Abstract
The increase in the human population has created pressure, due to the high consumption of natural resources, to meet basic needs. Poor waste management resulting from human activities has caused plastics to become pollutants that are present around the planet, including aquatic environments. The degradation of plastics through physicochemical processes has resulted in the presence of microplastics (particles < 5 mm), which have been found in species for human consumption and economic importance, including tilapia. In the last decade, research has shown the presence of microplastics in tilapia collected from different water bodies and aquaculture ponds, as well as in fish markets. In addition to this, there are studies that demonstrate that exposure to microplastics can have negative effects on the health of tilapia. The aim of this review is to compile and analyze the available information on microplastic contamination in Oreochromis spp., as well as in their environment, due to their importance as a species for human consumption.
1. Introduction
The increase in the world’s population and the poor management of waste have caused pressure on natural resources in recent years, as well as their pollution around the planet, including aquatic environments. Water, as a limited resource, is seriously affected due to the variety of contaminants that are directly or indirectly present in it. In recent decades, one of the pollutants that has been found to be present in all environments is plastic. Plastic is a material distributed worldwide due to its great utility, which ranges from household, industrial, medical and technological objects. Upon reaching their short but useful life cycle, they are mostly discarded without proper management. Once inside the different environmental matrices, they can degrade into small fragments called microplastics (less than 5 mm) through various physical, chemical and biological processes [1].
Microplastics (MPs) can be transported by the wind to the atmosphere and, from there, reach the soil and water bodies by being washed away by rain, thus entering the water indirectly, or, on the other hand, due to their manufacture for use in cosmetic and cleaning products or as waste from the manufacture of plastic products, where they can enter directly [2]. In this way, MPs are present in water bodies, including coastal areas used for aquaculture crops [3].
On the other hand, several studies agree that MPs function as vectors for other pollutants, such as persistent organic compounds and metals, facilitating their ingestion by aquatic organisms and climbing the food chain [4,5,6]. This could affect various species of crustaceans, bivalves and fish of commercial importance worldwide, as their presence has been proved around the world, and it has been concluded that the quality and quantity of aquaculture production could be affected due to the presence of MPs [3,7,8].
Among the fish produced for human consumption, tilapia stands out as a species of great economic importance worldwide. According to the FAO, in 2020, in continental aquaculture, 4407.2 thousand tons (live weight) of Oreochromis niloticus and 1069.9 of Oreochromis spp. were produced, while in marine and coastal aquaculture, 107.4 thousand tons (live weight) of O. niloticus was reported [9].
2. From Plastics to Microplastics
Plastics are synthetic organic polymers derived from fossil fuels that share the properties of elasticity and flexibility at certain temperatures, allowing them to be molded and adapted to various shapes [1]. Their lightness, durability, corrosion resistance and low cost have led to their domestic and worldwide use as industrial, medical and technological products [10]. By 2021, 390.7 million tons of plastics were produced, and 90.2% of them were based on fossil fuels, while only 8.3% came from recycling and 1.5% from bioplastics [11]. The main types of plastics produced today are polypropylene (PP; 19.3%), low-density polyethylene (PE-LD; 14.4%), polyvinyl chloride (PVC; 12.9%), high-density (PE HD) and medium-density (PE MD) polyethylene, both with 12.5%, polyethylene terephthalate (PET; 6.2%), polyurethane (PUR; 5.5%) and polystyrene (PS; 5.3%) [11]. Upon completing their useful life cycle, which is often short, plastic products are discarded, most of them without proper handling. Once inside the different environmental matrices, through various physical, chemical and biological processes, plastics are fractionated, and, by definition, when they reach a size less than 5 mm, they become MPs [1].
Microplastics can be classified as primary and secondary according to their origin. Primary MPs are manufactured in microscopic sizes and are commonly used in cosmetic products, medicine and detergents; they are also usually byproducts of the manufacture of other plastic products. Secondary MPs are the product of the degradation of larger plastics via physical, chemical and biological processes [1,12]. Another way to classify MPs is according to their shape: fragments, fibers or filaments, spheres, foams and pellets [13].
MPs can directly or indirectly enter water bodies, including coastal areas used for aquaculture crops [2,3], within which tools are used that can represent other sources of input of MPs to the water and, therefore, to the cultured organisms [13,14,15,16]. MPs have been studied since the 70s; however, since the 2000s, their study has gained greater relevance due to their impact on the environment [17].
3. Microplastics in Freshwater Systems
Environmental conditions within aquatic ecosystems, such as currents, solar radiation and interactions with organisms, cause plastic waste to slowly degrade and fragment into smaller particles [18]. MPs are present in freshwater bodies worldwide in remote lakes, estuaries and rivers [17,19]. Human population centers, especially those dedicated to industry, are the main contributors to the dispersion of plastic pollutants. The poor management of waste generated on land causes runoff, storm drains and wastewater to turn rivers into a method of transportation for plastics [17,20]. Wastewater discharges contain primary and secondary MPs. The distribution of MPs in freshwater bodies is associated with anthropogenic activities, so their concentration is influenced by the proximity of human populations and the number of inhabitants; the size of the water body and physical forces involved, such as wind and water currents, also have an influence [19]. MPs have higher rates of degradation, transportation and distribution in environmental compartments than larger plastics. Another important factor to consider is the absorption of other contaminants, since these affect mobility and bioavailability. Consequently, biota will be exposed to a complex mix of plastics and plastic-associated chemicals that change over time and space [17].
4. Tilapia
The cultivation of tilapia dates back approximately 4000 years. The genus Oreochromis belongs to cichlids, whose ideal temperature range is 20 to 30 °C. They are omnivores, feeding in their natural environment on phytoplankton, periphyton, aquatic plants, small invertebrates, benthic fauna, waste and bacterial layers associated with detritus. O. niloticus can live more than 10 years and reach a weight of 5 kg [21].
