Microplastic Pollution and Its Physiological Effects on the Top Fish Predator Dentex dentex from the Western Mediterranean
Abstract
1. Introduction
2. Materials and Methods
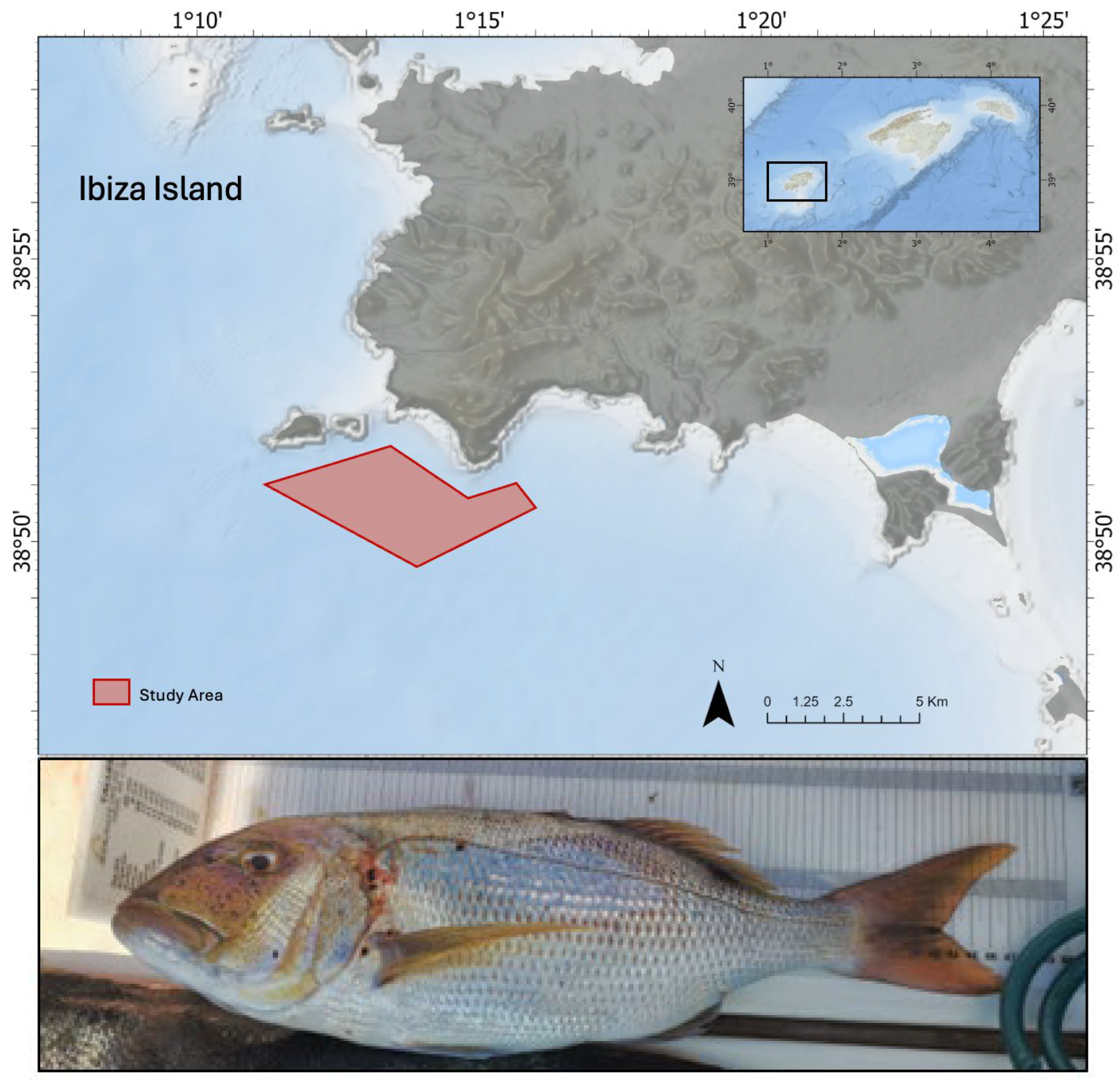
2.1. Study Area and Fish Sampling
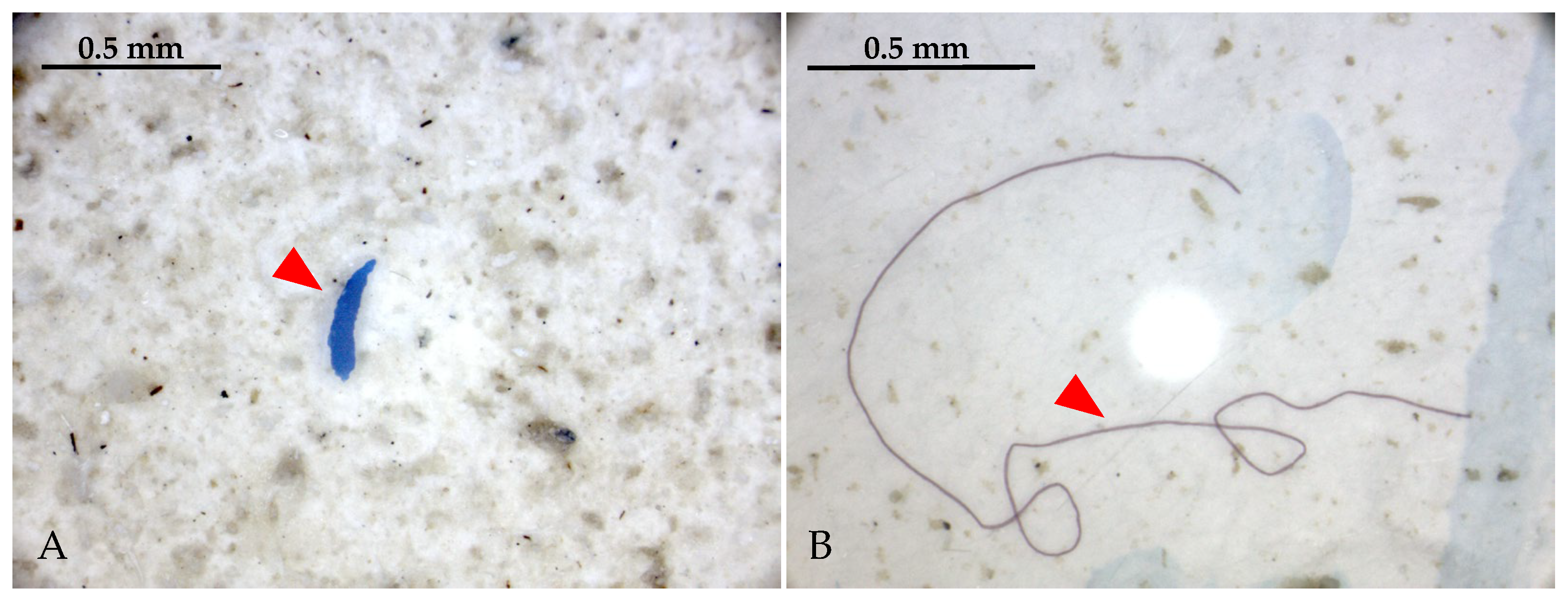
2.2. Microplastic Analysis
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Biometric Parameters
3.2. Microplastic Presence
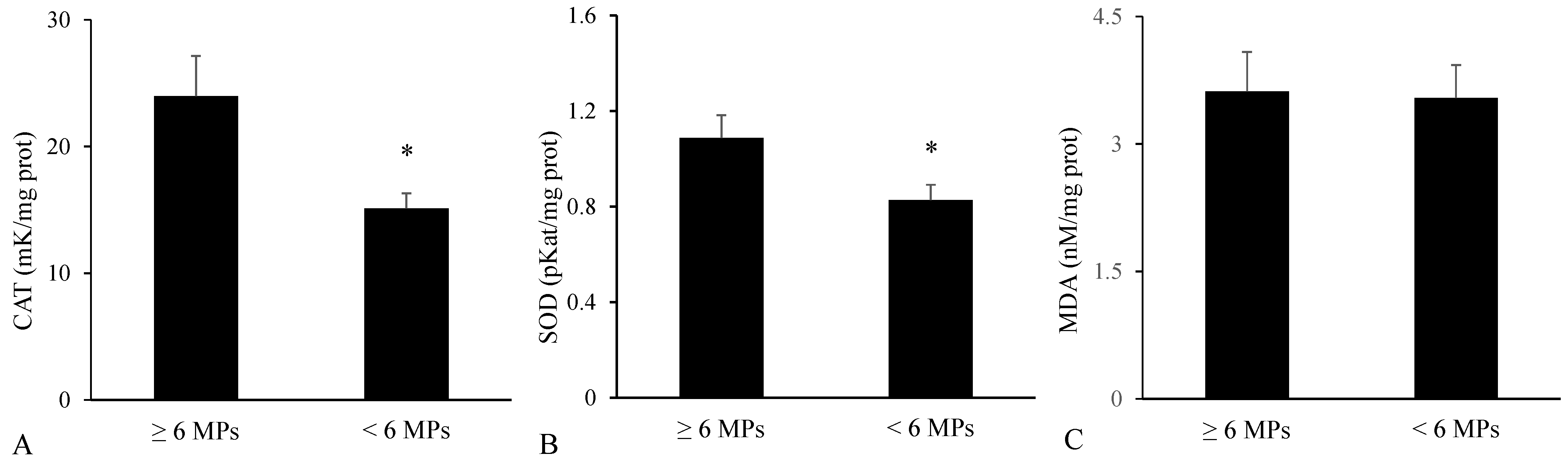
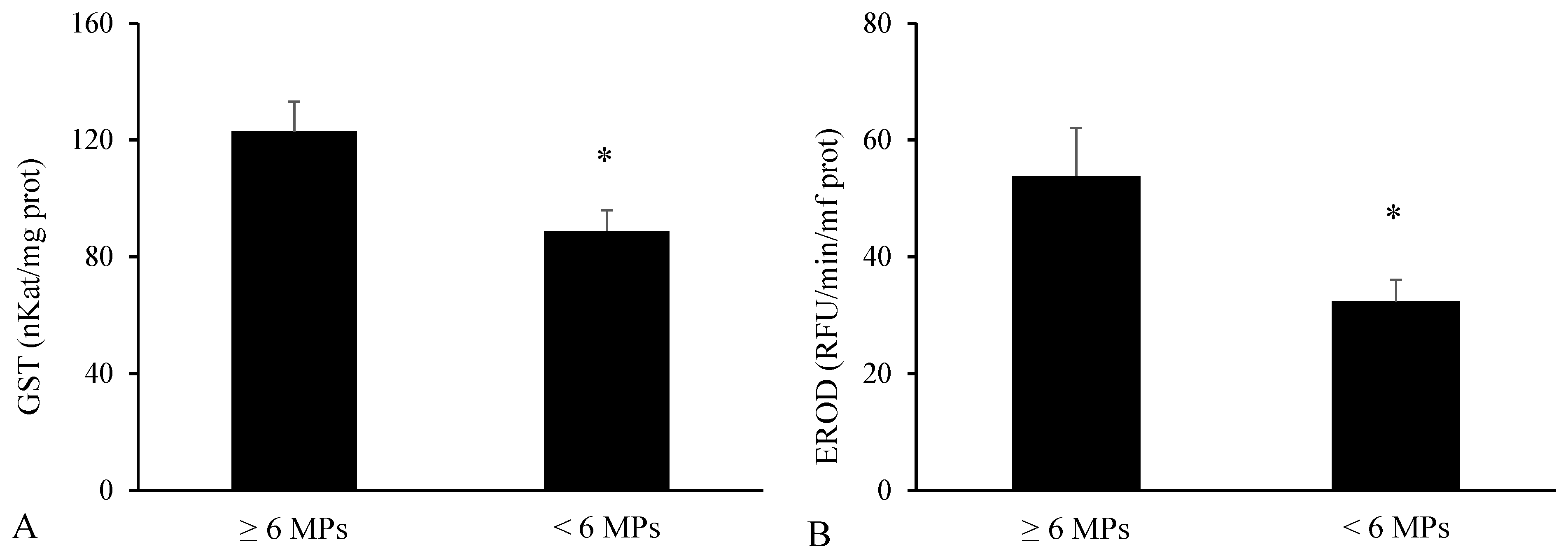
3.3. Biomarkers in the Digestive Tract
3.4. Biomarkers in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| EROD | Ethoxyresorufin-O-deethylase |
| GST | Glutathione s-transferase |
| MDA | Malondialdehyde |
| MP | Microplastic |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TL | Total length |
References
- Marcharla, E.; Vinayagam, S.; Gnanasekaran, L.; Soto-Moscoso, M.; Chen, W.-H.; Thanigaivel, S.; Ganesan, S. Microplastics in marine ecosystems: A comprehensive review of biological and ecological implications and its mitigation approach using nanotechnology for the sustainable environment. Environ. Res. 2024, 256, 119181. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Koelmans, A.A. Accumulation of Plastic Debris and Associated Contaminants in Aquatic Food Webs. Environ. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Compa, M.; Capó, X.; Alomar, C.; Deudero, S.; Sureda, A. A meta-analysis of potential biomarkers associated with microplastic ingestion in marine fish. Environ. Toxicol. Pharmacol. 2024, 107, 104414. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Akca, G.; Pekmez, T.; Kankılıç, G.B.; Çırak, T.; Çağan, A.S.; Kotiloğlu, S.Ö.; Grossart, H.-P. Increasing microplastics pollution: An emerging vector for potentially pathogenic bacteria in the environment. Water Res. 2025, 274, 123142. [Google Scholar] [CrossRef]
- Junaid, M.; Siddiqui, J.A.; Sadaf, M.; Liu, S.; Wang, J. Enrichment and dissemination of bacterial pathogens by microplastics in the aquatic environment. Sci. Total Environ. 2022, 830, 154720. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Sheng, Y.; Ye, X.; Zhou, Y.; Li, R. Microplastics (MPs) Act as Sources and Vector of Pollutants-Impact Hazards and Preventive Measures. Bull. Environ. Contam. Toxicol. 2021, 107, 722–729. [Google Scholar] [CrossRef]
