A Study on Microplastic Emission from Disposable Straws and Its Dietary Relevance
Abstract
1. Introduction
2. Materials and Methods
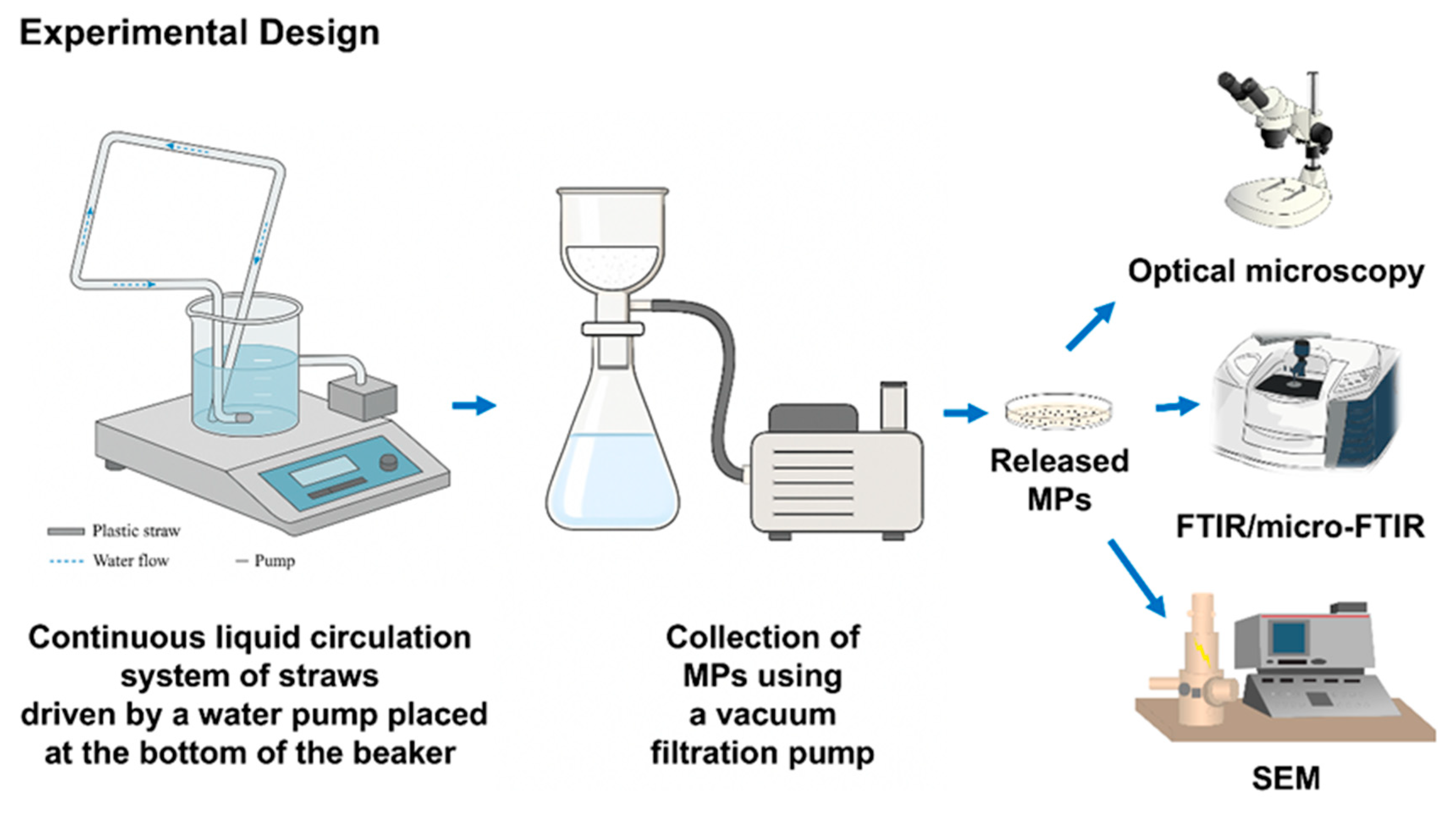
2.1. Experimental Design and Release of MPs from PP and PLA Straws
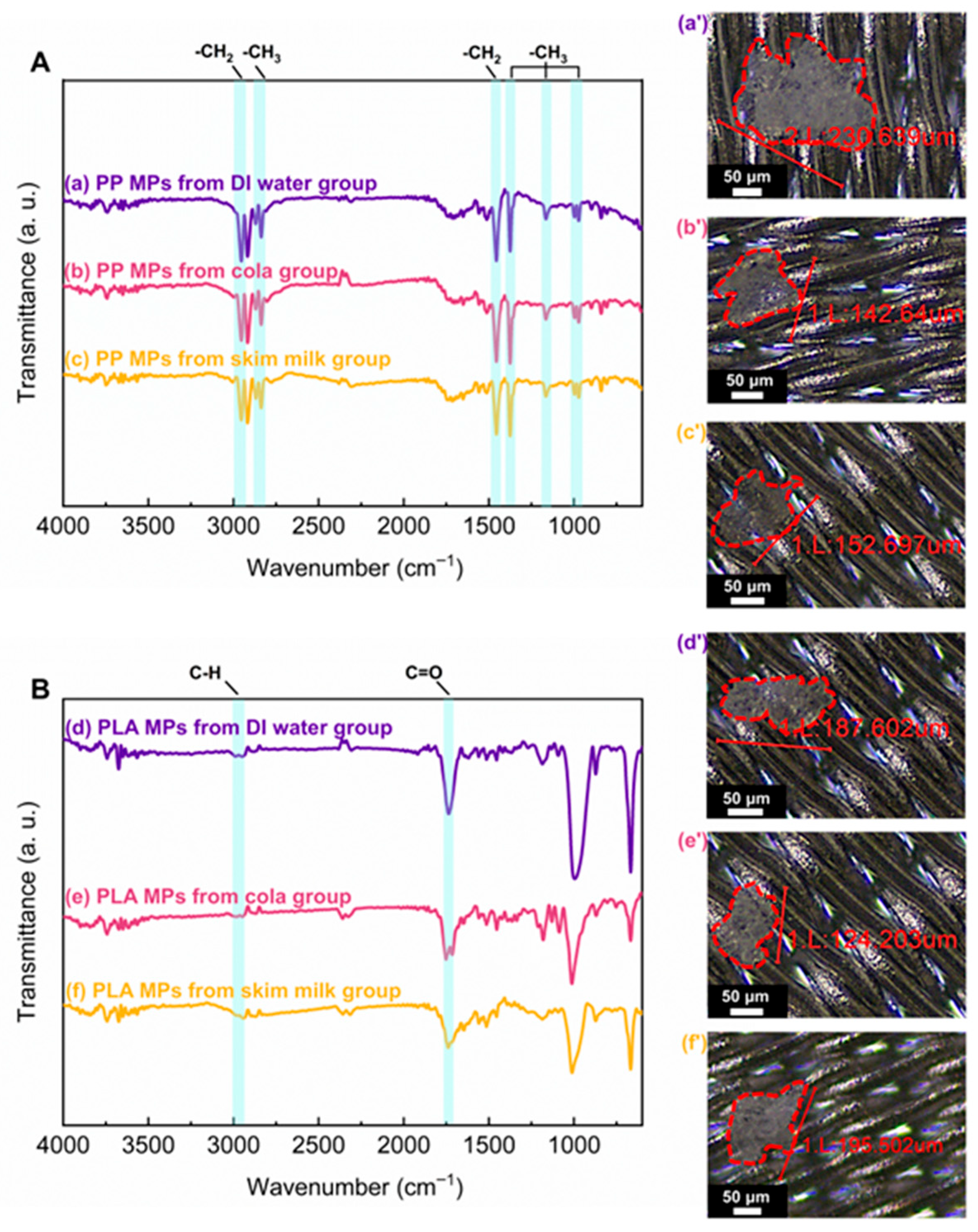
2.2. Identification of Released PP and PLA MPs
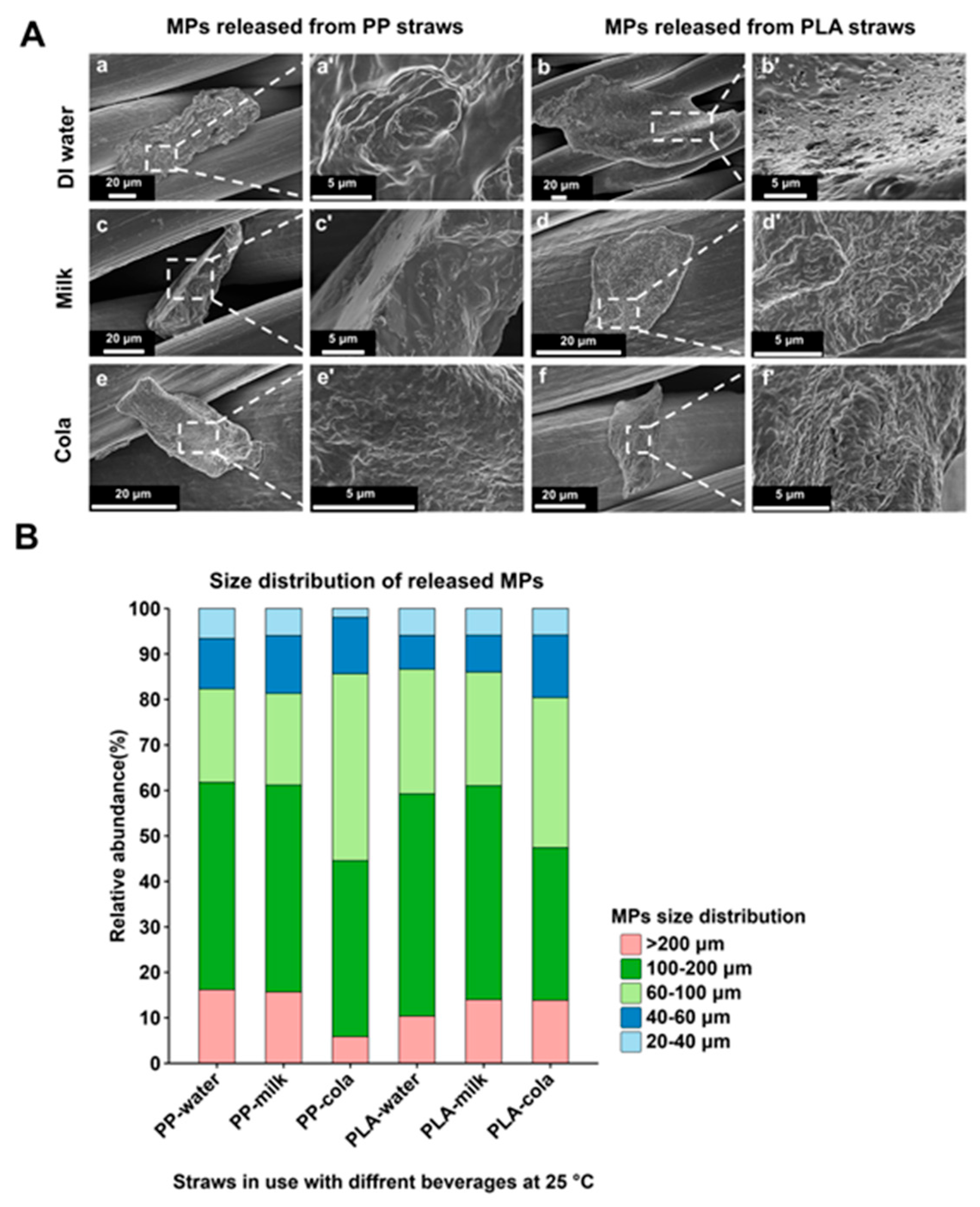
2.3. Morphological Characterization of Released MPs by SEM
2.4. Contamination Prevention and Control Experiment
3. Results
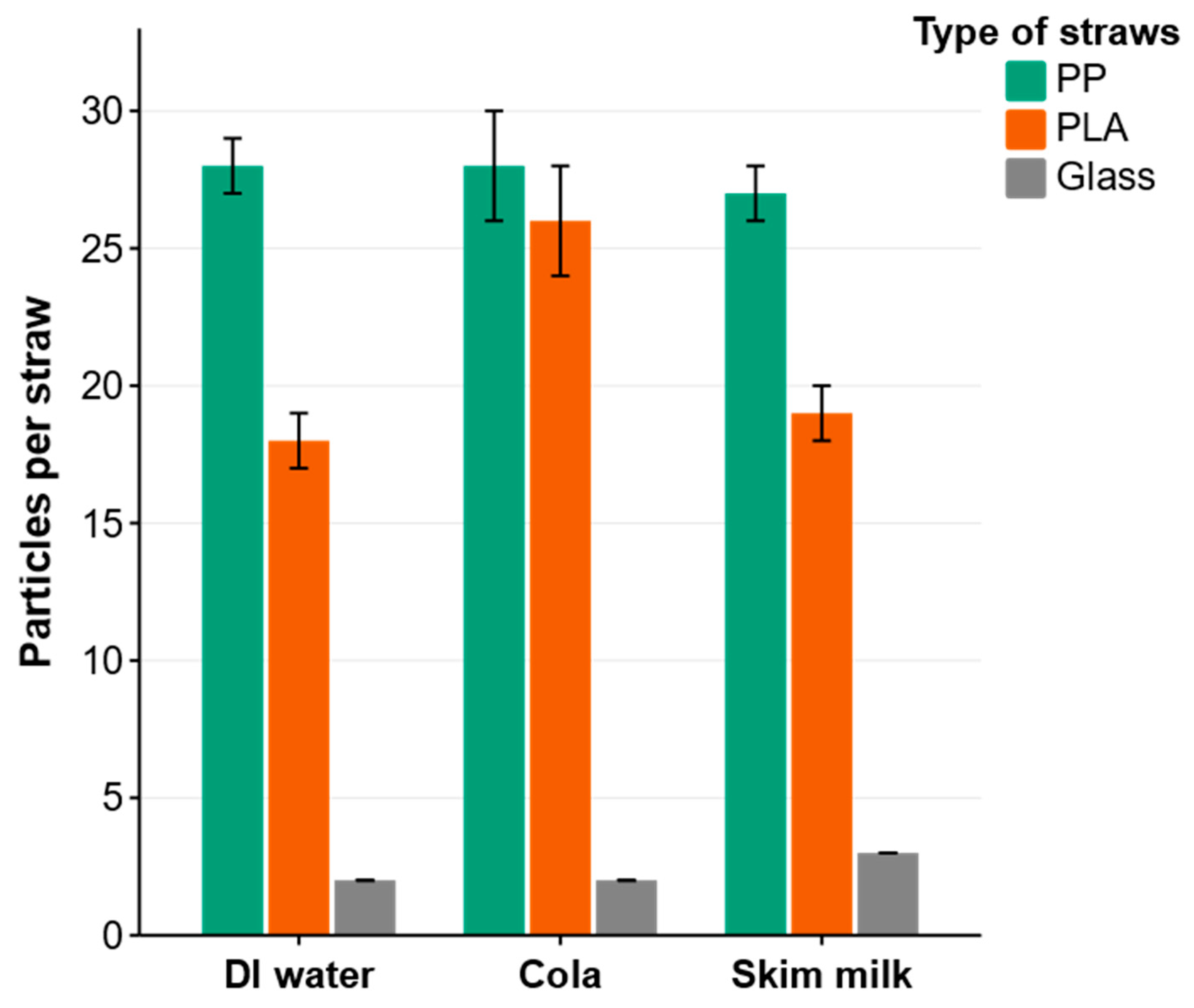
3.1. MPs Release from Disposable Straws and Micro-FTIR Identification of MPs
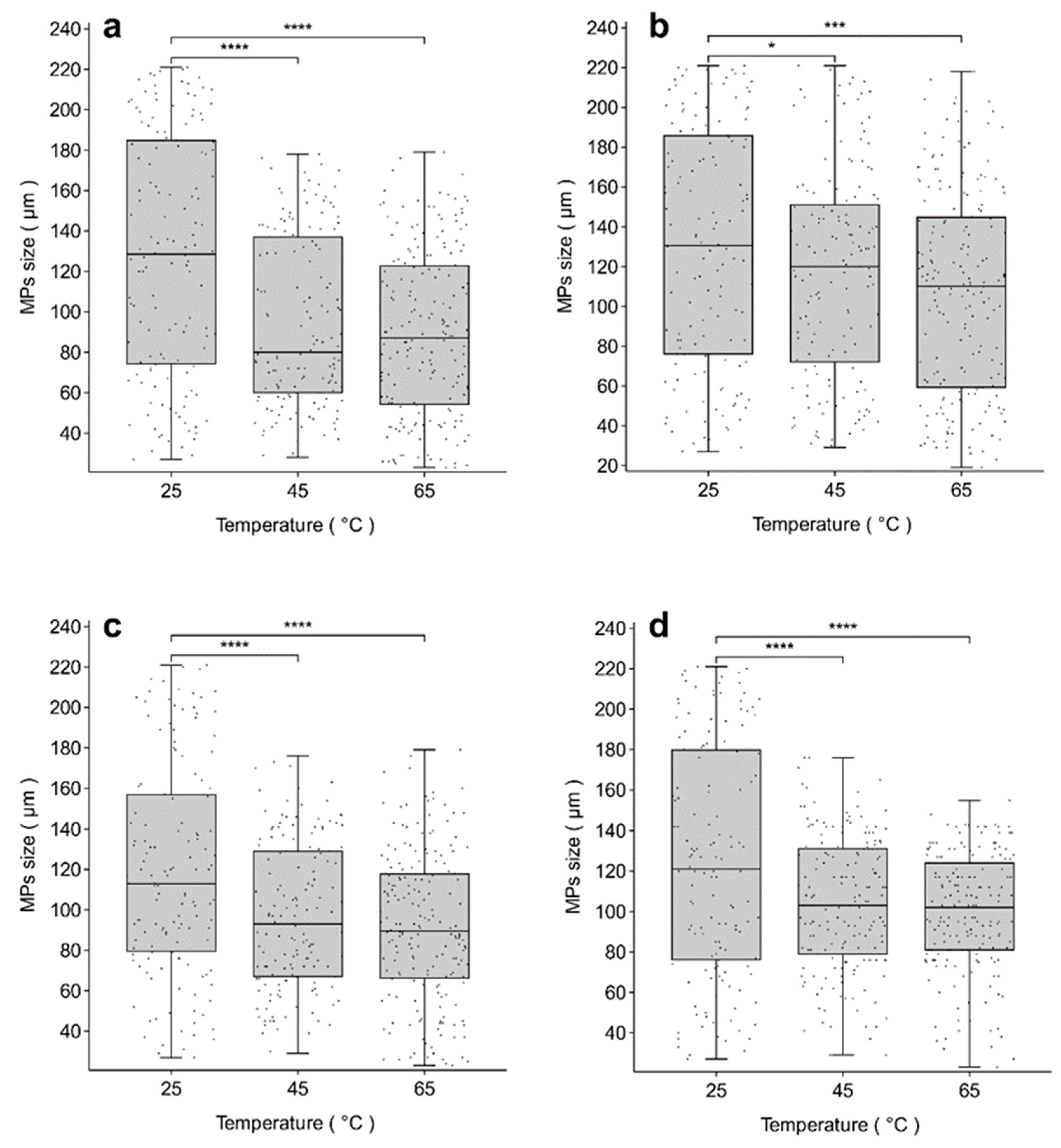
3.2. The Relationship Between the Size of MPs Released from PP/PLA Straws and the Temperature of Usage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zangmeister, C.D.; Radney, J.G.; Benkstein, K.D.; Kalanyan, B. Common Single-Use Consumer Plastic Products Release Trillions of Sub-100 nm Nanoparticles per Liter into Water during Normal Use. Environ. Sci. Technol. 2022, 56, 5448–5455. [Google Scholar] [CrossRef] [PubMed]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Ma, S.; Sun, Z.; Wang, Z. Microplastics May Be a Significant Cause of Male Infertility. Am. J. Men’s Health 2022, 16, 15579883221096549. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, B.; Sander, S.G.; Tolosa, I. A review on per- and polyfluorinated alkyl substances (PFASs) in microplastic and food-contact materials. Environ. Res. 2022, 206, 112595. [Google Scholar] [CrossRef] [PubMed]
- Sezer, M.; Isgoren, M.; Veli, S.; Topkaya, E.; Arslan, A. Removal of microplastics in food packaging industry wastewaters with electrocoagulation process: Optimization by Box-Behnken design. Chemosphere 2024, 352, 141314. [Google Scholar] [CrossRef] [PubMed]
