Abstract
Soil microplastic pollution poses a significant threat to the integrity of terrestrial ecosystems and agricultural sustainability. This review provides a comprehensive synthesis of recent progress on soil microplastic (MP) sources, ecological impacts, and separation technologies. Agricultural practices (e.g., residual plastic mulch and wastewater irrigation) and atmospheric deposition serve as primary drivers of contamination accumulation, with pronounced spatial heterogeneity observed across regions. Predominant MP types such as polyethylene, polystyrene, and polypropylene disrupt soil structure and biogeochemical processes through three core mechanisms: physical interference, chemical toxicity, and biological accumulation. These particles further form carrier–pollutant complexes, exacerbating ecotoxicological impacts across trophic levels. While emerging separation techniques like magnetic separation and solvent extraction demonstrate enhanced efficiency, their implementation faces challenges stemming from soil matrix complexity and high operational costs. This article underscores the need for global collaborative efforts to accelerate innovation in biodegradable polymers, offering practical pathways for sustainable soil management.
1. Introduction
Amid escalating global plastic pollution, microplastics (MPs)—defined as plastic particles typically ranging in size from 1 μm to 5 mm—have emerged as a critical environmental concern. These anthropogenic contaminants are characterized by diverse polymer compositions and varied physical morphologies, including fragments, fibers, films, and microbeads [1]. Plastic, a synthetic polymer renowned for its outstanding durability, lightweight characteristics, and cost-efficiency, has become ubiquitous in packaging, agriculture, and construction. However, a significant amount of plastic debris infiltrates ecosystems via surface runoff, atmospheric deposition, and biological carriers [2,3,4]. Recent research projects indicate that, without intervention, global annual mismanaged plastic waste will nearly double to 121 million tons by 2050 [5]. During the photooxidation process, plastics are irradiated by ultraviolet light. The polymer chains absorb light energy, causing the chemical bonds to break. This generates free radicals that trigger a chain reaction, gradually decomposing the plastics into smaller fragments [6]. In the mechanical fragmentation process, external forces such as wind, water flow scouring, and mechanical rolling break large plastic particles into smaller ones [7].
Although marine microplastic research has made remarkable progress, terrestrial ecosystems, particularly soil ecosystems, have been increasingly recognized as both significant reservoirs of microplastic pollution and a critical frontier for environmental research. The complex soil matrix hinders detection and isolation [8]. Soil organisms such as earthworms interact with MPs, altering the structural composition and ecological processes within the soil ecosystem [9]. Long-term MP accumulation impairs soil structure and functionality [10]. These challenges underscore the need for in-depth research on terrestrial MPs, which differ fundamentally from marine microplastics. The unique challenges posed by soil ecosystems highlight the importance of investigating terrestrial MPs separately from their marine counterparts, while acknowledging that both contribute to global microplastic pollution and may exhibit interconnected ecological impacts.
Recent research has reported that the average abundance of MPs in agricultural soils in China is 3.7 ± 11.9 particles/kg [11], and there is especially severe accumulation of plastic mulch in intensive farming systems. In the vegetable fields of the North China Plain, where continuous plastic mulching has been practiced for more than a decade, the microplastic concentration in the subsurface soils (0–20 cm) can reach 18.2 particles/kg, and the mulch residue rate exceeds 40% [12]. In a Chilean case study evaluating sludge application in Mellipilla County, 31 fields with varying sludge amendment histories were assessed. The results showed that microplastic counts increased with cumulative applications, with fibers as the dominant particle type and a median concentration of 3.5 particles/g in soil [13]. Regarding human health, soil MPs may enter the human body via the food chain. When plants absorb water and nutrients from MP-contaminated soil, MPs or the pollutants they carry can accumulate in plant tissues. Humans consuming these plants may be exposed to potential health risks [14]. Ecotoxicological research is essential for assessing these risks. Scientists employ diverse methodologies, including laboratory-based toxicity tests on soil organisms such as earthworms and plants [15], alongside the field-based monitoring of MP-contaminated sites. These investigations aid in understanding the dose–response relationships between MPs and organisms, as well as the long-term impacts of MP exposure on both ecosystems and human health.
Soil plays a dual and critical role in the microplastic lifecycle. On one hand, it acts as a primary sink for MPs. These particles enter soil through diverse pathways, including agricultural practices such as plastic mulching [16], wastewater irrigation [17], and atmospheric deposition [18]. Once in the soil, MPs accumulate over time due to their low degradation rates. For example, the continuous use of plastic mulch in agriculture results in a steady buildup of MPs in the topsoil. On the other hand, soil functions as a vector for further contamination: Through infiltration, MPs can migrate deeper into the soil profile, potentially reaching and contaminating groundwater sources [19]. Additionally, MPs in soil can exert substantial effects on soil microbial communities. They may alter the composition and functionality of these communities, thereby disrupting critical ecological processes such as nutrient cycling and organic matter decomposition [20]. Certain MPs can adsorb and transport harmful substances, which are subsequently released into the soil environment, posing risks to soil-dwelling organisms and plants [21].
Given the complexity and far-reaching implications of soil microplastic pollution, this review centers specifically on soil MPs. It presents an in-depth analysis of their diverse sources, recent advancements in separation technologies, and intricate ecotoxicological pathways affecting soil-dwelling organisms. The overarching aim is twofold: first, to systematically synthesize existing knowledge and identify specific, actionable knowledge gaps in understanding soil MP pollution, and second, to stimulate interdisciplinary collaboration among environmental scientists, material engineers, and policymakers, thereby facilitating the development of more effective, evidence-based strategies to mitigate this critical environmental challenge.
2. Sources and Input Pathways of Soil Microplastics
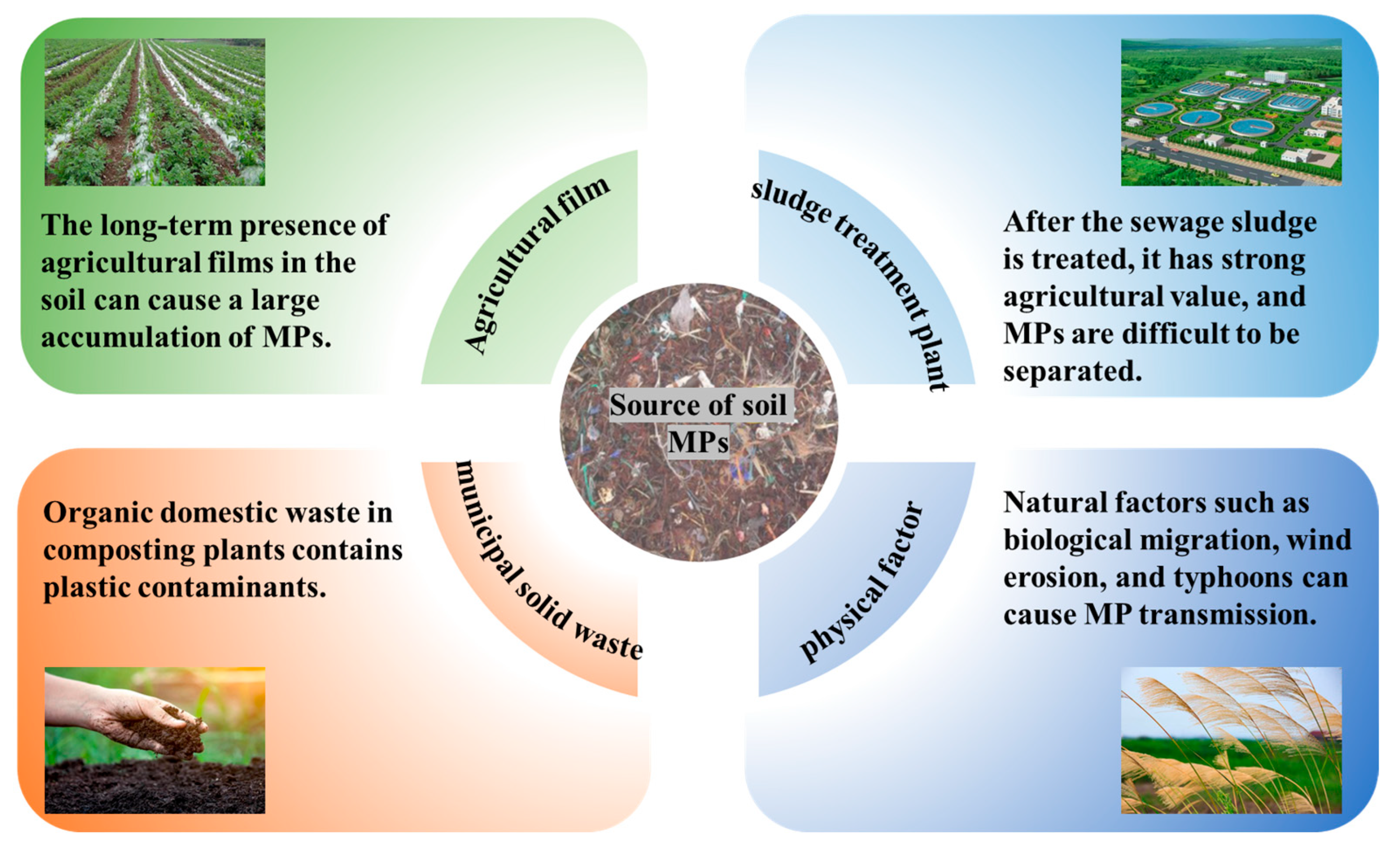
