2017, 9, 452–473. [Google Scholar] [CrossRef]
- Zhong, Z.; Huang, W.; Yin, Y.; Wang, S.; Chen, L.; Chen, Z.; Wang, J.; Li, L.; Khalid, M.; Hu, M.; et al. Tris(1-chloro-2-propyl)phosphate enhances the adverse effects of biodegradable polylatic acid microplastics on the mussel Mytilus coruscus. Environ. Pollut. 2024, 359, 124741. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 71. [Google Scholar] [CrossRef]
- Davos AM24—World Economic Forum. Landing an Ambitious Global Plastics Treaty. Available online: https://www.weforum.org/events/world-economic-forum-annual-meeting-2024/sessions/landing-an-ambitious-global-plastics-treaty/ (accessed on 3 December 2024).
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 5 December 2024).
- Pabortsava, K.; Lampitt, R. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254 Pt A, 113011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S.; et al. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Borah, S.J.; Gupta, A.K.; Kumar, V.; Jhajharia, P.; Singh, P.P.; Kumar, R. DubeThe Peril of Plastics: Atmospheric Microplastics in Outdoor, Indoor, and Remote Environments. Sustain. Chem. 2024, 5, 149–162. [Google Scholar] [CrossRef]
- Albazoni, H.J.; Al-Haidarey, M.J.S.; Nasir, A.S. A Review of Microplastic Pollution: Harmful Effect on Environment and Animals, Remediation Strategies. J. Ecol. Eng. 2024, 25, 140–157. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Yan, P.; Hao, X.; Xu, B.; Wang, W.; Aurangzeib, M. Non-biodegradable microplastics in soils: A brief review and challenge. J. Hazard. Mater. 2021, 409, 124525. [Google Scholar] [CrossRef]
- Nafea, T.H.; Al-Maliki, A.J.; Al-Tameemi, I.M. Sources, fate, effects, and analysis of microplastic in wastewater treatment plants: A review. Environ. Eng. Res. 2024, 29, 230040. [Google Scholar] [CrossRef]
- Bodor, A.; Feigl, G.; Kolossa, B.; Mészáros, E.; Laczi, K.; Kovács, E.; Perei, K.; Rákhely, G. Soils in distress: The impacts and ecological risks of (micro)plastic pollution in the terrestrial environment. Ecotoxicol. Environ. Saf. 2024, 269, 115807. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of Microplastic Accumulation in Agricultural Soils from Sewage Sludge Disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Loder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic Fertilizer as a Vehicle for the Entry of Microplastic into the Environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, B.; Khan, I.; Khan, A.R.; Jho, E.H.; Salam, A.; Zhou, H.; Zhao, X.; Li, G.; Du, D. Microplastic contamination in the agricultural soil-mitigation strategies, heavy metals contamination, and impact on human health: A review. Plant Cell Rep. 2024, 43, 65. [Google Scholar] [PubMed]
- Rillig, M.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Sacco, V.A.; Zuanazzi, N.R.; Selinger, A.; Costa, J.H.A.; Lemunie, É.S.; Comelli, C.L.; Abilhoa, V.; Sousa, F.C.; Fávaro, L.F.; Mendoza, L.M.R.; et al. What are the global patterns of microplastic ingestion by fish? A scientometric review. Environ. Pollut. 2024, 350, 123972. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Brown, E.; MacDonald, A.; Allen, S.; Allen, D. The potential for a plastic recycling facility to release microplastic pollution and possible filtration remediation effectiveness. J. Hazard. Mater. Adv. 2023, 10, 100309. [Google Scholar] [CrossRef]
- Li, P.; Liu, J. Micro(nano)plastics in the Human Body: Sources, Occurrences, Fates, and Health Risks. Environ. Sci. Technol. 2024, 58, 3065–3078. [Google Scholar]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food chain microplastics contamination and impact on human health: A review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Al Mamun, A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858 Pt 1, 159834. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; You, F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ. Sci. Technol. 2024, 58, 8709–8723. [Google Scholar] [CrossRef] [PubMed]