- Yin, L.-Z.; Luo, X.-Q.; Li, J.-L.; Liu, Z.; Duan, L.; Deng, Q.-Q.; Chen, C.; Tang, S.; Li, W.-J.; Wang, P. Deciphering the pathogenic risks of microplastics as emerging particulate organic matter in aquatic ecosystem. J. Hazard. Mater. 2024, 474, 134728. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Setién, E.; Rios-Fuster, B.; Alomar, C.; Fagiano, V.; Sánchez-García, N.; Bernal-Mondejar, I.; Deudero, S. Floating microplastics along the western Mediterranean Sea: Are we reaching a “Good Environmental Status” or drifting away? Mar. Pollut. Bull. 2025, 211, 117372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- Rodríguez-Alcántara, J.S.; Cruz-Pérez, N.; Rodríguez-Martín, J.; García-Gil, A.; Santamarta, J.C. Effect of tourist activity on wastewater quality in selected wastewater treatment plants in the Balearic Islands (Spain). Environ. Sci. Pollut. Res. 2024, 31, 15172–15185. [Google Scholar] [CrossRef]
- Solomando, A.; Cohen-Sánchez, A.; Box, A.; Montero, I.; Pinya, S.; Sureda, A. Microplastic presence in the pelagic fish, Seriola dumerili, from Balearic Islands (Western Mediterranean), and assessment of oxidative stress and detoxification biomarkers in liver. Environ. Res. 2022, 212, 113369. [Google Scholar] [CrossRef]
- Cohen-Sánchez, A.; Solomando, A.; Pinya, S.; Tejada, S.; Valencia, J.M.; Box, A.; Sureda, A. First detection of microplastics in Xyrichtys novacula (Linnaeus 1758) digestive tract from Eivissa Island (Western Mediterranean). Environ. Sci. Pollut. Res. 2022, 29, 65077–65087. [Google Scholar] [CrossRef]
- Torres, S.; Compa, M.; Box, A.; Pinya, S.; Sureda, A. Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands. Fishes 2024, 9, 55. [Google Scholar] [CrossRef]
- Saleh, S.M.M.; Abdel-Zaher, S.; Mohamed, M.S.; Sayed, A.E.H. Microplastics induced ileum damage: Morphological and immunohistochemical study. Microsc. Res. Tech. 2025, 88, 251–269. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Banaee, M.; Multisanti, C.R.; Impellitteri, F.; Piccione, G.; Faggio, C. Environmental toxicology of microplastic particles on fish: A review. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2025, 287, 110042. [Google Scholar] [CrossRef]