In culture, they reach sexual maturity between 5 and 6 months of age. Different growing techniques are used to produce tilapia, which have different characteristics and requirements; they can be grown in ponds, floating cages and recirculation systems. They eat balanced diets that provide complete nutrition (adequate levels of protein, lipids, carbohydrates, vitamins and minerals), with the main ingredients being balanced foods, such as soy or fish meal [21]. It must be considered that the fish meals used to feed farmed species, since they are mostly made with organisms from pelagic regions, areas where there is a greater accumulation of MPs, represent a direct source of input of MPs to culture ponds [14]. Anthropogenic polymer particles, such as polyamide, polyester, polyethylene, polypropylene and polystyrene, have been identified in both fish meal and soy meal [15].
Harvesting is carried out via capture with plastic seine nets in combination with drainage. To maximize production, partial harvests are usually carried out using sorters with graduated separators to capture the largest fish [21]. Currently, tilapia farming represents 88% of the total production [22].
Microplastics in Tilapia
In recent years, studies on the effects and accumulation of MPs in aquatic organisms have gained relevance [23]; however, most studies have focused on marine organisms, and fewer works focus on freshwater organisms [17].
Tilapia stands out among the fish species used for human consumption for its protein content, in addition to being an aquaculture product that is easy to handle and grow. Unfortunately, it has been shown that it is also susceptible to the ingestion of MPs and, therefore, to their accumulation (Figure 1) because it is an omnivorous species that usually feeds on zooplankton, phytoplankton and suspended matter. There are contradictory studies about the effects that ingesting microplastics can have on aquatic organisms and, in turn, the damage that their consumption can cause to human health. Some authors mention that MPs are accumulated and stored only in the digestive tract of organisms [13], so only the excessive consumption of some marine products (some mollusks) can be considered a considerable source of MPs for the humans [24]. There is research that shows the opposite; for example, a trial of O. niloticus exposure to polyethylene revealed its bioaccumulation of MPs in the blood, gills, gonads, intestines, kidneys, stomach and muscle [25].

Figure 1.
Routes of entry of MPs to tilapia in water bodies.
Despite the importance of tilapia in the world economy and human nutrition, research regarding the presence of MPs in this organism does not go beyond a decade; however, the presence of MPs has been recorded in fish from the genus Oreochromis around the world (Table 1).

Table 1.
Presence and concentration of MPs (microplastics) in the Oreochromis spp. in different studies. * Data are sorted from the highest- to lowest-reported abundance. GIT = gastrointestinal tract, FTIR= Fourier transform infrared spectroscopy, ATR-FTIR = attenuated total reflectance Fourier transform infrared, EVA = ethylene–vinyl acetate, HDPE = high-density polyethylene, LDPE = low-density polyethylene, PS = polystyrene, NY = nylon, PA = polyamide, PE = polyethylene, PES = polyester, PET = polyethylene terephthalate, PMMA = poly methyl methacrylate, PP = polypropylene, PP = polypropylene, PU = polyurethane, PVA = polyvinyl acetate, PVC = polyvinyl chloride, RY = rayon, and SC = synthetic cellulose.
5. Materials and Methods
This review considers peer-reviewed scientific literature and official government websites to present the state of the art regarding microplastic pollution in fish of the genus Oreochromis due to its commercial importance. A search of the literature was conducted using Google, Google Scholar, Elsevier-Science Direct Online, Redalyc and the Web of Science, utilizing the following keywords: microplastic*, pollution*, freshwater*, oreochromis* and aquaculture*. Additionally, an analysis of the reference database generated in Zotero was performed using the Research Rabbit application to find related bibliographies. Publications from the last 5 years were prioritized and were thoroughly reviewed to obtain the location, Oreochromis species, type of water body in which they were raised and analyzed organs, as well as the microplastic concentrations and classifications made by the authors. Finally, a bibliometric analysis was conducted using Vosviewer 1.6.20.
6. Results
6.1. Bibliometric Analysis
After searching and analyzing the retrieved articles, only those reporting the presence of microplastics in the organs and tissues of Oreochromis spp. were considered due to the limited information reported in the last 5 years. The year 2015 was established as the starting point for the reporting of these contaminants in this genus, with increased attention beginning in 2020 (Figure 2).
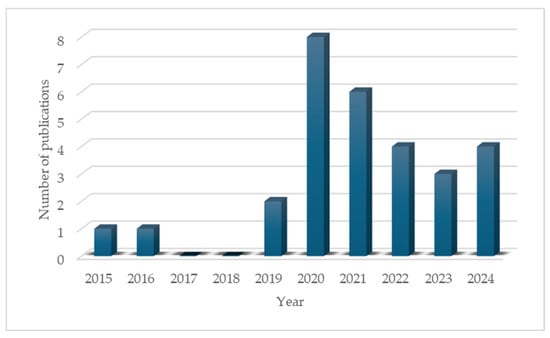
Figure 2.
Number of research articles reporting the presence of microplastics in Oreochromis spp. over the last decade.
The analysis of the keywords assigned by the authors indicates that “microplastics” is the most frequently used term, being primarily associated with the matrices in which the contaminants are found, namely “sediments” and “water”, which are further linked to “fish”. Additionally, it is observed that microplastic contamination is directly correlated with its ingestion by O. niloticus (Figure 3).
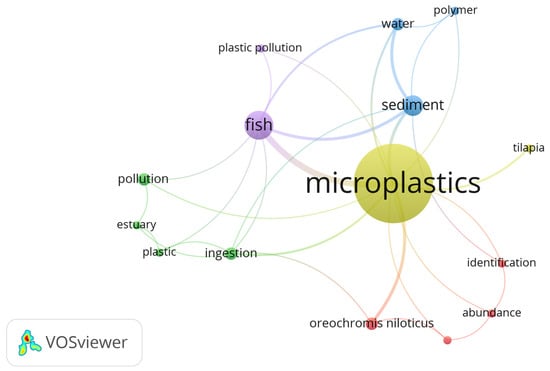
Figure 3.