- Fleury, J.B.; Baulin, V.A. Synergistic Effects of Microplastics and Marine Pollutants on the Destabilization of Lipid Bilayers. J. Phys. Chem. B 2024, 128, 8753–8761. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, L.; Simon-Sánchez, L.; Vianllo, A.; Nielsen, A.H.; Vollertsen, J. Every breath you take: High concentration of breathable microplastics in indoor environments. Chemosphere 2024, 20, 142553. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.; Li, K.; Zhang, G.; Zhang, M.; Hu, M.; Huang, Y. Human Exposure to Ambient Atmospheric Microplastics in a Megacity: Spatiotemporal Variation and Associated Microorganism-Related Health Risk. Environ. Sci. Technol. 2024, 58, 3702–3713. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Prata, J.; Costa, J.; Lopes, I.; Duarte, A.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2019, 702, 134455. [Google Scholar] [CrossRef]
- Hore, M.; Bhattacharyya, S.; Roy, S.; Sarkar, D.; Biswas, J.K. Human Exposure to Dietary Microplastics and Health Risk: A Comprehensive Review. Rev. Environ. Contam. Toxicol. 2024, 262, 14. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Bhutto, S.U.A.; You, X. Spatial distribution of microplastics in Chinese freshwater ecosystem and impacts on food webs. Environ. Pollut. 2022, 293, 118494. [Google Scholar] [CrossRef]
- Peters, C.A.; Thomas, P.A.; Rieper, K.B.; Bratton, S.P. Foraging preferences influence microplastic ingestion by six marine fish species from the Texas Gulf Coast. Mar. Pollut. Bull. 2017, 124, 82–88. [Google Scholar] [CrossRef]
- Oliveira, C.W.S.; Corrêa, C.S.; Smith, W.S. Food ecology and presence of microplastic in the stomach content of neotropical fish in an urban river of the upper Paraná River Basin. Rev. Ambient. Água 2020, 15, e255. [Google Scholar] [CrossRef]
- Pegado, T.S.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, P.; Abhishek, K. Sampling, separation, and characterization methodology for quantification of microplastic from the environment. J. Hazard. Mater. Adv. 2024, 14, 100416. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 15, 410–422. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Microplastics Research. Available online: https://www.epa.gov/water-research/microplastics-research (accessed on 20 December 2024).
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Rahman, L.; Williams, A.; Wu, D.; Halappanavar, S. Polyethylene Terephthalate Microplastics Generated from Disposable Water Bottles Induce Interferon Signaling Pathways in Mouse Lung Epithelial Cells. Nanomaterials 2024, 14, 1287. [Google Scholar] [CrossRef]
- Cesarini, G.; Secco, S.; Taurozzi, D.; Venditti, I.; Battocchio, C.; Marcheggiani, S.; Mancini, L.; Fratoddi, I.; Scalici, M.; Puccinelli, C. Teratogenic effects of environmental concentration of plastic particles on freshwater organisms. Sci. Total Environ. 2023, 898, 165564. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhu, L.; Chen, L.; Zhang, L.; Mao, L.; Wu, C.; Chang, Y.; Jiang, J.; Jiang, H.; Liu, X. Metabolomics and microbiomics revealed the combined effects of different-sized polystyrene microplastics and imidacloprid on earthworm intestinal health and function. Environ. Pollut. 2024, 361, 124799. [Google Scholar] [CrossRef] [PubMed]
- Vincoff, S.; Schleupner, B.; Santos, J.; Morrison, M.; Zhang, N.; Dunphy-Daly, M.M.; Eward, W.C.; Armstrong, A.J.; Diana, Z.; Somarelli, J.A. The Known and Unknown: Investigating the Carcinogenic Potential of Plastic Additives. Environ. Sci. Technol. 2024, 58, 10445–10457. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 28 December 2024).