- Hee, Y.Y.; Weston, K.; Suratman, S. The effect of storage conditions and washing on microplastic release from food and drink containers. Food Packag. Shelf Life 2022, 32, 100826. [Google Scholar] [CrossRef]
- Hussain, K.A.; Romanova, S.; Okur, I.; Zhang, D.; Kuebler, J.; Huang, X.; Wang, B.; Fernandez-Ballester, L.; Lu, Y.; Schubert, M.; et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health. Environ. Sci. Technol. 2023, 57, 9782–9792. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Malafeev, K.V.; Scarfato, P.; Incarnato, L. Generation of Microplastics from Biodegradable Packaging Films Based on PLA, PBS and Their Blend in Freshwater and Seawater. Polymers 2024, 16, 2268. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Kerpen, J.; Weber, F.; Prediger, J. A Preliminary Study of Microplastic Abrasion from the Screw Cap System of Reusable Plastic Bottles by Raman Microspectroscopy. ACS EST Water 2021, 1, 1363–1368. [Google Scholar] [CrossRef]
- Akbulut, S.; Akman, P.K.; Tornuk, F.; Yetim, H. Microplastic Release from Single-Use Plastic Beverage Cups. Foods 2024, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, L.; Yu, K.; Wei, F.; Zhang, M. Release of microplastics from disposable cups in daily use. Sci. Total Environ. 2023, 854, 158606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wu, Q.; Tang, P.; Chen, C.; Cheng, X.; Wei, X.-F.; Ma, J.; Liu, B. How many microplastics do we ingest when using disposable drink cups? J. Hazard. Mater. 2023, 441, 129982. [Google Scholar] [CrossRef]
- Mei, T.; Wang, J.; Xiao, X.; Lv, J.; Li, Q.; Dai, H.; Liu, X.; Pi, F. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods 2022, 11, 2871. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A. Sustainable management of drinking plastic straws is required to reduce plastic pollution: Are we using them more during COVID-19? J. Hazard. Mater. Adv. 2023, 12, 100328. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.M.; Gomes, T.S.; Pertel, M.; Vieira, L.A.V.P.; Pacheco, E.B.A.V. An overview of plastic straw policies in the Americas. Mar. Pollut. Bull. 2021, 172, 112813. [Google Scholar] [CrossRef] [PubMed]
- Fanini, L.; Guittard, A. On single use plastic straws: Pre-ban findings on touristic beaches in Crete. Mar. Pollut. Bull. 2021, 171, 112790. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, X.; Sarkar, B.; Ok, Y.S. The COVID-19 pandemic necessitates a shift to a plastic circular economy. Nat. Rev. Earth Environ. 2021, 2, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.L.; Wan, Y. Life cycle assessment of environmental impact of disposable drinking straws: A trade-off analysis with marine litter in the United States. Sci. Total Environ. 2022, 817, 153016. [Google Scholar] [CrossRef] [PubMed]
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878. [Google Scholar] [CrossRef] [PubMed]
- Chitaka, T.Y.; Russo, V.; von Blottnitz, H. In pursuit of environmentally friendly straws: A comparative life cycle assessment of five straw material options in South Africa. Int. J. Life Cycle Assess. 2020, 25, 1818–1832. [Google Scholar] [CrossRef]
- Dong, T.; Chen, W.; Cai, C.; Bai, F.; Zhou, Z.; Wang, J.; Li, X. Water-stable, strong, biodegradable lignocellulose straws replacement for plastic straws. Chem. Eng. J. 2023, 451, 138970. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Xiao, S.; Peng, B.-Y.; Chen, J.; Yang, L.; Zhou, X.; Zhang, Y. Distinct exposure impact of non-degradable and biodegradable microplastics on freshwater microalgae (Chlorella pyrenoidosa): Implications for polylactic acid as a sustainable plastic alternative. J. Hazard. Mater. 