Soil MPs display pronounced spatial heterogeneity. This is exemplified by the concentration levels varying from 4.94 to 252.70 items/kg across 85 agricultural sites in the lower Yangtze River Basin, where the 0.1–0.5 mm size range dominated the microplastic composition, particularly for polypropylene fragments [22]. This spatial distribution originates from three interlinked aspects (Figure 1): agronomic management practices, wastewater/compost additions, and atmospheric fallout. Agricultural practices, particularly plastic mulch deployment for soil moisture retention and thermal regulation, drive continuous microplastic generation via photooxidative degradation processes and the mechanical fragmentation of weathered polymer films. Concurrently, untreated municipal wastewater serves as a primary vector for initial MP introduction through irrigation infrastructure, whereas organic waste amendments induce secondary MP liberation from partially mineralized plastic residues (e.g., packaging substrates and single-use food-ware). Wind-borne transport contributes significantly to MP distribution through the atmospheric transport of particulates, followed by hydrologic deposition through precipitation events. These processes collectively establish a self-perpetuating contamination cycle, facilitating MP accumulation in soil matrices through cross-compartmental feedback mechanisms.
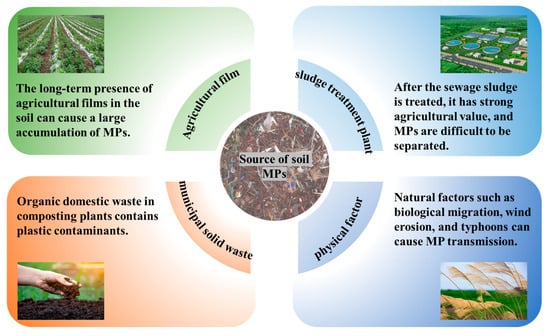
Figure 1.
The main sources of soil microplastics.
2.1. Microplastic Inputs from Agricultural Activities
Agricultural practices are the primary anthropogenic source of soil microplastic pollution, especially due to the agricultural use of wastewater sludge. A study in eastern Spain sampled four wastewater treatment plants and 16 agricultural fields, finding that sewage sludge contained 18,000 ± 15,940 light-density MP particles/kg and 32,070 ± 19,080 heavy-density MP particles/kg on average, with soils showing increased microplastic loads after sludge application [23]. In Mauritius, agricultural soils contained 320.0 ± 112.2 (shallow) and 420.0 ± 244.0 (deep) MP particles/kg, while sewage sludge and wastewater effluents had 14,750 ± 8612.9 MP particles/kg and 276.3 ± 137.3 particles/L, respectively [24]. A nine-year field investigation in Suzhou, China, confirmed that continuous sludge additions lead to exponential microplastic accumulation in topsoil [25]. National-level analysis of Chinese municipal sludge further identified microplastic concentrations of 1448–11,125 MP particles/kg DW [26].
Plastic mulching technology exacerbates microplastic pollution despite enhancing agricultural productivity and carbon sequestration [27]. Farmlands with three decades of mulching history contain MPs up to 40.35 mg/kg, dominated by 0.9–2.0 mm particles [28]. In Guangdong, plastic film mulching resulted in an average microplastic abundance of 22,675 MP particles/kg, primarily polyurethane [29]. Long-term mulching accelerates fragmentation, evidenced by decreasing particle size, rising abundance, and intensified FTIR oxidation peaks [30]. In addition to conventional mulch films, plastic shading nets, widely utilized as crop protection covers, make a significant contribution to soil contamination [31]. Computational modeling projects an annual microplastic release of 1629.68 metric tons from the degradation of shading nets in the Beijing–Tianjin–Hebei region, highlighting their underappreciated environmental consequences [32].
In summary, each of these agricultural practices contributes significantly to soil microplastic pollution. Plastic mulching directly adds MPs from degraded films, composting introduces MPs from incompletely decomposed waste, and wastewater irrigation brings in MPs from various sources in the wastewater. Understanding these differences is crucial for developing effective strategies to mitigate soil microplastic pollution.
2.2. Microplastic Accumulation Pathways via Solid Waste Disposal and Utilization
Agricultural practices and waste management systems significantly contribute to soil MP pollution through multiple pathways. Municipal solid waste composting, while enhancing soil organic matter [33], introduces MPs via contaminated waste streams. Compost samples from rural domestic waste facilities contain 2400 ± 358 MP particles/kg, predominantly fibers and films [34]. Similarly, livestock manure application, while enhancing phosphorus transformation efficiency [35], transmits MPs ingested by livestock to agricultural soils. In intensive vegetable farms in southeastern Spain, MP levels reach ~2 × 103 particles/kg in soils and ~103 particles/kg in sheep feces. Given that sheep graze on residual vegetables from mulch-covered fields, they ingest MPs; a herd of 1000 sheep transfers approximately 106 MPs per hectare per year through ingestion–excretion cycles [36]. This transfer mechanism is supported by the concurrent detection of MPs in topsoil and ovine fecal samples across pastoral systems, underscoring livestock as vectors of MPs. Specifically, herbivores such as sheep accumulate and redistribute MPs through manure, highlighting the necessity for enhanced livestock management practices (e.g., feed purification and manure treatment) to mitigate soil contamination.
Urban landfills act as persistent MPs reservoirs. Studies on municipal landfills in China detect MPs in all leachate samples (0.42–24.58 items/L), with inactive landfills continuously releasing MPs via waste degradation [37]. Informal landfills in southern China exhibit severe contamination, with MPs concentrations ranging from 590 to 103,080 particles/kg in waste, 570–14,200 particles/kg in underlying soils, and 3–25 particles/L in leachate and groundwater, highlighting leakage risks due to inadequate protective measures [38]. Globally, similar patterns emerge—Northern Indian MSW sites exhibit extreme soil MPs levels (53,580 ± 720 particles/kg) and hazardous polymer risks, with monsoonal runoff modulating contamination spread [39].
Emerging non-traditional sources, such as optical lens polishing and cigarette filters, challenge conventional paradigms of microplastic pollution. Optical lens polishing, a previously overlooked industrial process, generates acrylic particles at scale, with South Korea’s 140,000 optical shops releasing several metric tons of MPs annually through wastewater [40]. Cigarette filters, often misperceived as biodegradable, contain over 15,000 plastic filaments per butt, acting as hazardous point sources that persist in environments long after disposal [41]. These pathways not only expand the known inventory of MPs sources but also reveal critical gaps in regulatory frameworks. For instance, while agricultural and waste-derived MPs are increasingly monitored, industrial byproducts like lens polishing residues and consumer goods (e.g., cigarette filters) remain underregulated. Addressing these unconventional pathways requires sector-specific interventions, such as mandating acrylic particle filtration in optical industries and phasing out plastic-based filters in tobacco products.