- Busch, M.; Bredeck, G.; Kämpfer, A.; Schins, R. Investigations of acute effects of polystyrene and polyvinyl chloride micro- and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environ. Res. 2020, 193, 110536. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; Briesen, H.; Büchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Wang, J.; Yu, Y. Freeze-thaw aging increases the toxicity of microplastics to earthworms and enriches pollutant-degrading microbial genera. J. Hazard. Mater. 2024, 479, 135651. [Google Scholar] [CrossRef]
- Liu, C.; Zong, C.; Chen, S.; Chu, J.; Yang, Y.; Pan, Y.; Yuan, B.; Zhang, H. Machine learning-driven QSAR models for predicting the cytotoxicity of five common Microplastics. Toxicology 2024, 508, 153918. [Google Scholar] [CrossRef]
- Jones, L.R.; Wright, S.J.; Gant, T.W. A critical review of microplastics toxicity and potential adverse outcome pathway in human gastrointestinal tract following oral exposure. Toxicol. Lett. 2023, 385, 51–60. [Google Scholar] [CrossRef]
- Awet, T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.; Ruf, T.; Jost, C.; Drexel, R.; Tunç, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Dong, C.; Chen, C.; Chen, Y.; Chen, H.; Lee, J.; Lin, C. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2019, 385, 121575. [Google Scholar] [CrossRef]
- Danso, I.K.; Woo, J.H.; Lee, K. Pulmonary toxicity of polystyrene, polypropylene, and polyvinyl chloride microplastics in mice. Molecules 2022, 27, 7926. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-kappaB signal in mice. Environ. Pollut. 2023, 320, 121068. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Y.; Bai, H.; Shimizu, K.; Li, R.; Zhang, C. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: The potential protective role of the antioxidant N-acetylcysteine. Sci. Total Environ. 2023, 855, 158851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/beta-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef]
- Kuroiwa, M.; Yamaguchi, S.I.; Kato, Y.; Hori, A.; Toyoura, S.; Nakahara, M.; Morimoto, N.; Nakayama, M. Tim4, a macrophage receptor for apoptotic cells, binds polystyrene microplastics via aromatic-aromatic interactions. Sci. Total Environ. 2023, 875, 162586. [Google Scholar] [CrossRef]
- Garcia, M.M.; Romero, A.S.; Merkley, S.D.; Meyer-Hagen, J.L.; Forbes, C.; El Hayek, E.; Sciezka, D.P.; Templeton, R.; Gonzalez-Estrella, J.; Jin, Y.; et al. In vivo tissue distribution of polystyrene or mixed polymer microspheres and metabolomic analysis after oral exposure in mice. Environ. Health Perspect. 2024, 132, 47005. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, Y.; Chen, L.; Zhang, A.; Liang, T.; Low, J.H.; Liu, Z.; He, S.; Guo, Z.; Xie, J. Microplastics exposure disrupts nephrogenesis and induces renal toxicity in human iPSC-derived kidney organoids. Environ. Pollut. 2024, 360, 124645. [Google Scholar] [CrossRef]
- Wen, Y.; Cai, J.; Zhang, H.; Li, Y.; Yu, M.; Liu, J.; Han, F. The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice by Nanoplastics and Microplastics. Biomedicines 2024, 12, 1714. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, M.; Sun, X.; Wu, Z.; Yu, Y.; Kang, Y.; Liu, Z.; Xu, Q.; An, L. Microplastics Entry into the Blood by Infusion Therapy: Few but a Direct Pathway. Environ. Sci. Technol. Lett. 2024, 11, 67–72. [Google Scholar] [CrossRef]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Liu, R.; Nihart, A.; El Hayek, E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and identification of microplastics accumulation in human placental specimens using pyrolysis gas chromatography mass spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cao, M.; Peng, T.; Shan, H.; Lian, W.; Yu, Y.; Shui, G.; Li, R. Features, Potential Invasion Pathways, and Reproductive Health Risks of Microplastics Detected in Human Uterus. Environ. Sci. Technol. 