2024, 480, 136265. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.-H.; Satoh, T.; Tung, S.H.; Kuo, C.-C. Sustainable Alternatives to Nondegradable Medical Plastics. ACS Sustain. Chem. Eng. 2022, 10, 4792–4806. [Google Scholar] [CrossRef]
- Guan, Q.-F.; Yang, H.-B.; Han, Z.-M.; Ling, Z.-C.; Yu, S.-H. An all-natural bioinspired structural material for plastic replacement. Nat. Commun. 2020, 11, 5401. [Google Scholar] [CrossRef] [PubMed]
- Shlyannikov, V.N.; Zakharov, A.P.; Yarullin, R.R. Structural integrity assessment of turbine disk on a plastic stress intensity factor basis. Int. J. Fatigue 2016, 92, 234–245. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Zhang, X.; Wei, T.; Ma, M.; Sun, Q.; Li, M.; Xie, F. Eco-friendly drinking straws: Navigating challenges and innovations. Trends Food Sci. Technol. 2024, 148, 104511. [Google Scholar] [CrossRef]
- Meza Huaman, S.M.; Nicholson, J.H.; Brogan, A.P.S. A general route to retooling hydrolytic enzymes toward plastic degradation. Cell Rep. Phys. Sci. 2024, 5, 101783. [Google Scholar] [CrossRef]
- Colwell, J.; Pratt, S.; Lant, P.; Laycock, B. Hazardous state lifetimes of biodegradable plastics in natural environments. Sci. Total Environ. 2023, 894, 165025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Chen, J.; Ao, R.X.; Zhao, X.L. Degradation Processes of Biodegradable Plastics in Soil and Their Effects on Soil Animals. Huan Jing Ke Xue = Huanjing Kexue 2025, 46, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Markic, A.; Bridson, J.H.; Morton, P.; Hersey, L.; Maes, T.; Bowen, M. Microplastic pollution in the surface waters of Vava’u, Tonga. Mar. Pollut. Bull. 2022, 185, 114243. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cheng, S.; Wang, X.; Li, Z.; Zheng, L.; Lyu, Y.; Ao, X.; Wu, H. Characterization of microplastics in the septic tank via laser direct infrared spectroscopy. Water Res. 2022, 226, 119293. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and other harmful substances released from disposable paper cups into hot water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef] [PubMed]
- Corbari, L.; Maltese, A.; Capodici, F.; Mangano, M.C.; Sarà, G.; Ciraolo, G. Indoor spectroradiometric characterization of plastic litters commonly polluting the Mediterranean Sea: Toward the application of multispectral imagery. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.; Harrison, J.; Ju-Nam, Y.; Sapp, M.; Bradley, E.; Sinclair, C.; Ojeda, J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Huang, C.; Lee, M. Unveiling microplastic spectral signatures under weathering and digestive environments through shortwave infrared hyperspectral sensing. Environ. Pollut. 2024, 342, 123106. [Google Scholar] [CrossRef] [PubMed]
- Marsich, L.; Ferluga, A.; Cozzarini, L.; Caniato, M.; Sbaizero, O.; Schmid, C. The effect of artificial weathering on PP coextruded tape and laminate. Compos. Part A Appl. Sci. Manuf. 2017, 95, 370–376. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Electrospinning of gelatin/chitosan nanofibers incorporated with tannic acid and chitooligosaccharides on polylactic acid film: Characteristics and bioactivities. Food Hydrocoll. 2022, 133, 107916. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Prodpran, T.; Benjakul, S. Antimicrobial film based on polylactic acid coated with gelatin/chitosan nanofibers containing nisin extends the shelf life of Asian seabass slices. Food Packag. Shelf Life 2022, 34, 100941. [Google Scholar] [CrossRef]