- Capó, X.; Morató, M.; Alomar, C.; Rios-Fuster, B.; Valls, M.; Compa, M.; Deudero, S. A Biomarker Approach as Responses of Bioindicator Commercial Fish Species to Microplastic Ingestion: Assessing Tissue and Biochemical Relationships. Biology 2022, 11, 1634. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.; Rodil, R.; et al. Long-term exposure to virgin and seawater exposed microplastic enriched-diet causes liver oxidative stress and inflammation in gilthead seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, J.; Solomando, A.; Cohen-Sánchez, A.; Pinya, S.; Tejada, S.; Ferriol, P.; Mateu-Vicens, G.; Box, A.; Faggio, C.; Sureda, A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics. Int. J. Mol. Sci. 2022, 23, 9018. [Google Scholar] [CrossRef]
- Rueda, F.; Martínez, F. A review on the biology and potential aquaculture of Dentex dentex. Rev. Fish Biol. Fish. 2001, 11, 57–70. [Google Scholar] [CrossRef]
- Font, T.; Lloret, J. Biological implications of recreational shore angling and harvest in a marine reserve: The case of Cape Creus. Aquat. Conserv. 2011, 21, 210–217. [Google Scholar] [CrossRef]
- Chemmam-Abdelkader, B.; Ezzeddine-Najaî, S.; Kraiem, M. Etude de l’état du stock de Dentex dentex (Linnaeus, 1758) (Teleostei, Sparidae) des côtes sud tunisiennes. Bull. Inst. Natn Scien Tech. Tabarka 2007, 12, 55–59. [Google Scholar]
- Marengo, M.; Durieux, E.D.H.; Marchand, B.; Francour, P. A review of biology, fisheries and population structure of Dentex dentex (Sparidae). Rev. Fish Biol. Fish 2014, 24, 1065–1088. [Google Scholar] [CrossRef]
- Bauchot, M.L.; Hureau, J.C. Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nisen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; pp. 883–907. [Google Scholar]
- Morales-Nin, B.; Moranta, J. Life history and fishery of the common dentex (Dentex dentex) in Mallorca (Balearic Islands, western Mediterranean). Fish. Res. 1997, 30, 67–76. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. World Wide Web Electronic Publication. FishBase 2015. Available online: https://www.fishbase.se/manual/english/PDF/WELCOME_RFroese_etal2015.pdf (accessed on 3 March 2012).
- Bizsel, C.; Kara, M.H.; Pollard, D.; Yokes, B.; Goren, M.; Francour, P. Dentex dentex (Mediterranean assessment). IUCN Red List. Threat. Species 2011, 2011, e.T170245A6731474. [Google Scholar]