2024, 58, 10482–10493. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Hu, C.J.; Garcia, M.A.; Nihart, A.; Liu, R.; Yin, L.; Adolphi, N.; Gallego, D.F.; Kang, H.; Campen, M.J.; Yu, X. Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis. Toxicol. Sci. 2024, 206, 458–459. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Anuar, S.T.; Azmi, A.A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open. 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Cetin, M.; Miloglu, F.D.; Baygutalp, N.K.; Ceylan, O.; Yildirim, S.; Eser, G.; Gul, H.İ. Higher number of microplastics in tumoral colon tissues from patients with colorectal adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.; Yan, K.; Nguyen, W.; Rawle, D.; Tang, B.; Larcher, T.; Suhrbier, A. Microplastics dysregulate innate immunity in the SARS-CoV-2 infected lung. Front. Immunol. 2024, 15, 1382655. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.; Carvalho-Oliveira, R.; Júnior, G.; Galvão, L.; Ando, R.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Qiu, L.; Lu, W.; Tu, C.; Li, X.; Zhang, H.; Wang, S.; Chen, M.; Zheng, X.; Wang, Z.; Lin, M.; et al. Evidence of microplastics in bronchoalveolar lavage fluid among never-smokers: A prospective case series. Environ. Sci. Technol. 2023, 57, 2435–2444. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Quan, S.; Chen, L.; Shen, A.; Jiao, A.; Qi, H.; Yu, G. Microplastics in the bronchoalveolar lavage fluid of chinese children: Associations with age, city development, and disease features. Environ. Sci. Technol. 2023, 57, 12594–12601. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Zha, H.; Xia, J.; Li, S.; Lv, J.; Zhuge, A.; Tang, R.; Wang, S.; Wang, K.; Chang, K.; Li, L. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice. Chemosphere 2023, 310, 136764. [Google Scholar] [CrossRef]
- Tran, D.Q.; Stelflug, N.; Hall, A.; Nallan Chakravarthula, T.; Alves, N.J. Microplastic effects on thrombin-fibrinogen clotting dynamics measured via turbidity and thromboelastography. Tran. Biomol. Ther. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Christodoulides, A.; Hall, A.; Alves, N.J. Exploring microplastic impact on whole blood clotting dynamics utilizing thromboelastography. Front. Public Health 2023, 11, 1215817. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Marfella, R.; Paolisso, G.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Landmark study links microplastics to serious health problems. Nature 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Keenan, J.; Shaw, I.; Frizelle, F. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, H.; Shi, L.; Jia, Y.; Sheng, H. Detection and quantification of microplastics in various types of human tumor tissues. Ecotoxicol. Environ. Saf. 2024, 283, 116818. [Google Scholar] [CrossRef]
- Emecheta, E.E.; Pfohl, P.M.; Wohlleben, W.; Haase, A.; Roloff, A. Desorption of Polycyclic Aromatic Hydrocarbons from Microplastics in Human Gastrointestinal Fluid Simulants-Implications for Exposure Assessment. ACS Omega 2024, 9, 24281–24290. [Google Scholar] [CrossRef]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, S.; Liu, J.; Liu, Z. The effects of micro- and nanoplastic on the central nervous system: A New Threat to Humanity? Toxicol. 2024, 504, 153799. [Google Scholar] [CrossRef]