- Mauro, N.; Andrea Utzeri, M.; Sciortino, A.; Cannas, M.; Messina, F.; Cavallaro, G.; Giammona, G. Printable Thermo- and Photo-stable Poly(D,L-lactide)/Carbon Nanodots Nanocomposites via Heterophase Melt-Extrusion Transesterification. Chem. Eng. J. 2022, 443, 136525. [Google Scholar] [CrossRef]
- Ségard, E.; Benmedakhene, S.; Laksimi, A.; Laï, D. Influence of the fibre–matrix interface on the behaviour of polypropylene reinforced by short glass fibres above glass transition temperature. Compos. Sci. Technol. 2002, 62, 2029–2036. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, K.; Wang, L.; Yao, Y.; Sun, H. Strong but reversible sorption on polar microplastics enhanced earthworm bioaccumulation of associated organic compounds. J. Hazard. Mater. 2022, 423, 127079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, T.; Wang, X. Investigation of microplastics release behavior from ozone-exposed plastic pipe materials. Environ. Pollut. 2022, 296, 118758. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, S.; Yu, X.; Vogt, R.D.; Feng, J.; Zhai, L.; Ma, W.; Zhu, L.; Lu, X. Kinetics and Size Effects on Adsorption of Cu(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Water Air Soil Pollut. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Nakatani, H.; Ohshima, Y.; Uchiyama, T.; Motokucho, S. Degradation and fragmentation behavior of polypropylene and polystyrene in water. Sci. Rep. 2022, 12, 18501. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, D.; Shi, Y.; Hill, C.; Pilliadugula, R.; Page, L.; Wang, J.J.; Boland, J.J.; Xiao, L. High levels of microparticles release from biodegradable polylactic acid paper cups compared with polyethylene-lined cups. Chem. Eng. J. 2023, 468, 143620. [Google Scholar] [CrossRef]
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does mechanical stress cause microplastic release from plastic water bottles? Water Res. 2019, 166, 115082. [Google Scholar] [CrossRef] [PubMed]