- Arbulú, I.; Rey-Maquieira, J.; Sastre, F. The impact of tourism and seasonality on different types of municipal solid waste (MSW) generation: The case of Ibiza. Heliyon 2024, 10, e33894. [Google Scholar] [CrossRef] [PubMed]
- Katircioglu, S.T. International tourism, energy consumption, and environmental pollution: The case of Turkey. Renew. Sustain. Energy Rev. 2014, 36, 180–187. [Google Scholar] [CrossRef]
- Wilson, S.P.; Verlis, K.M. The ugly face of tourism: Marine debris pollution linked to visitation in the southern Great Barrier Reef, Australia. Mar. Pollut. Bull. 2017, 117, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Espinosa Díaz, L.F.; Pereira Cardoso, R.; Costa Muniz, M. The impact of tourism on marine litter pollution on Santa Marta beaches, Colombian Caribbean. Mar. Pollut. Bull. 2020, 160, 111558. [Google Scholar] [CrossRef]
- Martinez-Ribes, L.; Basterretxea, G.; Palmer, M.; Tintoré, J. Origin and abundance of beach debris in the Balearic Islands. Sci. Mar. 2007, 71, 305–314. [Google Scholar] [CrossRef]
- Carreras, M.; Coll, M.; Quetglas, A.; Goñi, R.; Pastor, X.; Cornax, M.J.; Iglesia, M. Estimates of Total Fisheries Removals for the Balearic Islands 1950–2010; The University of British Columbia: Vancouver, BC, Canada, 2015. [Google Scholar]
- Klangnurak, W.; Chunniyom, S. Screening for microplastics in marine fish of Thailand: The accumulation of microplastics in the gastrointestinal tract of different foraging preferences. Environ. Sci. Pollut. Res. 2020, 27, 27161–27168. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assay. Methods Enzymol. 1984, 105, 93–104. [Google Scholar]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Burke, M.D.; Mayer, R. Ethoxyresorufin: Direct fluorimetric assay of a microsomal-O-deethylation which is preferencially inducible by 3-methylcholantrene. Drug Metab. Dispos. 1974, 2, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Fast Facts 2023; Plastics Europe: Bruxelles, Belgium, 2023. [Google Scholar]