2.3. Environmental Transport and Redistribution of Microplastics
In agricultural regions of the eastern Qinghai–Tibet Plateau, land-use patterns explained 51.35% of the variance in soil MP abundance according to statistical modeling, with grassland and cropland MPs showing positive associations with precipitation and negative associations with average wind speed [42]. A survey of 62 surface soil samples and 8 freshwater sediment samples across five land-use categories (urban areas, tea plantations, drylands, paddy fields, and woodlands) detected MPs in all samples, with abundance in descending order as follows: urban areas > paddy fields > drylands > tea plantations > woodlands [43].
Atmospheric deposition contributes significantly to soil MP pollution. In Beijing, plant leaves accumulate 3.62 ± 1.29 microplastic particles/cm2, mainly < 80 μm, which enter the soil upon decomposition [44]. Research in Shanghai during the rainy season indicates rainfall washes MPs from the air into the soil, with these MPs originating from industrial activities, plastic product usage, and daily life [45]. Wind-mediated transport plays a key role in MP redistribution. In Swiss nature reserves, 90% of 29 floodplain soil samples contained MPs, with concentrations positively correlated with watershed population density [46]. Research in Fars Province, Iran, demonstrated that wind erosion serves as a key pathway for terrestrial MP dispersion. Industrial zones, urban areas, and agricultural fields act as MP sources susceptible to wind transport. Wind entrains MPs attached to dust particles and carries them across landscapes. In Fars Province, this process has resulted in the widespread presence of MPs across diverse regions, highlighting wind erosion as a significant driver of MP contamination [47]. Typhoon impacts were evidenced by increased MP abundance and altered polymer composition in Shenzhen coastal areas after Typhoon Wipha [48].
3. Ecological Impacts of Microplastics in Soil Ecosystems
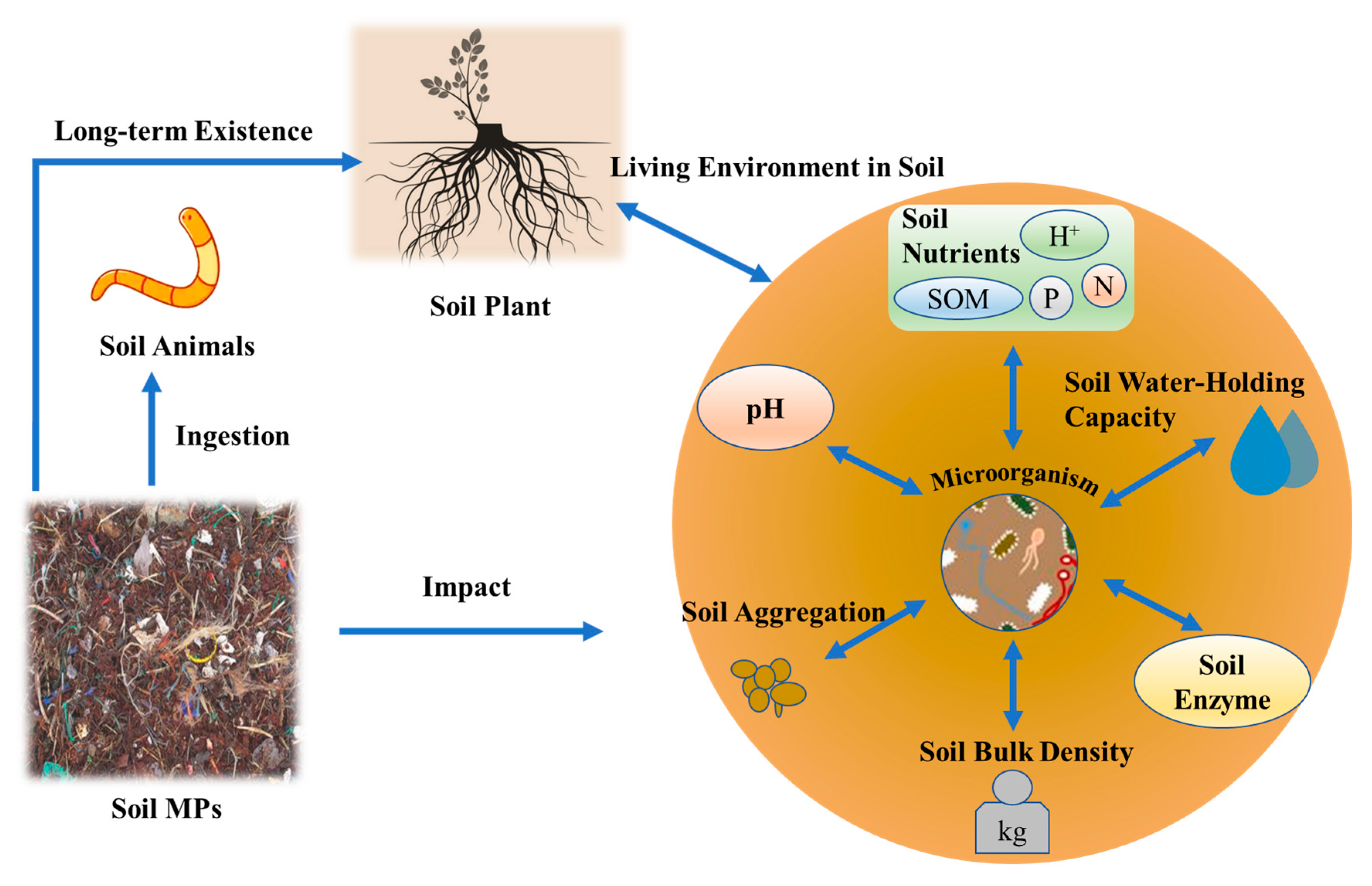
MPs have permeated terrestrial ecosystems, with soils acting as long-term reservoirs for these persistent contaminants. Their ecological impacts stem from three synergistic mechanisms: physical interference (e.g., altered soil structure), chemical toxicity (e.g., additive leaching and pollutant co-transport), and biotic interactions (e.g., trophic transfer), collectively undermining soil health and ecosystem services (Figure 2) [49]. Notably, although marketed as sustainable alternatives, biodegradable polymers like polyhydroxyalkanoates (PHAs) exhibit unanticipated persistence and disrupt soil biogeochemical processes. This discovery challenges the core assumptions underpinning their eco-design [50].
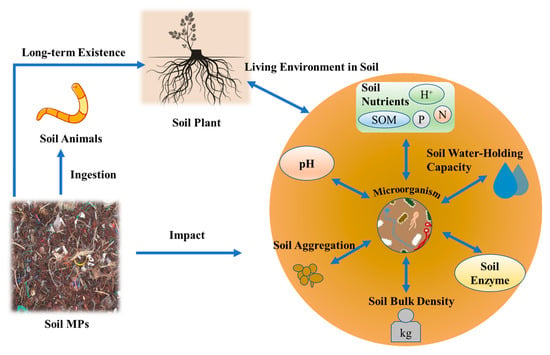
Figure 2.
The impact of microplastics on soil ecosystems [48].
This section synthesizes MPs’ cascading effects across hierarchical levels: from physicochemical property alterations (e.g., nutrient cycling and hydraulic conductivity) to microbial community shifts, faunal physiological disruptions, and plant growth inhibition. By integrating polymer-specific toxicodynamics and co-contaminant interactions, we elucidate how MPs reshape soil ecosystems at multiple scales.
3.1. Alterations in Physical and Chemical Properties of Soil
Polyethylene microplastics (PE-MPs) have been shown to significantly degrade the physicochemical properties of soil aggregates, inhibit soil enzyme activity, and impair soil fertility. A soil incubation experiment showed that while plant residues like wheat straw increased water-stable aggregates (WSAs) by 281.65% and soil enzyme activities by 55.45%, coexisting microplastic fibers reduced these benefits by 37.57% (WSAs) and 26.11% (enzyme activities) in agricultural soil contexts [51]. Batch experiments showed that environment-exposed PE-MPs enhance Cu/TC adsorption, with air-exposed fitting Freundlich and water/soil-exposed Langmuir models, influenced by biofilm and DOM [52]. Aging and particle size further modulate the environmental behavior of PE-MPs. Aged PE-MPs exhibit heightened adsorption capacity, acting as efficient carriers for hydrophobic pesticides [53]. Notably, smaller PE-MPs (<50 μm) demonstrate significantly greater adsorption capacity for organochlorine pesticides such as DDT compared to larger particles (>500 μm), effectively concentrating low-concentration pollutants within soil suspensions [54]. This size-dependent adsorption highlights the critical role of particle morphology in contaminant transport and accumulation, underscoring the need to account for weathering processes and physical characteristics when assessing ecological risks of PE-MP.