- Savuca, A.; Curpan, A.S.; Hritcu, L.D.; Buzenchi Proca, T.M.; Balmus, I.M.; Lungu, P.F.; Jijie, R.; Nicoara, M.N.; Ciobica, A.S.; Solcan, G.; et al. Do Microplastics Have Neurological Implications in Relation to Schizophrenia Zebrafish Models? A Brain Immunohistochemistry, Neurotoxicity Assessment, and Oxidative Stress Analysis. Int. J. Mol. Sci. 2024, 25, 8331. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef] [PubMed]
- Dris RGasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, J.; Lan, W.; Yang, Z.; Li, M.; Li, J.; Yu, J.; Yang, S.; Du, J.; Dong, R.; et al. Microplastics exposed by respiratory tract and exacerbation of community-acquired pneumonia: The potential influences of respiratory microbiota and inflammatory factors. Environ. Int. 2025, 199, 109485. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhan, D.; Fang, Y.; Li, L.; Chen, G.; Chen, S.; Wang, L. Microplastics, potential threat to patients with lung diseases. Front. Toxicol. 2022, 4, 958414. [Google Scholar] [CrossRef]
- Goodman, K.; Hare, J.; Khamis, Z.; Hua, T.; Sang, Q. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ. Sci. Eur. 2021, 34, 25. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Cao, Q.; Tu, C.; Zhong, C.; Qiu, L.; Li, S.; Zhang, H.; Lan, M.; Qiu, L.; et al. Microplastics in the Lung Tissues Associated with Blood Test Index. Toxics 2023, 11, 759. [Google Scholar] [CrossRef]
- Xu, X.; Goros, R.A.; Dong, Z.; Meng, X.; Li, G.; Chen, W.; Liu, S.; Ma, J.; Zuo, Y.Y. Microplastics and Nanoplastics Impair the Biophysical Function of Pulmonary Surfactant by Forming Heteroaggregates at the Alveolar-Capillary Interface. Environ. Sci. Technol. 2023, 57, 21050–21060. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Li, X.; Sun, H.; Xia, X.; Ji, X.; Zhang, J.; Liu, M.; Lin, Y.; Zhang, R.; et al. Inhaled tire-wear microplastic particles induced pulmonary fibrotic injury via epithelial cytoskeleton rearrangement. Environ. Int. 2022, 164, 107257. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, J.; Na, J.; Fang, J.; Qi, C.; Lu, J.; Liu, X.; Zhou, C.; Feng, J.; Zhu, W.; et al. Exposure to microplastics in the upper respiratory tract of indoor and outdoor workers. Chemosphere 2022, 307, 136067. [Google Scholar] [CrossRef]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Xu, E.G.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Zhu, M.; Li, P.; Xu, T.; Zhang, G.; Xu, Z.; Wang, X.; Zhao, L.; Yang, H. Combined exposure to lead and microplastics increased risk of glucose metabolism in mice via the Nrf2/NF-κB pathway. Environ. Toxicol. 2024, 39, 2502–2511. [Google Scholar] [CrossRef]
- Bhatnagar, A. Cardiovascular pathophysiology of environmental pollutants. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H479–H485. [Google Scholar] [CrossRef]
- Bhatnagar, A. Studying Mechanistic Links Between Pollution and Heart Disease. Circ. Res. 2006, 99, 692–705. [Google Scholar] [CrossRef]
- Guo, Q.; Deng, T.; Du, Y.; Yao, W.; Tian, W.; Liao, H.; Wang, Y.; Li, J.; Yan, W.; Li, Y. Impact of DEHP on mitochondria-associated endoplasmic reticulum membranes and reproductive toxicity in ovary. Ecotoxicol. Environ. Saf. 2024, 282, 116679. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, N.X.; Huang, L.; Wu, M.; Li, C.X.; Zhang, F.; Huang, Y.; Cai, Q.Y.; Xiang, L.; Li, Y.W.; et al. Improving key gene expression and di-n-butyl phthalate (DBP) degrading ability in a novel Pseudochrobactrum sp. XF203 by ribosome engineering. Sci. Total Environ. 2024, 946, 174207. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Machado, M.R.; Costa, G.G.; Oliveira, G.A.R.; Nunes, H.F.; Veloso, D.F.M.C.; Ishizawa, T.A.; Pereira, J.; Oliveira, T.F. Influence of different concentrations of plasticizer diethyl phthalate (DEP) on toxicity of Lactuca sativa seeds, Artemia salina and Zebrafish. Heliyon 2023, 9, e18855. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Elanjickal, A.; Mankar, J.; Krupadam, R. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Cheng, W.; Zhou, Y.; Xie, Y.; Li, Y.; Zhou, R.; Wang, H.; Feng, Y.; Wang, Y. Combined effect of polystyrene microplastics and bisphenol A on the human embryonic stem cells-derived liver organoids: The hepatotoxicity and lipid accumulation. Sci. Total Environ. 2023, 854, 158585. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Rawle, D.J.; Dumenil, T.; Tang, B.; Bishop, C.R.; Yan, K.; Le, T.T.; Suhrbier, A. Microplastic consumption induces inflammatory signatures in the colon and prolongs a viral arthritis. Sci. Total Environ. 2022, 809, 152212. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2021, 302 Pt A, 113995. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Wang, J.; Dai, Y.; Li, H.; Li, J.; Liu, X.; Chen, X.; Wang, Z.; Zhang, P. Interactions Between Microplastics and Heavy Metals in Aquatic Environments: A Review. Front. Microbiol. 2021, 12, 652520. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255 Pt 3, 113363. [Google Scholar] [CrossRef]
- Sun, J.; Qu, H.; Ali, W.; Chen, Y.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z.; et al. Co-exposure to cadmium and microplastics promotes liver fibrosis through the hemichannels-ATP-P2×7 pathway. Chemophere 2023, 344, 140372. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Ali, W.; Zhuang, T.; Sun, J.; Wang, T.; Song, J.; Ma, Y.; Yuan, Y.; Bian, J.; et al. Co-exposure of polyvinyl chloride microplastics with cadmium promotes non-alcoholic fatty liver disease in female ducks through oxidative stress and glycolipid accumulation. Poult. Sci. 2024, 103, 104152. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, H.; Zhou, Y.; You, Y.; Lei, D.; Li, Y.; Feng, Y.; Wang, Y. Aged fragmented-polypropylene microplastics induced ageing statues-dependent bioenergetic imbalance and reductive stress: In vivo and liver organoids-based in vitro study. Environ. Int. 2024, 191, 108949. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics and human health: Integrating pharmacokinetics. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1489–1511. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Dipl-Ing Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]


| Sample | MP/NP Polymers | Analysis Method | Concentration or Particle Size | Reference |
|---|---|---|---|---|
| Sperm mice | PS | - | 80 nm (NP) 5 μm (MP) | Wen et al. [75] |
| Human blood from a transfusion therapies | PE, PA, PS, and PC | - | 4 to 148 μm (MP) | Zhu et al. [76] |
| Human amniotic fluid and/or placenta | PVC and calcium–zinc PVC | Attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) | 10 to 50 μm (MP) | Halfar et al. [77] |
| Human placenta and fecal meconium | Ten types; mainly PE, PP, and PS | Fourier transform infrared microspectroscopy | Qualitative | Braun et al. [78] |
| Human placenta | PP, PVC, and PBS | Laser direct infrared spectroscopy (LD-IR) | 0.28 to 9.55 particles/g; 20.34 to 307.29 μm | Zhu et al. [79] |
| Human breast milk | PE, PVC, and PP | Raman Microspectroscopy Detection | 2 to 12 μm (MP) | Ragusa et al. [80] |
| Human endometrium | PA, PU, PET, PP, PS, and PE | Raman Microspectroscopy Detection | 2 to 200 μm (MP) | Qin et al. [81] |
| Residues from human cardiac surgery | Nine types of MPs detected | Laser direct infrared chemical imaging and scanning electron microscopy | 184 to 469 μm (MP) | Yang et al. [82] |