- Graupner, N.; Müssig, J. Cellulose Fiber-Reinforced PLA versus PP. Int. J. Polym. Sci. 2017, 2017, 6059183. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, X.; Lu, Y.; Qiu, Y.; Zeng, Q. Review of the Synthesis and Degradation Mechanisms of Some Biodegradable Polymers in Natural Environments. Polymers 2025, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Vaid, R.; Yildirim, E.; Pasquinelli, M.A.; King, M.W. Hydrolytic Degradation of Polylactic Acid Fibers as a Function of pH and Exposure Time. Molecules 2021, 26, 7554. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Lee, K.S.; Kim, S.J.; Choi, B.I.; Go, B.R.; Rhu, C.J.; Han, T.H. Effect of Chemical Agents on the Morphology and Chemical Structures of Microplastics. Polymers 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste-Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, J. Exposure sources and pathways of micro- and nanoplastics in the environment, with emphasis on potential effects in humans: A systematic review. Integr. Environ. Assess. Manag. 2023, 19, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Craig, N.; Su, L. A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use. Toxics 2023, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Curto, M.; Le Gall, M.; Demeyer, E.; Asselman, J.; Janssen, C.R.; Dhakal, H.N.; Davies, P.; Catarino, A.I.; Everaert, G. Accelerated fragmentation of two thermoplastics (polylactic acid and polypropylene) into microplastics after UV radiation and seawater immersion. Ecotoxicol. Environ. Saf. 2024, 271, 115981. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Vašíček, A.; Lenfeld, P.; Běhálek, L. Degradation of Polylactic Acid Polymer and Biocomposites Exposed to Controlled Climatic Ageing: Mechanical and Thermal Properties and Structure. Polymers 2023, 15, 2977. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Rubin, A.E.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Bikiaris, D.; Lambropoulou, D. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Li, D.; Li, P.; Shi, Y.; Sheerin, E.D.; Zhang, Z.; Yang, L.; Xiao, L.; Hill, C.; Gordon, C.; Ruether, M.; et al. Stress-induced phase separation in plastics drives the release of amorphous polymer micropollutants into water. Nat. Commun. 2025, 16, 3814. [Google Scholar] [CrossRef] [PubMed]
- Ristenpart, W.D.; Cotter, A.R.; Guinard, J.-X. Impact of beverage temperature on consumer preferences for black coffee. Sci. Rep. 2022, 12, 20621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Agumba, D.O.; Pham, D.H.; Kim, J. Ultrastrong, Hydrostable, and Degradable Straws Derived from Microplastic-Free Thermoset Films for Sustainable Development. ACS Omega 2023, 8, 7968–7977. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Yu, S. A Study on Microplastic Emission from Disposable Straws and Its Dietary Relevance. Microplastics 2025, 4, 42. https://doi.org/10.3390/microplastics4030042
Peng B, Yu S. A Study on Microplastic Emission from Disposable Straws and Its Dietary Relevance. Microplastics. 2025; 4(3):42. https://doi.org/10.3390/microplastics4030042
Chicago/Turabian StylePeng, Bangyuan, and Shengwang Yu. 2025. "A Study on Microplastic Emission from Disposable Straws and Its Dietary Relevance" Microplastics 4, no. 3: 42. https://doi.org/10.3390/microplastics4030042
APA StylePeng, B., & Yu, S. (2025). A Study on Microplastic Emission from Disposable Straws and Its Dietary Relevance. Microplastics, 4(3), 42. https://doi.org/10.3390/microplastics4030042