- Jansen, M.A.K.; Barnes, P.W.; Bornman, J.F.; Rose, K.C.; Madronich, S.; White, C.C.; Zepp, R.G.; Andrady, A.L. The Montreal Protocol and the fate of environmental plastic debris. Photochem. Photobiol. Sci. 2023, 22, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, B.S. Review of microplastic distribution, toxicity, analysis methods, and removal technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Fagiano, V.; Alomar, C.; Compa, M.; Soto-Navarro, J.; Jordá, G.; Deudero, S. Neustonic microplastics and zooplankton in coastal waters of Cabrera Marine Protected Area (Western Mediterranean Sea). Sci. Total Environ. 2022, 804, 150120. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic ingestion and diet composition of planktivorous fish. Limnol. Oceanogr. Lett. 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Fagiano, V.; Compa, M.; Alomar, C.; Rios-Fuster, B.; Morató, M.; Capó, X.; Deudero, S. Breaking the paradigm: Marine sediments hold two-fold microplastics than sea surface waters and are dominated by fibers. Sci. Total Environ. 2023, 858, 159722. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F. Marine debris review for Latin America and the Wider Caribbean Region: From the 1970s until now, and where do we go from here? Mar. Pollut. Bull. 2007, 54, 1087–1104. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Bayo, J.; Rojo, D.; Martínez-Baños, P.; López-Castellanos, J.; Olmos, S. Commercial gilthead seabream (Sparus aurata L.) from the mar menor coastal lagoon as hotspots of microplastic accumulation in the digestive system. Int. J. Environ. Res. Public Health 2021, 18, 6844. [Google Scholar] [CrossRef] [PubMed]
- Iveša, N.; Turković, D.; Jelenović, R.; Zanchi, E.; Markić, A.; Buršić, M.; Pustijanac, E.; Kovačić, I.; Burić, P.; Paliaga, P. Prevalence of Fibers as the Dominant Microplastic Fraction in the Digestive Tract of Three Commercially Important Fish Species (Sparus aurata Linneaeus 1758, Pagellus erythrinus Linneaeus 1758 and Chelon auratus Risso, 1810) from the Southeastern Coast of Istria, Northern Adriatic, Croatia. Nase More 2024, 71, 131–138. [Google Scholar] [CrossRef]