Results of the bibliometric analysis based on keywords conducted with VOSviewer.
Most of the studies reported in this document were conducted in Asia, followed by Africa. To a lesser extent, studies have been recorded in the Americas, primarily in the southern region of the continent, and, finally, a single study has been reported in Oceania (Figure 4).
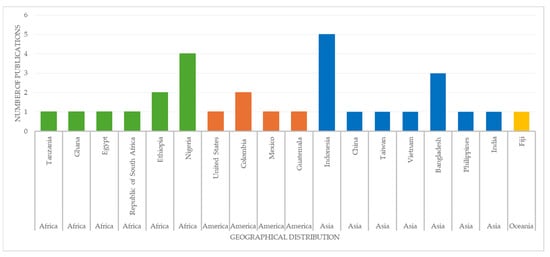
Figure 4.
Geographical distribution of studies reporting the presence of microplastics in Oreochromis spp. over the last decade.
6.2. Concentrations of Microplastics in Oreochromis spp.
Most of the tilapia for human consumption is obtained from aquaculture [9]; however, until now, only 12% of the authors cited in this work carried out sampling in aquaculture ponds, and most of the monitoring was performed in natural water bodies (Figure 5).
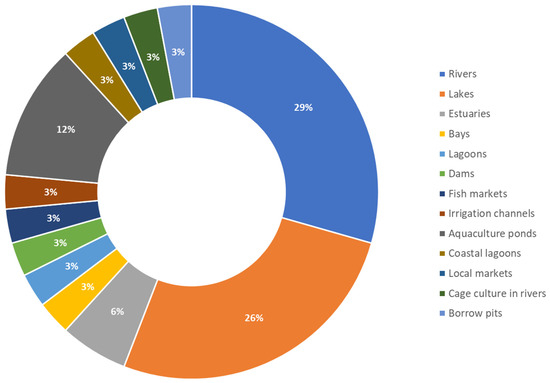
Figure 5.
Systems where the presence of MPs has been identified in fish of the genus Oreochromis.
Most of the samples carried out belong to countries on the Asian continent, and in all the studies, the presence of MPs in tilapia was detected in a greater or lesser proportion and/or concentration. In Indonesia, sampling carried out in the Pelayaran River showed 100% positivity for the presence of MPs in all the O. niloticus samples collected, reaching up to 970 particles/individual in the gastrointestinal tract (GIT) [39], while in the Surabaya River, the maximum value recorded was 303.59 MPs/individual in the gills and 265.71 MPs/individual in the GIT [39], and Ikhtiar et al., in 2020 [36], found the presence of MPs in O. mossambicus in the Tallo River. In the Fengshan River, Taiwan, O. niloticus had a concentration of 46 MPs/fish [37], while in the Philippines, in the Pasig River, at two sampling points near densely populated areas, abundances of 0.3 and 0.08 MPs/individual of O. niloticus were found [49]. Regarding Asian lakes, similar quantities were reported in the GIT of O. mossambicus and O. niloticus in the lakes Dhanmondi in Bangladesh and Towuti in Indonesia, respectively [43,48]. Similarly, the occurrence of MPs has been reported in the GIT, gills and muscle of O. mossambicus in lakes of India [50]. When analyzing commercial fish species in Jakarta, Indonesia, MPs were found in the GIT of O. mossambicus [28]. Pollution by MPs in Chinese mariculture shows accumulation in the GIT and gills of Oreochromis spp. [29]. Other works that show the presence of MPs in tilapia for human consumption are the samples obtained by Parvin et al., in 2021 [42], from a fish market in Dhaka, Bangladesh, in an analysis of several species of fish, and the authors found that O. mossambicus had an average concentration of three MPs/individual, similar to what Mai et al. [46] reported, who, in 2022, in tilapia from markets in the Bac Ninh Province of Vietnam, found MPs in the intestines of O. niloticus; in addition to this, they also sampled fish from cages and culture ponds, showing the same result. It should be noted that in the organisms collected from markets, the concentrations are below the average reported in the rest of the studies consulted; however, the presence of microplastics is reported in 100% of the fish, which could mean that transport from the place where they are obtained and their handling for sale may be influencing the increase in contamination, and although the authors did not analyze edible tissue, other works have reported that MPs can lodge in the muscle. Finally, a recent study indicates the presence of MPs in culture ponds of O. niloticus in the Bay of Bengal, Bangladesh [32,53].
In the African continent, most of the studies carried out have been on the species O. niloticus. In the Nile River of Egypt, around 75% of the tilapia sampled presented MPs in the GIT [32]. In Tanzania, sampling in Lake Victoria, 35% of the O. niloticus specimens analyzed contained MPs in the intestinal tract [27]. In Ethiopia, in Lake Ziway, 22% of the tilapias presented MPs in the GIT [33], while in Lake Hawassa of Sidama, the presence of MPs was found in 86.66% of the collected individuals [51]. In Nigeria, in Lake Eleyele, the presence of MPs was reported in 34% of the fish analyzed [30]; meanwhile, other studies conducted in the Nigerian rivers, specifically those of Benin City and Ondo State, demonstrated the presence of MPs in the GIT of Nile tilapia [53,54]. In Ghana, O. niloticus from the Accra-Tema lagoon presented a concentration of 2.10 ± 1.73 MPs/individual in the GIT [31]. Regarding the species O. mossambicus in the KwaZulu-Natal estuary in the Republic of South Africa, Naidoo et al., in 2020, reported a concentration of 0.41 ± 0.57 particles in the intestine [34].