Polypropylene microplastics (PP-MPs) exert distinct effects on soil properties. Long-term PP-MP pollution alters soil physical characteristics: at 2% w/w, PP-MPs increase soil contact angle and saturated hydraulic conductivity, while at 4.88% w/w, bulk density decreases by 12%, accompanied by moderate reductions in water-holding capacity [55]. Chemically, PP-MPs enhance soil enzyme activity and cation exchange capacity (CEC), with CEC exhibiting an extremely strong positive correlation with PP-MP concentration (r = 0.9944). Total organic carbon (TOC) content also rises significantly when PP-MP concentration exceeds 0.3% w/w, demonstrating a robust positive relationship (r = 0.9827) [56]. The coexistence of PE-MPs and PP-MPs synergistically alters soil biogeochemical processes. Comparative studies show that these MPs collectively reduce available phosphate content by 48%while increasing ammonium nitrogen levels by 63% (from 0.94 to 1.53 mg N L−1), indicative of disrupted nitrogen and phosphorus cycling [57].
Microplastic-induced structural changes manifest in soil porosity and hydrological properties. PP-MPs disrupt pore-size distribution and impair pore connectivity across loam, clay, and sandy soils, leading to substantial declines in saturated hydraulic conductivity and more steeply inclined soil water characteristic curves [58]. MPs also influence soil thermal properties: polyethylene and polyvinyl chloride reduce the thermal conductivity of sandy soils by up to 38%, a phenomenon attributed to their hydrophobicity altering soil water distribution patterns [59,60]. Notably, polyester microfibers (PMFs) exert unique effects, increasing macroporosity (>30 μm) and promoting the formation of water-stable macroaggregates (>2 mm) by 39–44% at concentrations of 0.1–0.3%—markedly higher than the 31% observed in control soils. This contrasting response highlights the potential for specific microfiber types to influence soil structural properties in manners distinct from granular MPs, underscoring the need to differentiate MP morphology when assessing ecological impacts [61].
3.2. Impacts on Soil Microbial Community and Its Functions
PE-MPs exacerbate cadmium contamination by diminishing soil’s adsorption capacity, thereby enhancing cadmium mobility in agroecosystems [62]. In paddy systems, PE-MPs further induce microbial functional genes associated with hemicellulose and lignin degradation, accelerating rates of organic carbon mineralization and potentially increasing N2O emissions [63]. PP-MPs drive time-dependent shifts in bacterial community composition. A 90-day soil column experiment revealed the progressive enrichment of Proteobacteria in topsoil layers, contrasting with declining abundances in middle soil horizons. This vertical differentiation suggests that PP-MPs may alter microbial niche partitioning across soil profiles, potentially disrupting biogeochemical cycles mediated by specific bacterial lineages [64]. Short-term exposure (28 days) to 0.25% (w/w) PP-MPs (13 μm) significantly decreases bacterial diversity, driving community reorganization through increased abundances of Aeromonadaceae and Pseudomonadaceae (opportunistic heterotrophs) and reduced Nitrososphaeraceae (ammonia oxidizers) [65]. Polyvinyl chloride microplastics (PVC-MPs) selectively regulate phosphorus dynamics. Over 35 days at 0.1–1% concentrations, PVC-MPs reshape bacterial genera associated with phosphate solubilization, thereby altering available phosphorus levels without impacting overall bacterial diversity [66].
Polystyrene microplastics (PS-MPs) in conjunction with heavy metals (Cd, Pb, and Zn) exacerbate microbial disruptions through interactive effects. Combined exposure alters soil pH, electrical conductivity, and organic matter content, collectively perturbing microbial community structure and functional diversity. This synergistic pollution pathway underscores the need to assess microplastic impacts within multi-contaminant contexts, as co-pollutant interactions may amplify ecological risks beyond individual stressor effects [67]. Cross-polymer interactions exhibit far-reaching functional implications. PA-6, PE, and PET MPs collectively enhance soil CO2 emissions and humification potential across four distinct soil types. Although microbial diversity remains relatively stable, these MPs reduce network complexity and elevate stochasticity in bacterial community assembly, indicating altered microbial ecological processes under multi-polymer exposure conditions [68]. PET-MPs specifically elevate antibiotic resistance gene (ARG) abundance and metabolite deficiency gene (MDG) levels, with structural equation models confirming indirect regulatory roles of MPs [69].
3.3. Physiological and Ecological Effects on Soil Fauna
The exposure of earthworms to PE-MPs and heavy metal ions (Cu2+ or Ni2+) induces dose-dependent metal accumulation, with smaller-sized PE-MPs demonstrating higher adsorption capacity due to their larger surface area-to-volume ratio [70]. Both pristine and aged PE-MPs at 0.1–10% concentrations efficiently adsorb Zn, Pb, and Cd ions, with 1% PE-MPs significantly increasing metal bioavailability and inducing synergistic toxicity in earthworms [71]. PS-MPs in coexistence with heavy metals (Cd, Pb) further modify soil enzymatic activities and reshape the trophic structure of soil nematode communities, reducing nematode diversity and shifting the ratios of bacterivorous to fungivorous nematodes [72]. PS-MPs increase pyrene accumulation in earthworms during initial exposure [73] and exacerbate metal bioaccumulation by disrupting intestinal barriers and inducing immunosuppression [74].
Furthermore, low-concentration PS-MPs (0.02% w/w) disrupt osmoregulatory metabolism in earthworms, altering gut metabolite profiles without impacting microbiota diversity [75]. Laser scanning confocal microscopy (LSCM) and scanning electron microscopy (SEM) confirm that physical abrasion serves as a primary toxicity mechanism of PS-MPs on epidermal tissues [76]. Nile red fluorescence staining revealed earthworm ingestion of both 25 μm HDPE and 13 μm PP MPs. Physiological analyses of earthworms showed that concurrent exposure to PP-MPs and PE-MPs perturbs lipid metabolism, induces oxidative stress, and impairs digestive and immune functions [77].
3.4. Effects on Plant Growth and Material Cycling in Ecosystems
MPs hinder plant growth and disrupt biogeochemical cycles through direct toxicity and indirect nutrient regulation. In strawberry agroecosystems, co-exposure to PE-MPs and copper nanoparticles increases copper bioavailability, leading to root accumulation and reductions in plant height and stem diameter [78]. Hydroponic studies on Vallisneria natans (water celery) show that PE-MPs decrease leaf nitrogen-to-carbon ratios by inhibiting nitrogen-related enzymes and photosynthetic pathways, ultimately suppressing biomass production [79]. Chinese cabbage exposed to nano-polystyrene (nano-PS) exhibits shoot nitrogen deficiency but root nitrogen accumulation, accompanied by reduced soil nitrous oxide (N2O) and ammonia (NH3) emissions [80]. Diatom experiments (Chaetoceros neogracile) further validate PSMP-induced growth inhibition: a 64.4% reduction in chlorophyll a content, a 35.5% decline in protein content, and the lowest photosynthetic efficiency (Fv/Fm) observed after 72–96 h of exposure to 200 mg L−1 PSMPs. Molecular docking simulations revealed strong affinity between PSMPs and the extrinsic protein in photosystem II, potentially disrupting photosynthetic machinery, consistent with the observed declines in photosynthetic efficiency [81]. MPs also alter microbial-driven nutrient cycling. Elevated PVC-MP concentrations restructure microbial communities and heighten maize seedling susceptibility to shoot phytotoxicity [82]. Polyamide MPs reduce microbial diversity and selectively inhibit nitrifying bacteria, thereby disrupting carbon-nitrogen cycling [83].