- Hamed, M.; Martyniuk, C.J.; Lee, J.S.; Shi, H.; Sayed, A.E.D.H. Distribution, abundance, and composition of microplastics in market fishes from the Red and Mediterranean seas in Egypt. J. Sea Res. 2023, 194, 102407. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Nadal, M.; Alomar, C.; Deudero, S. High levels of microplastic ingestion by the semipelagic fish bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016, 214, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- López-Martínez, S.; Morales-Caselles, C.; Kadar, J.; Rivas, M.L. Overview of global status of plastic presence in marine vertebrates. Glob. Change Biol. 2021, 27, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wu, J.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Accumulation of microplastics in fish guts and gills from a large natural lake: Selective or non-selective? Environ. Pollut. 2022, 309, 119785. [Google Scholar] [CrossRef]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Bi, L.; Liu, Y.; Jin, L.; Peng, R. Immunotoxicity of microplastics in fish. Fish Shellfish Immunol. 2024, 150, 109619. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, F.; Lama, A.; Monnolo, A.; Pirozzi, C.; Piccolo, G.; Vozzo, S.; De Biase, D.; Riccio, L.; Fusco, G.; Mercogliano, R.; et al. Subchronic Exposure to Polystyrene Microplastic Differently Affects Redox Balance in the Anterior and Posterior Intestine of Sparus aurata. Animals 2023, 13, 606. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.H. Adsorption of environmental contaminants on micro- and nano-scale plastic polymers and the influence of weathering processes on their adsorptive attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 82728. [Google Scholar] [CrossRef]
- Fröhlich, E. Local and systemic effects of microplastic particles through cell damage, release of chemicals and drugs, dysbiosis, and interference with the absorption of nutrients. J. Toxicol. Environ. Health Part B 2024, 27, 315–344. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Hoyo-Alvarez, E.; Arechavala-Lopez, P.; Jiménez-García, M.; Solomando, A.; Alomar, C.; Sureda, A.; Moranta, D.; Deudero, S. Effects of pollutants and microplastics ingestion on oxidative stress and monoaminergic activity of seabream brains. Aquat. Toxicol. 2022, 242, 106048. [Google Scholar] [CrossRef]
- Pei, X.; Heng, X.; Chu, W. Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microb. Pathog. 2022, 163, 105387. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; De la Vieja, A.; de Alba Gonzalez, M.; Esteban Lopez, M.; Castaño Calvo, A.; Cañas Portilla, A.I. Toxicity of nanoplastics for zebrafish embryos, what we know and where to go next. Sci. Total Environ. 2021, 797, 149125. [Google Scholar] [CrossRef] [PubMed]