On the American continent, as in Africa, O. niloticus continues to be the most studied tilapia species. In Guatemala, in Lake Amatitlan, 65 specimens of O. niloticus contained MPs (9.71 MPs/individual) [41]. In Mexico, in the Atoyac River, Puebla, of a total of 15 tilapia of the species O. niloticus, 53.67% showed a prevalence of MPs [40]. In Colombia, the species was sampled in aquaculture ponds and in the Bache River, finding that in ponds, about half of the organisms had the presence of MPs in contrast to the 75% presence in fish from the natural environment [35]. Also in Colombia, in the area of Cienega Grande de Santa Marta, a coastal lagoon system, a sample of 63 tilapias presented an average of 14 MPs per organism in the GIT [45], while Phillips and Bonner, in 2015, in a study that involved several species of fish, made one of the first reports of MPs in the genus Oreochromis in the species of O. aureus [26].
And finally, on the island of Viti Levu, Fiji, in Oceania, in a study carried out in culture ponds, the presence of MPs was found in 20% of the O. niloticus individuals sampled [44].
As can be seen in the studies cited in this article, there is no standard regarding the way of reporting research results. The authors express their findings in percentages of MPs present in the sampled organisms, the number of MPs/individual and the particles/g, and others report the maximum figures found, which makes it difficult to make a reliable comparison in terms of the concentrations of MPs in fish. Furthermore, there are variations in the methodologies for the quantification and extraction of MPs; an example is the use of rose Bengal staining to rule out particles initially identified as MPs that are parasites [31], which, according to the authors, significantly changes the proportions of concentrations and colors [16]. Almost all studies conducted include organic matter degradation before proceeding to observation with stereoscopic microscopes. The most used chemical compounds for digestion are KOH and H2O2, with NaOH being used to a lesser extent. In this analysis, it was observed that the average concentration of MPs in most works is reported as MPs/individual, and the average value is around 6.6, except for two studies carried out in Indonesian rivers, whose concentrations are above 100 MPs/individual. (Table 1). In the same way, the results of predominant MPs usually vary, and the classification includes at least some of the following categories: type, size, shape and color. The evaluated aspect in which there seems to be greater consensus is in the organs of the fish where the MPs are evaluated, where almost 80% consider the GIT as an object of study. The study is accompanied by the analysis of the gills, while only 7.1% report that the intestine was analyzed, and only 3.6% consider the edible part. The articles that have evaluated the GIT and gills together report different proportions of concentration and/or diversity of MPs between both organs [29,35,42]. It is important to highlight that although the muscle is the most consumed part of the fish, it is also the least analyzed part, and the little information available is that bioaccumulation is lower compared to the gills and GIT [33,40].
Regarding the types of polymers found, it can be observed that polyethylene is the most frequently and abundantly mentioned in the samples collected in different regions of the world, being reported as the most abundant (38.46% of the works consulted), followed by rayon and polyethylene terephthalate; PES is also frequently reported, although in a lesser proportion (Figure 6). The characterization of polymer types is predominantly carried out using FTIR (Table 1). No relationship is observed with the type of polymer and countries in which the studies have been carried out; however, when relating the type of polymer with the body of water where the fish samples were collected, it can be observed that polystyrene was located in rivers and lakes, polyethylene terephthalate was reported in samples from aquaculture ponds, and rayon was obtained in bodies of water closer to coasts, such as estuaries and bays.
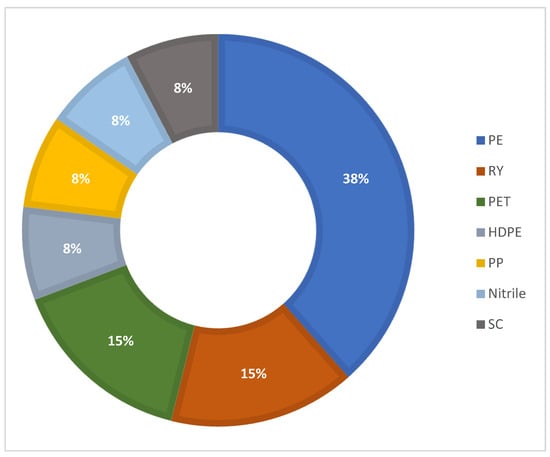
Figure 6.
Most abundant polymer type in Oreochromis samples collected from different water bodies.
In the last decade, some scientists have begun to focus on the effects that MPs can have on tilapia exposed to these contaminants in laboratory conditions. There are studies where the presence and bioaccumulation of MPs in the blood, gills, gonads, intestines, kidney, stomach and muscle of O. niloticus have been documented, showing potential risks to human health [25,55,56].
It has been reported that MP exposure of O. niloticus for 9 weeks through diet decreases the growth and activity of digestive enzymes, as well as causes an increase in pathogenic bacteria in the intestine [57]. In contrast, a recent study that evaluates the impacts of exposure of O. niloticus to polystyrene within a biofloc culture found that this relationship significantly increased the hepato-somatic index, reducing crude protein and lipids in muscle, without finding an effect on the growth. The order of polystyrene accumulation for the organs analyzed is as follows: liver, gills and intestine; however, the exposure time was only 28 days [58].
It has been shown that exposure for only 15 days of O. niloticus juveniles to MPs (1 mg/L, 10 mg/L and 100 mg/L) can induce the overproduction of reactive oxygen, altering antioxidant parameters, which can result in oxidative stress and DNA damage [59], as well as histopathological lesions in the kidneys, liver, pancreas, muscle, gills and intestine, damage that, after a clearance period of 15 days, shows that some lesions can be reversed [60]; in addition, it was shown that the damage caused by MPs is dose dependent [61]. However, a study carried out on Oreochromis urolepis larvae with an exposure time of 65 days and a clearance period of 60 days demonstrates that MPs cause degeneration in the wall of the small intestine with a slow recovery and may affect digestive functions, growth and reproduction [61].