| Human cirrhotic liver | Six types of MPs detected | Fluorescence microscopy and Raman spectroscopy | 4 to 30 μm (MP) | Horvatits et al. [83] |
| Sperm from dogs and humans | PE | Pyrolysis gas chromatography associated with mass spectrometry (Py-GC/MS) | 112.63 mg/g in dogs; 328.44 mg/g in humans | Hu et al. [84] |
| Human colectomy | Polycarbonate, PA, and PP | Stereo and FTIR microscopes | 28.1 particles/g of tissue | Ibrahim et al. [85] |
| Human colorectal adenocarcinoma | PE, MMA, and PA | ATR-FTIR and Raman spectroscopy | 1 nm (NP) to 1.2 mm (MP) | Cetin et al. [86] |
| Neuronal tissues | PS | - | 5 μm to 20 μm | Prüst et al. [87] |
| Human pulmonary tissues | PS | - | 20 to 100 μm (MP); 2.2 particles per gram of lung | Bishop et al. [88] |
| Human pulmonary tissues | PE and PP | Raman spectroscopy | <5.5 μm (MP particles); 8.12 to 16.8 μm (MP fibers) | Amato-Lourenço et al. [89] |
| Human bronchoalveolar lavage fluid (BALF) | PP, PE, and PS | LD-IR combined with scanning electron microscopy | <20 μm (MP); 4.31 particles per 10 mL | Qiu et al. [90], Chen et al. [91] |
| Human pulmonary tissue | PET and PP | μFTIR spectroscopy | 3 μm (MP); 1.42 particles per gram of lung | Jenner et al. [92] |
| Nasal and pulmonary samples | - | - | - | Zha et al. [93] |
| Human blood | Amino-polystyrene and PS | Thrombin/fibrinogen clot model, characterized by turbidity and thromboelastography | <100 μg/mL 50, 100, and 500 nm (NP) | Tran et al. [94], Christodoulides et al. [95] |
| Cardiovascular system | PET, PA-66, PE-vinyl chloride, and PE | Py-GC/MS | 118.66 μg MP/g of tissue | Liu et al. [96] |
| Atheroma plaque from the human carotid arteries | PE | Py-GC/MS, stable isotope analysis, and electronic microscopy | 21.7 μg/mg of plaque (MP) | Marfella et al. [97], Kozlov et al. [98] |
| Colorectal cancer in rats | 80 μg/kg/day | - | 1 μg | Li et al. [99] |
| Human pancreatic, esophageal, lung, stomach, colon, and cervical tumors | PS, PVC, and PE | Gas chromatography and mass spectrometry (CG/MS) | MPs detected; 18.4 to 427.1 ng/g | Zhao et al. [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.S.; Ferreira, P.G.; Pereira, P.R.; de Jesus, I.S.; de Oliveira, R.P.R.F.; de Carvalho, A.S.; Rodrigues, L.C.D.; Paschoalin, V.M.F.; Futuro, D.O.; Ferreira, V.F. Micro- and Nanoplastics on Human Health and Diseases: Perspectives and Recent Advances. Microplastics 2025, 4, 64. https://doi.org/10.3390/microplastics4030064
de Souza AS, Ferreira PG, Pereira PR, de Jesus IS, de Oliveira RPRF, de Carvalho AS, Rodrigues LCD, Paschoalin VMF, Futuro DO, Ferreira VF. Micro- and Nanoplastics on Human Health and Diseases: Perspectives and Recent Advances. Microplastics. 2025; 4(3):64. https://doi.org/10.3390/microplastics4030064
Chicago/Turabian Stylede Souza, Acácio S., Patricia G. Ferreira, Patricia Ribeiro Pereira, Iva S. de Jesus, Rafael P. R. F. de Oliveira, Alcione S. de Carvalho, Leandro C. D. Rodrigues, Vania Margaret Flosi Paschoalin, Debora O. Futuro, and Vitor F. Ferreira. 2025. "Micro- and Nanoplastics on Human Health and Diseases: Perspectives and Recent Advances" Microplastics 4, no. 3: 64. https://doi.org/10.3390/microplastics4030064
APA Stylede Souza, A. S., Ferreira, P. G., Pereira, P. R., de Jesus, I. S., de Oliveira, R. P. R. F., de Carvalho, A. S., Rodrigues, L. C. D., Paschoalin, V. M. F., Futuro, D. O., & Ferreira, V. F. (2025). Micro- and Nanoplastics on Human Health and Diseases: Perspectives and Recent Advances. Microplastics, 4(3), 64. https://doi.org/10.3390/microplastics4030064