4. Recent Advances in Separation Technologies for Soil Microplastics
Extensive research has focused on MP separation in marine environments [84], sediments [85], and marine organisms [86]. However, soil MPs pose unique challenges that demand separation methodologies distinct from or adapted to those used in marine or sedimentary settings [87]. In marine and sediment systems, MPs typically originate from more defined sources and exhibit relatively homogeneous compositions. By contrast, soil MPs enter ecosystems through diverse pathways, resulting in heterogeneous mixtures encompassing multiple polymer types (e.g., PE, PS, PP, PVC, and PA), morphological variations, and broad size distributions, which are key factors that impede the development of universal separation protocols. Furthermore, soil organic matter (SOM) displays dynamic physicochemical properties and recalcitrant characteristics [88], along with soil environmental factors (e.g., humidity), which consistently interfere with MP extraction efficiency. This section critically assesses existing separation methods that demonstrate relative effectiveness under these complex constraints. To facilitate comparative analysis, Table 1 summarizes key separation technologies, their underlying principles, essential reagents, advantages, and inherent limitations.

Table 1.
Comparative overview of soil microplastic separation technologies.
4.1. Density Separation
Density separation exploits density disparities between MPs (0.89–1.58 g/cm3) and soil particles (2.65–2.86 g/cm3). Sodium chloride (NaCl, 1.2 g/cm3) effectively floats common polymers but is not effective for high-density PVC and PET [89,90], while sodium bromide (NaBr, 1.55 g/cm3) and zinc chloride (ZnCl2, 1.6 g/cm3) extend density coverage under conditions of increased environmental risk [91,92]. Lipophilic enhancements using olive oil achieve polymer-specific recoveries (90–97%) [93], with canola oil outperforming NaCl/ZnCl2 in low-SOM soils [94]. PDMS-coated nickel foams broaden applicability (0.94–2.2 g/cm3) through hydrophobic adsorption [95]. Although density separation techniques utilize density differences between MPs and soil particles for extraction, the complexity of the soil matrix presents significant challenges. Soil is a complex mixture of organic matter, microorganisms, and diverse compounds, with some MPs potentially trapped within soil aggregates, hindering access by extraction agents. Additionally, the high cost of salts like sodium bromide (NaBr) and zinc chloride (ZnCl2) for separation along with secondary pollution risks from waste salt solutions escalates operational expenses, limiting the large-scale applicability of this technique.
4.2. Organic Matter Digestion Pretreatment
Organic digestion pretreatments target SOM interference, with pre-digestion using 30% hydrogen peroxide (H2O2) at 70 °C significantly boosting the extraction of MPs from soils and sewage sludge [96,97], while NaOCl oxidation improves detection in SOM-rich soils [98]. Fenton reagent achieves 90% SOM removal in estuarine sediments [99], but risks MP degradation unless reaction time is controlled [100]. Sequential digestion using H2O2 followed by NaOCl balances efficiency and MP preservation; however, the presence of humic acid–MP complexes necessitates additional enzymatic treatment steps [101,102]. Organic matter digestion pretreatment facilitates MP separation but encounters challenges, notably that aggressive chemicals (e.g., NaOCl, Fenton’s reagent, and H2O2) degrade MP polymers via chemical bond cleavage. Mitigation includes optimizing reagent concentration/treatment time via spiking experiments, adopting milder sequential treatments, and using protective agents to balance organic removal and MP structural integrity.
4.3. Centrifugal Gradient Separation
Centrifugal techniques using CsCl gradients (1.1–1.5 g/cm3) recover 86–99% of MPs across polymer densities [103], while simplified protocols achieve 90% recovery in sandy soils with <0.8% residual soil mass [104]. Fenton-assisted centrifugation combines digestion and isolation, attaining 94% recovery through multi-step cycles [105] yet requiring prolonged processing times. Centrifugal gradient separation has the potential for MP extraction but is broadly limited by the complex soil matrix, where overlapping size and density ranges of soil particles and MPs, along with interference from organic matter and microorganisms, complicate precise separation. The method requires expensive ultracentrifuges, lengthy processing, and specialized maintenance, with high costs from equipment, upkeep, and energy consumption compounded by inefficient large-scale sample handling, thereby restricting its broad adoption.
4.4. Magnetic/Electrostatic Sorting
Protocols tailored for soil applications consist of three consecutive operational phases: (1) magnetic soil fraction elimination through high-gradient magnetic separation (HGMS); (2) microplastic tagging using surface-modified iron nanoparticles; and (3) labeled MP recovery via HGMS, achieving recovery rates exceeding 95% for both low- and high-density polymers [106]. Recent studies demonstrate that Fe3O4-modified biochar enhances polystyrene (PS) microplastic retention—achieving a maximum efficiency of 92.36%—through synergistic electrostatic adsorption and surface complexation. Notably, humic acid (HA) significantly reduces retention efficacy via competitive adsorption under 1 mM Na+ conditions, whereas Ca2+ exhibits stronger transport inhibition than Na+ across different ionic strengths [107]. Emerging techniques such as magnetism-assisted density gradient separation (Mag-DG-Sep) enable precise particle differentiation, successfully isolating 180–212 μm PE-MPs across 0.98–2.20 g cm−3 density gradients using paramagnetic manganese chloride (MnCl2) solutions. This method proves particularly effective for heterogeneous soil–glass mixtures, significantly streamlining spectroscopic pre-treatment workflows [108]. Evaluation of the Korona–Walzen–Scheider system demonstrates pronounced size-dependent recovery efficiency: particulates ≥ 2 mm attain near-total recovery, whereas 20 μm MPs show markedly reduced recovery rates (45%) [109].
While magnetic/electrostatic methods minimize chemical use, challenges persist in organic matter interference, size-dependent efficiency, and high operational costs. Future developments require optimized surface modifiers to mitigate SOM effects and automated workflows to enhance throughput. These innovations align separation advances with ecological remediation needs, offering scalable solutions for soil MP pollution.
5. Conclusions and Future Perspectives
Soil microplastic pollution poses a multifaceted threat to global agricultural sustainability. First, intertwined multi-source contamination pathways, including agricultural plastic residues, wastewater irrigation, and wind-borne depositions create self-perpetuating networks that drive continuous MP accumulation in soil-crop systems. This buildup then causes a series of problems for ecosystems. MPs exert multifaceted impacts on soil ecosystems: disrupting structural stability, enhancing contaminant co-accumulation with heavy metals/antibiotics, and triggering cascading toxic effects across biological hierarchies. Due to the complex soil environment, current analytical and separation approaches face persistent technological challenges. These methods are challenged by both the physicochemical heterogeneity of MPs and the multidimensional interference from the soil matrix, underscoring the complexity of mitigating microplastic pollution.
From a strategic perspective, future research should prioritize three key focal areas. First, investigations must aim to develop eco-friendly polymers and enhance the degradation rates of biodegradable materials across diverse soil conditions [110]. Second, improving the accuracy and sensitivity of microplastic detection techniques [111], especially for low-concentration samples, is of vital importance. Third, optimizing the efficiency and selectivity of advanced separation methods tailored to soil characteristics will foster more effective pollution control strategies [112]. These priorities will guide future studies and underpin sustainable soil management frameworks. To bridge the gap between laboratory-scale separation technologies and field implementation [113], future work should emphasize scaling up promising methodologies such as density-based separation and surfactant-assisted approaches for large-scale soil treatments.