In addition to causing damage directly, the presence of MPs can enhance the damage of other contaminants present in the aquatic environment. Exposure in laboratory conditions of O. niloticus to MPs together with copper increases its potential to cause damage to fish (alters the intestinal microbiota and causes histopathological damage in the liver, intestine and gills), contributes to greater bioaccumulation and, consequently, represents a greater risk to human health [5]. Similarly, the co-exposure of O. niloticus to MPs and roxithromycin (ROX) has shown that MPs function as a vehicle to transfer it to aquatic organisms, increasing its bioaccumulation in the digestive tract, gills, brain and liver, in addition to altering the antioxidant enzyme response, affecting drug toxicity [6]. Also, the degree of aging of MPs can change, increasing the degree of adsorption of sulfamethoxazole in the gills of O. niloticus [62].
There are environmental factors that can increase the intake of MPs and their toxicity in tilapia. In a trial involving exposure to polyamide under different temperatures (30, 33 and 36 °C), the fish at the highest temperature showed a greater intake of the contaminant [63].
Although some of the damage due to exposure to MPs can be reversible to a certain extent after periods of purification [64], factors such as diet can influence the reduction in damage. The supplementation of O. niloticus with the diatom Amphora coffeaeformis improves detoxification and prevents testicular damage caused by exposure to MPs [65].
7. Future Perspectives
Among the 2030 sustainable development goals, there is the prevention and reduction of pollution, as well as protecting aquatic ecosystems and seeking to understand and mitigate the repercussions of MPs on aquatic animals and human health [9]. It is important to consider that the problem of pollution by MPs in the current context of the global increase in temperature may be even more worrying than is thought due to the relationship between the increase in the intake of these pollutants and the increase in temperature [63].
Currently, global actions to face the plastic problem are based on action plans to implement a circular economy, thereby minimizing plastic waste production and promoting recycling and reuse [64,66]. Several countries have taken measures to ban cosmetic and hygiene products that contain MPs, including the United States [67], France [68], Belgium, Canada [69], England [70] and Sweden [71]; this represents an important step toward the initial regulation of MPs.
There is poor regulation of these contaminants within aquatic systems in countries like Mexico, which does not even consider MPs as a problem within its official standard NOM-127-SSA1-2021, which regulates the permissible limits and treatments to which tap water must be subjected to for human use and consumption [72], and with regard to measures taken to control their intake through the consumption of aquatic products, the Codex Alimentarius does not yet consider standards or codes that contemplate reference values or precautionary measures regarding the handling of fish and fishery products [73]. On the contrary, the European Union has regulations for the use of this material and its contact with food [74].
8. Conclusions
Tilapia has great commercial importance; however, few studies have focused on monitoring the presence of MPs in these organisms directly in aquaculture crops.
After the analysis of the data collected for this document, which includes studies conducted over the past decade, it was observed that the presence of microplastics has been reported in Oreochromis spp. specimens at percentages ranging from 22% to 100%. This should constitute a matter of significant concern not only for researchers but also for producers and consumers.
Moreover, a significant number of the studies referenced in this document were conducted in countries that are global leaders in tilapia production. In Asia, which accounts for 45.8% of the research, China, Indonesia and Bangladesh stand out, as they are among the top five tilapia producers in the region. Africa is the second region with the highest number of studies, with Egypt, Nigeria and Ghana occupying the first, second and fourth positions in aquaculture production on the continent. In the Americas, reports about the presence of microplastics in tilapia originate from Mexico and Colombia, which hold the fifth and sixth positions in aquaculture production, respectively. Finally, in Oceania, Fiji, ranked fifth in aquaculture production, has also documented the presence of microplastics in Oreochromis spp.
Another crucial aspect to highlight is that most of the fish analyzed in these studies originated from water bodies such as rivers or lakes. Given that most of the tilapia consumed globally is sourced from aquaculture, conducting further studies directly on aquaculture farms is of vital importance to prevent economic losses and ensure production safety.
Furthermore, although only a limited number of studies have examined tilapia muscle to assess the presence of MPs, researchers have detected these particles in this edible tissue. It is essential to conduct further research to evaluate the presence of MPs in this important species for human consumption, particularly in muscle tissue, to assess the potential risk of contamination for the end consumers of tilapia.
On the other hand, it is observed that the methods for the extraction and identification of MPs have not yet been standardized. However, polyethylene was the most often identified polymer in the analyzed organisms, as it is one of the most common and widely used plastics worldwide, and studies have shown its potential toxicological effects.
It is of utmost importance for environmental, economic and food safety reasons that more studies be conducted on the presence and consequences of MPs within aquaculture, considering all sources of input, the effects on organisms, the impact on the economy and the health of consumers. This is particularly critical for species of global importance, such as Oreochromis spp.
Author Contributions
D.G.M.-L.: conceptualization, investigation, resources, writing—original draft, writing—review and editing and visualization. M.d.R.C.-C.: conceptualization, investigation, resources, writing—review and editing, visualization and supervision. L.M.-C.: review and editing and translation. E.F.C.-M.: investigation and review and editing. F.L.-R.: conceptualization, investigation, resources, writing—original draft, writing—review and editing, visualization, supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We acknowledge the Instituto Tecnológico Nacional de México/Instituto Tecnológico de Boca del Río (TecNM/ITBOCA) and the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CO-NAHCYT) for the posdoctoral fellowship (CVU: 418944).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, G.; Xu, K.; Huang, K.; Wang, J. Microplastics Environmental Effect and Risk Assessment on the Aquaculture Systems from South China. Int. J. Environ. Res. Public Health 2021, 18, 1869. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Ruiz-Fernández, A.C.; Frías-Espericueta, M.G.; Rivera-Hernández, J.R.; Green-Ruiz, C.R.; Páez-Osuna, F. Microplastics in the Tissues of Commercial Semi-Intensive Shrimp Pond-Farmed Litopenaeus vannamei from the Gulf of California Ecoregion. Chemosphere 2022, 297, 134194. [Google Scholar] [CrossRef]
- Zhang, F.; Li, D.; Yang, Y.; Zhang, H.; Zhu, J.; Liu, J.; Bu, X.; Li, E.; Qin, J.; Yu, N.; et al. Combined Effects of Polystyrene Microplastics and Copper on Antioxidant Capacity, Immune Response and Intestinal Microbiota of Nile Tilapia (Oreochromis niloticus). Sci. Total Environ. 2022, 808, 152099. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Razanajatovo, R.M.; Jiang, H.; Zou, H.; Zhu, W. Interactive Effects of Polystyrene Microplastics and Roxithromycin on Bioaccumulation and Biochemical Status in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 648, 1431–1439. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Xie, S.; Chen, Y.; Li, X.; Wang, J.; Zou, J. Microplastics and Their Potential Effects on the Aquaculture Systems: A Critical Review. Rev. Aquac. 2021, 13, 719–733. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and Ecological Impact of Microplastics in Aquaculture Ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de La Pesca y La Acuicultura 2022. Hacia La Transformación Azul.; FAO: Rome, Italy, 2022; ISBN 978-92-5-136464-2. [Google Scholar]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Plastics Europe Plastics—The Facts 2023 • Plastics Europe. 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 4 April 2025).