| Total Number of Fish | Fish with MPs | MPs/Ind. (Mean ± S.E.M.) (Range) | MPs Identified Fibres/Fragments | Fibres/Fragments per Ind. (Range) | Frequencies (%) Fibres/Fragments |
|---|---|---|---|---|---|
| 22 | 20 | 6.6 ± 1.2 (0–20) | 116/31 | 5.2 ± 0.9/1.4 ± 0.4 (0–17)/(0–8) | 78.9/21.1 |
| CAT (mK/mg) | SOD (pKat/mg) | MDA (nM/mg) | GST (nKat/mg) | EROD (RFU/min/prot) | |
|---|---|---|---|---|---|
| ≥6 MPs | 27.5 ± 3.8 | 0.81 ± 0.06 | 1.46 ± 0.07 | 120.4 ± 13.2 | 67.2 ± 8.35 |
| <6 MPs | 23.1 ± 2.9 | 0.77 ± 0.06 | 1.42 ± 0.12 | 101.7 ± 10.1 | 61.0 ± 10.0 |
| p value | p = 0.188 | p = 0.321 | p = 0.416 | p = 137 | p = 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen-Sánchez, A.; Solomando, A.; Compa, M.; Box, A.; Montero, I.; Tejada, S.; Pinya, S.; Quetglas-Llabrés, M.M.; Sureda, A. Microplastic Pollution and Its Physiological Effects on the Top Fish Predator Dentex dentex from the Western Mediterranean. Microplastics 2025, 4, 28. https://doi.org/10.3390/microplastics4020028
Cohen-Sánchez A, Solomando A, Compa M, Box A, Montero I, Tejada S, Pinya S, Quetglas-Llabrés MM, Sureda A. Microplastic Pollution and Its Physiological Effects on the Top Fish Predator Dentex dentex from the Western Mediterranean. Microplastics. 2025; 4(2):28. https://doi.org/10.3390/microplastics4020028
Chicago/Turabian StyleCohen-Sánchez, Amanda, Antònia Solomando, Montserrat Compa, Antonio Box, Inmaculada Montero, Silvia Tejada, Samuel Pinya, Maria Magdalena Quetglas-Llabrés, and Antoni Sureda. 2025. "Microplastic Pollution and Its Physiological Effects on the Top Fish Predator Dentex dentex from the Western Mediterranean" Microplastics 4, no. 2: 28. https://doi.org/10.3390/microplastics4020028
APA StyleCohen-Sánchez, A., Solomando, A., Compa, M., Box, A., Montero, I., Tejada, S., Pinya, S., Quetglas-Llabrés, M. M., & Sureda, A. (2025). Microplastic Pollution and Its Physiological Effects on the Top Fish Predator Dentex dentex from the Western Mediterranean. Microplastics, 4(2), 28. https://doi.org/10.3390/microplastics4020028