Author Contributions
All authors participated in conceptualization, literature search, and writing of original draft, revision, and final article. Conceptualization, K.C., Y.H. and Y.M.; methodology, K.C. and Y.M.; investigation, K.C.; data curation, K.C.; writing—original draft preparation, K.C., Y.H. and Y.M.; writing—review and editing, K.C., Y.H. and Y.M.; visualization, K.C. and Y.H.; supervision, Y.M. and Y.H.; project administration, Y.H. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research—What have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.J.; Lechthaler, S. Plastics as a stratigraphic marker in fluvial deposits. Anthropocene 2021, 36, 100314. [Google Scholar] [CrossRef]
- Özgenç, E.; Keleş, E.; Buluş, E.; Buluş, G.S.; Töre, G.Y. Evaluating Ecological Impacts and Atmospheric Fate of Microplastics: Ecological Perspectives and Challenges. Environ. Eng. Manag. J. 2024, 23, 1285–1304. [Google Scholar] [CrossRef]
- Luna, Á.; Moreno, E.; Pinzolas, J.A.; Oliver, S.; Meyer, S.; Brodermann, O.; Merino, C.; Karaardıç, H.; da Silva, L.P.; Chatton, C.; et al. Anthropogenic debris as nest material in three swift species: New insights into the interactions of atmospheric pollution with wildlife. Sci. Total Environ. 2024, 949, 175171. [Google Scholar] [CrossRef]
- Pottinger, A.S.; Geyer, R.; Biyani, N.; Martinez, C.C.; Nathan, N.; Morse, M.R.; Liu, C.; Hu, S.; de Bruyn, M.; Boettiger, C.; et al. Pathways to reduce global plastic waste mismanagement and greenhouse gas emissions by 2050. Science 2024, 386, 1168–1173. [Google Scholar] [CrossRef]
- Bonyadinejad, G.; Salehi, M.; Herath, A. Investigating the sustainability of agricultural plastic products, combined influence of polymer characteristics and environmental conditions on microplastics aging. Sci. Total Environ. 2022, 839, 156385. [Google Scholar] [CrossRef]
- Berenstein, G.; Córdoba, P.; Díaz, Y.B.; González, N.; Ponce, M.B.; Montserrat, J.M. Macro, meso, micro and nanoplastics in horticultural soils in Argentina: Abundance, size distribution and fragmentation mechanism. Sci. Total Environ. 2024, 906, 167672. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Taey, D.K.A.; Al-Mammori, H.Z. Earthworms and eco-consequences: Considerations to soil biological indicators and plant function: A review. Acta Ecol. Sin. 2021, 41, 512–523. [Google Scholar] [CrossRef]
- Liu, Y.; He, B.; Xiao, Q.; Wang, X.; Lin, X.; Hu, J. Earthworms facilitated pepper (Capsicum annuum L.) growth via enhancing the population and function of arbuscular mycorrhizal fungi in a low-density polyethylene-contaminated soil. Chem. Biol. Technol. Agric. 2023, 10, 115. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef]
- Harms, I.K.; Diekotter, T.; Troegel, S.; Lenz, M. Amount, distribution and composition of large microplastics in typical agricultural soils in Northern Germany. Sci. Total Environ. 2021, 758, 143615. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Hao, T.; Zhang, J.; Müller, C.; Florent, P.; Yan, H.; Ren, S.; Qu, K.; Ren, K.; et al. Long-term plastic film mulching promotes microplastic accumulation and alters gross nitrogen transformation in soil. Appl. Soil Ecol. 2025, 208, 106007. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Xu, L.; Li, R.; Zhang, R.; Li, M.; Ran, C.; Rao, Z.; Wei, X.; Chen, M.; et al. Leaf absorption contributes to accumulation of microplastics in plants. Nature 2025, 614, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Saf. 2020, 187, 109788. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Tian, J.; Niu, F.; Xing, Y.; Wu, Y.; Zhang, R.; Zheng, J.; Xu, L. Accumulation of microplastics in greenhouse soil after long-term plastic film mulching in Beijing, China. Sci. Total Environ. 2022, 828, 154544. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]
- Szewc, K.; Graca, B.; Dołęga, A. Atmospheric deposition of microplastics in the coastal zone: Characteristics and relationship with meteorological factors. Sci. Total Environ. 2021, 761, 143272. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.; Cao, X.; Yeh, T.-c.J.; Zhang, J.; Zhan, Z.; Cui, Y.; Li, H. Modeling of Microplastics Migration in Soil and Groundwater: Insights into Dispersion and Particle Property Effects. Environ. Sci. Technol. 2024, 58, 15224–15235. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Shirkhorshidi, B.; Ghanatghestani, M.D.; Moeinpour, F.; Parvaresh, H. Exploring the interaction between microplastics and heavy metals: Unveiling the impact of microplastics on lead sorption and desorption in soil. Environ. Monit. Assess. 2023, 195, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, D.; Liu, P.; Hu, W.; Xu, L.; Sun, Y.; Wu, Q.; Tian, K.; Huang, B.; Yoon, S.J.; et al. Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Sci. Total Environ. 2021, 794, 148694. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Ragoobur, D.; Huerta-Lwanga, E.; Somaroo, G.D. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci. Total Environ. 2021, 798, 149326. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef]
- Li, X.-y.; Liu, H.-t.; Wang, L.-x.; Guo, H.-n.; Zhang, J.; Gao, D. Effects of typical sludge treatment on microplastics in China—Characteristics, abundance and micro-morphological evidence. Sci. Total Environ. 2022, 826, 154206. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, B.; Li, Z.; Huang, F.; Zhao, C.; Zhang, P.; Jia, Z. Plastic film mulch combined with adding biochar improved soil carbon budget, carbon footprint, and maize yield in a rainfed region. Field Crops Res. 2022, 284, 108574. [Google Scholar] [CrossRef]
- Li, W.; Wufuer, R.; Duo, J.; Wang, S.; Luo, Y.; Zhang, D.; Pan, X. Microplastics in agricultural soils: Extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ. 2020, 749, 141420. [Google Scholar] [CrossRef]
- Long, B.; Li, F.; Wang, K.; Huang, Y.; Yang, Y.; Xie, D. Impact of plastic film mulching on microplastic in farmland soils in Guangdong province, China. Heliyon 2023, 9, e16587. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.Q.; Yan, C.R.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Stevens, C.J. A nationwide assessment of microplastic abundance in agricultural soils: The influence of plastic crop covers within the United Kingdom. Plants People Planet 2024, 6, 304–314. [Google Scholar] [CrossRef]
- Mo, X.; Li, H.; Lian, Y.; Zheng, B.; Dong, J.; Lu, X. Estimation of soil microplastic input derived from plastic gauze using a simplified model. Sci. Total Environ. 2021, 793, 148577. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Prit, H.; Maturi, K.C.; Kalamdhad, A.S. Amendment of Nutrient-deficient Alluvial Soil by Municipal Solid Waste Char and Compost for the Remediation of Contaminants and Enhancement of Plant Growth. Water Air Soil Pollut. 2025, 236, 238. [Google Scholar] [CrossRef]
- Gui, J.; Sun, Y.; Wang, J.; Chen, X.; Zhang, S.; Wu, D. Microplastics in composting of rural domestic waste: Abundance, characteristics, and release from the surface of macroplastics. Environ. Pollut. 2021, 274, 116553. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, J.; Chen, H.; Zhao, X.; Wang, S.; Zhu, Y.; Wang, Y. Animal manures promoted soil phosphorus transformation via affecting soil microbial community in paddy soil. Sci. Total Environ. 2022, 831, 154917. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lu, F. Municipal solid waste (MSW) landfill: A source of microplastics?-Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, X.; Liu, Q.; Hu, H.; Wu, C.; Xue, Q. Informal landfill contributes to the pollution of microplastics in the surrounding environment. Environ. Pollut. 2022, 293, 118586. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, B.K.; Krause, S. Spatiotemporal distribution and ecological hazards of microplastic pollution in soil water resources around a wastewater treatment plant and municipal solid waste site. J. Contam. Hydrol. 2025, 269, 104515. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Jeong, J.; Chae, K.J. Eye-glass polishing wastewater as significant microplastic source: Microplastic identification and quantification. J. Hazard. Mater. 2021, 403, 123991. [Google Scholar] [CrossRef]