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.C.H.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; ISBN 978-92-5-109882-0. [Google Scholar]
- Mahamud, A.G.M.S.U.; Anu, M.S.; Baroi, A.; Datta, A.; Khan, M.S.U.; Rahman, M.; Tabassum, T.; Tanwi, J.T.; Rahman, T. Microplastics in Fishmeal: A Threatening Issue for Sustainable Aquaculture and Human Health. Aquac. Rep. 2022, 25, 101205. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and Characterisation of Microplastics and Microfibres in Fishmeal and Soybean Meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hou, J.; Wang, X. A Review of Microplastic Pollution in Aquaculture: Sources, Effects, Removal Strategies and Prospects. Ecotoxicol. Environ. Saf. 2023, 252, 114567. [Google Scholar] [CrossRef] [PubMed]
- Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2018; Volume 58, ISBN 978-3-319-61614-8. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Elías, R. Mar del plástico: Una revisión del plástico en el mar. In Plastic Sea: A Review of Plastic at Sea; Cornell Lab Publishing Group: New York, NY, USA, 2015. [Google Scholar]
- FAO. Oreochromis Niloticus; Cultured Aquatic Species Fact Sheets; FAO: Rome, Italy, 2009. [Google Scholar]
- FAO. El Estado Mundial de la Pesca y la Acuicultura 2020. La Sostenibilidad En Acción; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; ISBN 978-92-5-132756-2. [Google Scholar]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of Microplastics in Typical Commercial Aquatic Species: A Case Study at a Productive Aquaculture Site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef]
- COFI. Los Microplásticos en la Pesca y la Acuicultura: Resumen de un Estudio de la FAO Comité de Pesca. 33 ° Periodo de Sesiones; FAO: Roma, Italy, 2018. [Google Scholar]
- Aryani, D.; Khalifa, M.A.; Herjayanto, M.; Solahudin, E.A.; Rizki, E.M.; Halwatiyah, W.; Istiqomah, H.; Maharani, S.H.; Wahyudin, H.; Pratama, G. Penetration of Microplastics (Polyethylene) to Several Organs of Nile Tilapia (Oreochromis niloticus). IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012061. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and Amount of Microplastic Ingested by Fishes in Watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First Evidence of Microplastics in the African Great Lakes: Recovery from Lake Victoria Nile Perch and Nile Tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Hastuti, A.R.; Lumbanbatu, D.T.; Wardiatno, Y. The Presence of Microplastics in the Digestive Tract of Commercial Fishes off Pantai Indah Kapuk Coast, Jakarta, Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 1233–1242. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Li, Y.; Tan, S.; Kang, Z.; Yu, X.; Lan, W.; Cai, L.; Wang, J.; Shi, H. Microplastic Pollution in the Maowei Sea, a Typical Mariculture Bay of China. Sci. Total Environ. 2019, 658, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.O.; Ibor, O.R.; Khan, E.A.; Chukwuka, A.V.; Omogbemi, E.D.; Arukwe, A. Detection and Occurrence of Microplastics in the Stomach of Commercial Fish Species from a Municipal Water Supply Lake in Southwestern Nigeria. Env. Sci. Pollut. Res. 2020, 27, 31035–31045. [Google Scholar] [CrossRef] [PubMed]
- Gbogbo, F.; Takyi, J.B.; Billah, M.K.; Ewool, J. Analysis of Microplastics in Wetland Samples from Coastal Ghana Using the Rose Bengal Stain. Env. Monit Assess 2020, 192, 208. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Shashoua, Y.; Crawford, A.; Drury, A.; Sheppard, K.; Stewart, K.; Sculthorp, T. ‘The Plastic Nile’: First Evidence of Microplastic Contamination in Fish from the Nile River (Cairo, Egypt). Toxics 2020, 8, 22. [Google Scholar] [CrossRef]
- Merga, L.B.; Redondo-Hasselerharm, P.E.; Van den Brink, P.J.; Koelmans, A.A. Distribution of Microplastic and Small Macroplastic Particles across Four Fish Species and Sediment in an African Lake. Sci. Total Environ. 2020, 741, 140527. [Google Scholar] [CrossRef]
- Naidoo, T.; Sershen; Thompson, R.C.; Rajkaran, A. Quantification and Characterisation of Microplastics Ingested by Selected Juvenile Fish Species Associated with Mangroves in KwaZulu-Natal, South Africa. Environ. Pollut. 2020, 257, 113635. [Google Scholar] [CrossRef]
- Garcia, A.G.; Suárez, D.C.; Li, J.; Rotchell, J.M. A Comparison of Microplastic Contamination in Freshwater Fish from Natural and Farmed Sources. Env. Sci. Pollut. Res. 2020, 28, 14488–14497. [Google Scholar] [CrossRef]
- Ikhtiar, M.; Baharuddin, A.; Habo Abbas, H. Identification of Microplastic in Tilapia Fish (Oreochromis mossambicus) at Tallo River in Macassart. Int. J. Healthc. Res. 2020, 5, 406–411. [Google Scholar]