- Shen, M.; Li, Y.; Song, B.; Zhou, C.; Gong, J.; Zeng, G. Smoked cigarette butts: Unignorable source for environmental microplastic fibers. Sci. Total Environ. 2021, 791, 148384. [Google Scholar] [CrossRef] [PubMed]
- Haixin, Z.; Yimei, H.; Shaoshan, A.; Haohao, L.; Xiaoqian, D.; Pan, W.; Mengyuan, F. Land-use patterns determine the distribution of soil microplastics in typical agricultural areas on the eastern Qinghai-Tibetan Plateau. J. Hazard. Mater. 2022, 426, 127806. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, S.; Qin, W.; Zhang, C.; Lv, C.; Zou, M. Effects of land use on the distribution of soil microplastics in the Lihe River watershed, China. Chemosphere 2023, 324, 138292. [Google Scholar] [CrossRef]
- Xu, L.; Li, K.; Bai, X.; Zhang, G.; Tian, X.; Tang, Q.; Zhang, M.; Hu, M.; Huang, Y. Microplastics in the atmosphere: Adsorb on leaves and their effects on the phyllosphere bacterial community. J. Hazard. Mater. 2024, 462, 132789. [Google Scholar] [CrossRef]
- Jia, Q.; Duan, Y.; Han, X.; Sun, X.; Munyaneza, J.; Ma, J.; Xiu, G. Atmospheric deposition of microplastics in the megalopolis (Shanghai) during rainy season: Characteristics, influence factors, and source. Sci. Total Environ. 2022, 847, 157609. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Rezaei, M.; Riksen, M.; Sirjani, E.; Sameni, A.; Geissen, V. Wind erosion as a driver for transport of light density microplastics. Sci. Total Environ. 2019, 669, 273–281. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Tang, Y.; Wang, S.; Lu, X.; Cheng, Z.; Zhang, X.; Wu, P.; Chang, X.; Xia, Y. Typhoon-induced turbulence redistributed microplastics in coastal areas and reformed plastisphere community. Water Res. 2021, 204, 117580. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Liang, Y.; Lehmann, A.; Yang, G.; Leifheit, E.F.; Rillig, M.C. Effects of Microplastic Fibers on Soil Aggregation and Enzyme Activities Are Organic Matter Dependent. Front. Environ. Sci. 2021, 9, 650155. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Lan, T.; Wang, T.; Cao, F.; Yu, C.; Chu, Q.; Wang, F. A comparative study on the adsorption behavior of pesticides by pristine and aged microplastics from agricultural polyethylene soil films. Ecotoxicol. Environ. Saf. 2021, 209, 111781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, Y.; Qian, J.; Qiao, Y.; Liu, J.; Li, S.; Dai, L.; Sun, K.; Guo, H.; Sui, G.; et al. Sorption of organochlorine pesticides on polyethylene microplastics in soil suspension. Ecotoxicol. Environ. Saf. 2021, 223, 112591. [Google Scholar] [CrossRef]
- Yu, Y.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowdhury, I.; Flury, M. Minimal Impacts of Microplastics on Soil Physical Properties under Environmentally Relevant Concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Zhang, Z.; Cui, M. The positive effects of polypropylene and polyvinyl chloride microplastics on agricultural soil quality. J. Soils Sediments 2022, 23, 1304–1314. [Google Scholar] [CrossRef]
- Li, H.; Liu, L. Short-term effects of polyethene and polypropylene microplastics on soil phosphorus and nitrogen availability. Chemosphere 2022, 291, 132984. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.; Zhao, Z.; Liu, G.; et al. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef]
- Aminzadeh, M.; Kokate, T.; Shokri, N. Microplastics in sandy soils: Alterations in thermal conductivity, surface albedo, and temperature. Environ. Pollut. 2025, 372, 125956. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Liu, Y.; Deng, Y.; Liu, S.; Nan, J.; Lyu, C. Polyethylene microplastic pollution changes the electrical resistance and thermal conductivity of loess soil. J. Environ. Manag. 2024, 371, 123127. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, X.; Feng, Z.; Xiao, M.; Ge, T.; Li, Y.; Yao, H. Polyethylene microplastics alter the microbial functional gene abundances and increase nitrous oxide emissions from paddy soils. J. Hazard. Mater. 2022, 432, 128721. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yue, L.; Zhao, Y.; Li, J.; Fu, Y.; Deng, H.; Feng, D.; Li, Q.; Yu, H.; Zhang, Y.; et al. Changes in bacterial community structures in soil caused by migration and aging of microplastics. Sci. Total Environ. 2022, 848, 157790. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, W.; Tian, H.; Zhang, K.; Li, B.; Du, Z.; Zhang, W.; Wang, J.; Wang, J.; Zhu, L. The effects of high-density polyethylene and polypropylene microplastics on the soil and earthworm Metaphire guillelmi gut microbiota. Chemosphere 2021, 267, 129219. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of Polyvinyl Chloride Microplastics on Bacterial Community and Nutrient Status in Two Agricultural Soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Qiu, T.; Cui, Q.; Yang, Y.; Li, L.; Chen, J.; Huang, M.; Zhan, A.; Fang, L. Interaction of microplastics with heavy metals in soil: Mechanisms, influencing factors and biological effects. Sci. Total Environ. 2024, 918, 170281. [Google Scholar] [CrossRef]
- Shi, J.; Sun, Y.; Wang, X.; Wang, J. Microplastics reduce soil microbial network complexity and ecological deterministic selection. Environ. Microbiol. 2022, 24, 2157–2169. [Google Scholar] [CrossRef]
- Li, Y.; Qin, W.; Xin, X.; Tang, C.; Huang, Y.; He, X.; Chen, L.; Yu, G.; Yu, F. Dynamic impact of polyethylene terephthalate nanoplastics on antibiotic resistance and microplastics degradation genes in the rhizosphere of Oryza sativa L. J. Hazard. Mater. 2025, 487, 137173. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xu, G.; Wang, Y.; Yu, Y. Impacts of polyethylene microplastics on bioavailability and toxicity of metals in soil. Sci. Total Environ. 2021, 760, 144037. [Google Scholar] [CrossRef]
- Li, M.; Jia, H.; Gao, Q.; Han, S.; Yu, Y.; Sun, L. Influence of aged and pristine polyethylene microplastics on bioavailability of three heavy metals in soil: Toxic effects to earthworms (Eisenia fetida). Chemosphere 2023, 311, 136833. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, J.; Chen, X.; Dong, X.; Liu, S.; Anderson, C.W.N.; Zhou, M.; Gao, X.; Tang, X.; Zhao, D.; et al. Interactive effects of microplastics and cadmium on soil properties, microbial communities and bok choy growth. Sci. Total Environ. 2024, 955, 176831. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, G.; Yu, Y. Effects of polystyrene microplastics on accumulation of pyrene by earthworms. Chemosphere 2022, 296, 134059. [Google Scholar] [CrossRef]
- Xu, G.; Yang, Y.; Yu, Y. Size effects of polystyrene microplastics on the accumulation and toxicity of (semi-)metals in earthworms. Environ. Pollut. 2021, 291, 118194. [Google Scholar] [CrossRef]
- Tang, R.; Ying, M.; Luo, Y.; El-Naggar, A.; Palansooriya, K.N.; Sun, T.; Cao, Y.; Diao, Z.; Zhang, Y.; Lian, Y.; et al. Microplastic pollution destabilized the osmoregulatory metabolism but did not affect intestinal microbial biodiversity of earthworms in soil. Environ. Pollut. 2023, 320, 121020. [Google Scholar] [CrossRef]
- Liu, J.; Qin, J.; Zhu, L.; Zhu, K.; Liu, Z.; Jia, H.; Lichtfouse, E. The protective layer formed by soil particles on plastics decreases the toxicity of polystyrene microplastics to earthworms (Eisenia fetida). Environ. Int. 2022, 162, 107158. [Google Scholar] [CrossRef]
- Chen, K.; Tang, R.; Luo, Y.; Chen, Y.; Ei-Naggar, A.; Du, J.; Bu, A.; Yan, Y.; Lu, X.; Cai, Y.; et al. Transcriptomic and metabolic responses of earthworms to contaminated soil with polypropylene and polyethylene microplastics at environmentally relevant concentrations. J. Hazard. Mater. 2022, 427, 128176. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Impact of polyethylene microplastics and copper nanoparticles: Responses of soil microbiological properties and strawberry growth. Appl. Soil Ecol. 2023, 184, 104773. [Google Scholar] [CrossRef]
- Yu, H.; Liu, M.; Gang, D.; Peng, J.; Hu, C.; Qu, J. Polyethylene microplastics interfere with the nutrient cycle in water-plant-sediment systems. Water Res. 2022, 214, 118191. [Google Scholar] [CrossRef]
- Zou, Z.; Li, S.; Wu, J.; Guo, S.; Zhang, Y.; Huang, M.; Valsami-Jones, E.; Lynch, I.; Liu, X.; Wang, J.; et al. Effects of nanopolystyrene addition on nitrogen fertilizer fate, gaseous loss of N from the soil, and soil microbial community composition. J. Hazard. Mater. 2022, 438, 129509. [Google Scholar] [CrossRef]
- Mojiri, A.; Vishkaei, M.N.; Zhou, J.L.; Trzcinski, A.P.; Lou, Z.; Kasmuri, N.; Rezania, S.; Gholami, A.; Vakili, M.; Kazeroon, R.A. Impact of polystyrene microplastics on the growth and photosynthetic efficiency of diatom Chaetoceros neogracile. Mar. Environ. Res. 2024, 194, 106343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, N.; Li, Y.; Dou, S.; Liu, Z.; Chen, Y.; Ma, C.; Zhang, H. Responses of maize (Zea mays L.) seedlings growth and physiological traits triggered by polyvinyl chloride microplastics is dominated by soil available nitrogen. Ecotoxicol. Environ. Saf. 2023, 252, 114618. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, T.; Feng, Z.; Li, B.; Cai, Y.; Ouyang, D.; Gustave, W.; Ying, C.; Zhang, H. Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups. J. Hazard. Mater. 2023, 453, 131391. [Google Scholar] [CrossRef] [PubMed]
- Stile, N.; Raguso, C.; Pedruzzi, A.; Cetojevic, E.; Lasagni, M.; Sanchez-Vidal, A.; Saliu, F. Extraction of microplastic from marine sediments: A comparison between pressurized solvent extraction and density separation. Mar. Pollut. Bull. 2021, 168, 112436. [Google Scholar] [CrossRef]
- Halbach, M.; Baensch, C.; Dirksen, S.; Scholz-Böttcher, B.M. Microplastic extraction from sediments established?–A critical evaluation from a trace recovery experiment with a custom-made density separator. Anal. Methods 2021, 13, 5299–5308. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Yu, J.; Ni, F.; Sheng, Y.; Scircle, A.; Cizdziel, J.V.; Zhou, Y. Separation and identification of microplastics in marine organisms by TGA-FTIR-GC/MS: A case study of mussels from coastal China. Environ. Pollut. 2021, 272, 115946. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, P.; Zhang, R.; Qu, C.; Yuan, Y.; Hui, K.; Sun, J.; Tan, W. Separation and degradation techniques for microplastics in soil environments. J. Water Process Eng. 2025, 71, 107302. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, B. Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil. Sustainability 2023, 15, 1537. [Google Scholar] [CrossRef]
- Schütze, B.; Thomas, D.; Kraft, M.; Brunotte, J.; Kreuzig, R. Comparison of different salt solutions for density separation of conventional and biodegradable microplastic from solid sample matrices. Environ. Sci. Pollut. Res. 2022, 29, 81452–81467. [Google Scholar] [CrossRef]
- Ren, S.; Graf, M.; Wang, K.; Zhang, J.; Zhang, H.; Liu, X.; Li, J.; Zhu, T.; Ren, K.; Sun, Y.; et al. Separation and Identification of Conventional Microplastics from Farmland Soils. JoVE 2025, 217, e67064. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Zhang, M.; Vogt, R.D.; Lu, X. A commonly available and easily assembled device for extraction of bio/non-degradable microplastics from soil by flotation in NaBr solution. Sci. Total Environ. 2021, 759, 143482. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Pearce, C.I.; Sanguinet, K.A.; Bary, A.I.; Chowdhury, I.; Eggleston, I.; Xing, B.; Flury, M. Accumulation of microplastics in soil after long-term application of biosolids and atmospheric deposition. Sci. Total Environ. 2024, 912, 168883. [Google Scholar] [CrossRef] [PubMed]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leinio, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.-J.; Park, J.-W.; Jung, S.M. Repeatable separation of microplastics integrating mineral oil extraction and a PDMS-Ni foam adsorbent in real soil. Chem. Eng. J. 2022, 429, 132517. [Google Scholar] [CrossRef]
- Gupta, E.; Mishra, V.K.; Patel, A.; Srivastava, P.K. A modified methodology for extraction and quantification of microplastics in soil. NanoImpact 2024, 35, 100525. [Google Scholar] [CrossRef]
- Li, G.; Pei, Z.; Li, Y.; Yang, R.; Wang, P.; Liang, Y.; Zhang, J.; Zhang, Q.; Jiang, G. A high-precision, effective method for extraction and identification of small-sized microplastics from soil. Talanta 2024, 272, 125802. [Google Scholar] [CrossRef]
- Bottone, A.; Boily, J.F.; Shchukarev, A.; Andersson, P.; Klaminder, J. Sodium hypochlorite as an oxidizing agent for removal of soil organic matter before microplastics analyses. J. Environ. Qual. 2022, 51, 112–122. [Google Scholar] [CrossRef]
- Vermeiren, P.; Munoz, C.; Ikejima, K. Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Onandia, G.; Maaß, S.; Zhao, T.; Rillig, M.C. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. J. Appl. Ecol. 2021, 58, 988–996. [Google Scholar] [CrossRef]
- Liu, C.; Jiao, Y.; Yang, C.; Li, B.; Li, W.; Qian, T.; Liu, X. Interfacial interactions of submicron plastics with carbon dots: Insights into the interface properties of microplastic weathering. Water Res. 2025, 277, 123377. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Huang, Y.; Chen, Y.; He, X.; Chen, Z.; Lu, X.; Wu, M.; Wanger, T.C. Non-Destructive Extraction and Separation of Nano- and Microplastics from Environmental Samples by Density Gradient Ultracentrifugation. Anal. Chem. 2022, 94, 15280–15287. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, P.; Roth, C.; Wohlleben, W. The power of centrifugation: How to extract microplastics from soil with high recovery and matrix removal efficiency. MethodsX 2024, 12, 102598. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.F.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef]
- Ramage, S.; Pagaling, E.; Haghi, R.K.; Dawson, L.A.; Yates, K.; Prabhu, R.; Hillier, S.; Devalla, S. Rapid extraction of high- and low-density microplastics from soil using high-gradient magnetic separation. Sci. Total Environ. 2022, 831, 154912. [Google Scholar] [CrossRef]
- Wang, X.; Dan, Y.; Diao, Y.; Liu, F.; Wang, H.; Sang, W.; Zhang, Y. Transport characteristics of polystyrene microplastics in saturated porous media with biochar/Fe3O4-biochar under various chemical conditions. Sci. Total Environ. 2022, 847, 157576. [Google Scholar] [CrossRef]
- Ren, X.; Breadmore, M.C.; Maya, F. Magnetism-Assisted Density Gradient Separation of Microplastics. Anal. Chem. 2022, 94, 17947–17955. [Google Scholar] [CrossRef]
- Enders, K.; Tagg, A.S.; Labrenz, M. Evaluation of Electrostatic Separation of Microplastics From Mineral-Rich Environmental Samples. Front. Environ. Sci. 2020, 8, 112. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and the response of bacterial community in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Geng, Y.; Zhang, Y.; Gao, X.; Xu, J.; Liu, X. Integrating automated machine learning and metabolic reprogramming for the identification of microplastic in soil: A case study on soybean. J. Hazard. Mater. 2024, 478, 135555. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, Z.; Ye, C.; Zhang, Z.; Wang, J. A performance optimization method of multi-phase extraction for soil remediation based on multi-loops coordinated control. Environ. Eng. Res. 2025, 30, 163–179. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Santás-Miguel, V.; Rodríguez-Seijo, A. Current Methodology for Extraction, Separation, Identification, and Quantification of Microplastics in Terrestrial Systems. In Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2023; Volume 114, pp. 267–287. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).