- Tien, C.-J.; Wang, Z.-X.; Chen, C.S. Microplastics in Water, Sediment and Fish from the Fengshan River System: Relationship to Aquatic Factors and Accumulation of Polycyclic Aromatic Hydrocarbons by Fish. Environ. Pollut. 2020, 265, 114962. [Google Scholar] [CrossRef]
- Al-Fatih, A.N.F. Identifikasi Mikroplastik Pada Sistem Pencernaan Ikan Nila (Oreochromis niloticus) Di Kali Pelayaran Kabupaten Sidoarjo. Environ. Pollut. J. 2021, 1, 237–244. [Google Scholar] [CrossRef]
- Lestari, P.; Trihadiningrum, Y.; Firdaus, M.; Warmadewanthi, I.D.A.A. Microplastic Pollution in Surabaya River Water and Aquatic Biota, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012054. [Google Scholar] [CrossRef]
- Martinez-Tavera, E.; Duarte-Moro, A.M.; Sujitha, S.B.; Rodriguez-Espinosa, P.F.; Rosano-Ortega, G.; Expósito, N. Microplastics and Metal Burdens in Freshwater Tilapia (Oreochromis niloticus) of a Metropolitan Reservoir in Central Mexico: Potential Threats for Human Health. Chemosphere 2021, 266, 128968. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Hernández, B.E.; Santos-Ruiz, F.M.; Muñoz-Wug, M.A.; Pérez-Sabino, J.F. Microplastics in Nile Tilapia (Oreochromis niloticus) from Lake Amatitlán. Rev. Ambient. Água 2021, 16, e2754. [Google Scholar] [CrossRef]
- Parvin, F.; Jannat, S.; Tareq, S.M. Abundance, Characteristics and Variation of Microplastics in Different Freshwater Fish Species from Bangladesh. Sci. Total Environ. 2021, 784, 147137. [Google Scholar] [CrossRef]
- Yusuf, M.; Tahir, A. Study of The Abundance and Characteristics of Microplastic Contamination in The Fish of Capture Results of Fishermen in The Lake Towuti Waters, East Luwu, South Sulawesi. Int. J. Sci. Res. Publ. 2021, 11, 699–704. [Google Scholar] [CrossRef]
- Dehm, J.; Volau, M.; Ledua, E.; Hewavitharane, C. Occurrence of Microplastics within a Freshwater Aquaculture System in the Pacific Islands, Viti Levu, Fiji. Environ. Monit Assess 2022, 194, 624. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Saldarriaga-Vélez, J.F.; Espinosa-Díaz, L.F.; Patiño, A.D.; Cusba, J.; Canals, M.; Mejía-Esquivia, K.; Fragozo-Velásquez, L.; Sáenz-Arias, S.; Córdoba-Meza, T.; et al. Microplastic Pollution in Water, Sediments and Commercial Fish Species from Ciénaga Grande de Santa Marta Lagoon Complex, Colombian Caribbean. Sci. Total Environ. 2022, 829, 154643. [Google Scholar] [CrossRef]
- Mai, H.; Thu-Huong, H.T.; Ngo, T.T.N.; Bui, T.T.U.; Dao, T.D.; Bui, V.H.; Nguyen, D.T.; Vu, T.K.; Chu, N.H.A. Baseline Assessment of Microplastic Concentration in Different Freshwater Bodies and in Local Fish of Bac Ninh Province. Vietnam J. Sci. Technol. 2022, 60, 63–72. [Google Scholar] [CrossRef]
- Sani, A.; Darma, A.I.; Abba Diso, F. Microplastics Profile in Fishes from Selected Burrow Pits: A Case of Plastic Pollution in Kano Metropolis, Nigeria. Environ. Forensics 2022, 25, 81–91. [Google Scholar] [CrossRef]
- Mercy, F.T.; Alam, A.K.M.R.; Akbor, M.d.A. Abundance and Characteristics of Microplastics in Major Urban Lakes of Dhaka, Bangladesh. Heliyon 2023, 9, e14587. [Google Scholar] [CrossRef]
- Espiritu, E.Q.; Rodolfo, R.S.; Evangelista, S.M.J.; Feliciano, J.J.G.; Sumaway, A.M.N.; Pauco, J.L.R.; Alvarez, K.V.N.; Enriquez, E.P. Microplastics Contamination in the Fishes of Selected Sites in Pasig River and Marikina River in the Philippines. Mar. Pollut. Bull. 2023, 187, 114573. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, K.; Thangal, S.H.; Yogeshwaran, A.; Kaaran, S.; Ajith Kumar, T.T.; Muralisankar, T. Microplastics Contamination in the Edible Fish Mozambique Tilapia (Oreochromis mossambicus) from the Selvampathy Wetland of Coimbatore, Tamil Nadu, India. Bull. Environ. Contam. Toxicol. 2023, 112, 7. [Google Scholar] [CrossRef] [PubMed]
- Demsie, A.F.; Yimer, G.T. Occurrence of Microplastics in Commercial Fish Species from the Ethiopian Rift Valley’s Lake Hawassa, Ethiopia. Environ. Sci. Pollut. Res. 2024, 31, 48641–48649. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.M.; Hossain, I.; Sunji, M.d.M.R.; Tahsin, T.; Walker, T.R.; Rahman, M.S. Characterization, Source Identification and Hazard Index Assessment of Ingested Microplastics in Farmed Tilapia Oreochromis niloticus. Ecol. Indic. 2024, 158, 111334. [Google Scholar] [CrossRef]
- Wangboje, O.M.; Adeghor, K.A. Baseline Investigation of Microplastic Levels in the Nile Tilapia (Oreochromis niloticus, Linnaeus, 1758) from River Okhuo, Benin City, Nigeria. Eur. J. Sci. Innov. Technol. 2024, 4, 335–345. [Google Scholar]
- Olanipekun, O.; Samuel, O.O.; Kazeem, G.O. Investigation of Microplastics Contamination in African Catfish Clarias Gariepinus and Nile Tilapia Oreochromis niloticus Fish Species in Owe River Ile-Oluji, Ondo State, Nigeria. J. Appl. Life Sci. Int. 2024, 27, 29–46. [Google Scholar]
- Aryani, D.; Khalifa, M.A.; Herjayanto, M.; Pratama, G.; Rahmawati, A.; Putra, R.D.; Munandar, E. Correlation of Water Quality with Microplastic Exposure Prevalence in Tilapia (Oreochromis niloticus). E3S Web Conf. 2021, 324, 03008. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, J.-X.; Zhang, L.; Wu, D.; Tian, J.; Yu, L.-J.; He, L.; Zhong, S.; Du, H.; Deng, D.-F.; et al. Comprehensive Understanding the Impacts of Dietary Exposure to Polyethylene Microplastics on Genetically Improved Farmed Tilapia (Oreochromis niloticus): Tracking from Growth, Microbiota, Metabolism to Gene Expressions. Sci. Total Environ. 2022, 841, 156571. [Google Scholar] [CrossRef]
- Hu, X.; Meng, L.-J.; Liu, H.-D.; Guo, Y.-S.; Liu, W.-C.; Tan, H.-X.; Luo, G.-Z. Impacts of Nile Tilapia (Oreochromis niloticus) Exposed to Microplastics in Bioflocs System. Sci. Total Environ. 2023, 901, 165921. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Antioxidants and Molecular Damage in Nile Tilapia (Oreochromis niloticus) after Exposure to Microplastics. Environ. Sci. Pollut. Res. 2020, 27, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Badrey, A.E.A.; Osman, A.G.M. Microplastics Induced Histopathological Lesions in Some Tissues of Tilapia (Oreochromis niloticus) Early Juveniles. Tissue Cell 2021, 71, 101512. [Google Scholar] [CrossRef] [PubMed]
- Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Kimaro, W.; Joseph, D.; Khan, F.R. Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis Urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics 2022, 1, 240–253. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive Effects of Microplastics and Selected Pharmaceuticals on Red Tilapia: Role of Microplastic Aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Hasan, J.; Siddik, M.A.; Ghosh, A.K.; Mesbah, S.B.; Sadat, M.A.; Shahjahan, M. Increase in Temperature Increases Ingestion and Toxicity of Polyamide Microplastics in Nile Tilapia. Chemosphere 2023, 327, 138502. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the Effect of Long-Term Exposure to Microplastics and Depuration Period in Sparus aurata Linnaeus, 1758: Liver and Blood Biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Ismail, R.F.; Saleh, N.E.; Sayed, A.E.-D.H. Impacts of Microplastics on Reproductive Performance of Male Tilapia (Oreochromis niloticus) Pre-Fed on Amphora Coffeaeformis. Environ. Sci. Pollut. Res. 2021, 28, 68732–68744. [Google Scholar] [CrossRef]
- Ley 7/2022, de 8 de Abril, de Residuos y Suelos Contaminados Para Una Economía Circular, Pub. L. No. Ley 7/2022, BOE-A-2022-5809 48578. 2022. Available online: https://www.boe.es/eli/es/l/2022/04/08/7 (accessed on 18 September 2023).
- U.S. Government Text—H.R.1321—114th Congress (2015-2016): Microbead-Free Waters Act of 2015. Available online: https://www.congress.gov/bill/114th-congress/house-bill/1321/text (accessed on 18 September 2023).
- Légifrance. Journal officiel “Lois et Décrets” JORF n° 0057 du 8 mars 2017. JORF N° 0057 Du 8 Mars 2017—Légifrance. 2017. Available online: https://www.legifrance.gouv.fr/jorf/jo/2017/03/08/0057 (accessed on 20 September 2024).
- Minister of Justice, L.S. Consolidated Federal Laws of Canada, Microbeads in Toiletries Regulations. Available online: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2017-111/index.html (accessed on 18 September 2023).
- UK Legislation. The Environmental Protection (Microbeads) (England) Regulations 2017. King’s Printer of Acts of Parliament. 2017. Available online: https://www.legislation.gov.uk/ukdsi/2017/9780111162118 (accessed on 20 September 2023).
- Löfven, S. Statement of Government Policy 10 September 2019. Regeringen och Regeringskansliet. 2019. Available online: https://www.government.se/speeches/20192/09/statement-of-government-policy-10-september-2019/ (accessed on 18 September 2023).
- NORMA Oficial Mexicana NOM-127-SSA1-2021, Agua Para Uso y Consumo Humano. Límites Permisibles de La Calidad Del Agua., NOM-127-SSA1-2021. 2023. Available online: https://sidof.segob.gob.mx/notas/5650705 (accessed on 18 September 2023).
- CODEXALIMENTARIUS FAO-WHO Normas Conexas. Available online: https://www.fao.org/fao-who-codexalimentarius/committees/committee-detail/related-standards/es/?committee=CCFFP (accessed on 18 September 2023).
- UNION EUROPEA. DOUE-L-2022-81372 Reglamento (UE) 2022/1616 de la Comisión de 15 de septiembre de 2022 relativo a los materiales y objetos de plástico reciclado destinados a entrar en contacto con alimentos y por el que se deroga el Reglamento (CE) no. 282/2008. 2022. Available online: https://www.boe.es/doue/2022/243/L00003-00046.pdf (accessed on 18 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
