Microplastics in Farmed Animals—A Review
Abstract
1. Introduction
2. Plastic Products—Trailing Micro- and Nanoplastic Particles
2.1. Plastics
2.2. Global Production of Plastics
3. Different Forms of Microplastic Materials in the Environment
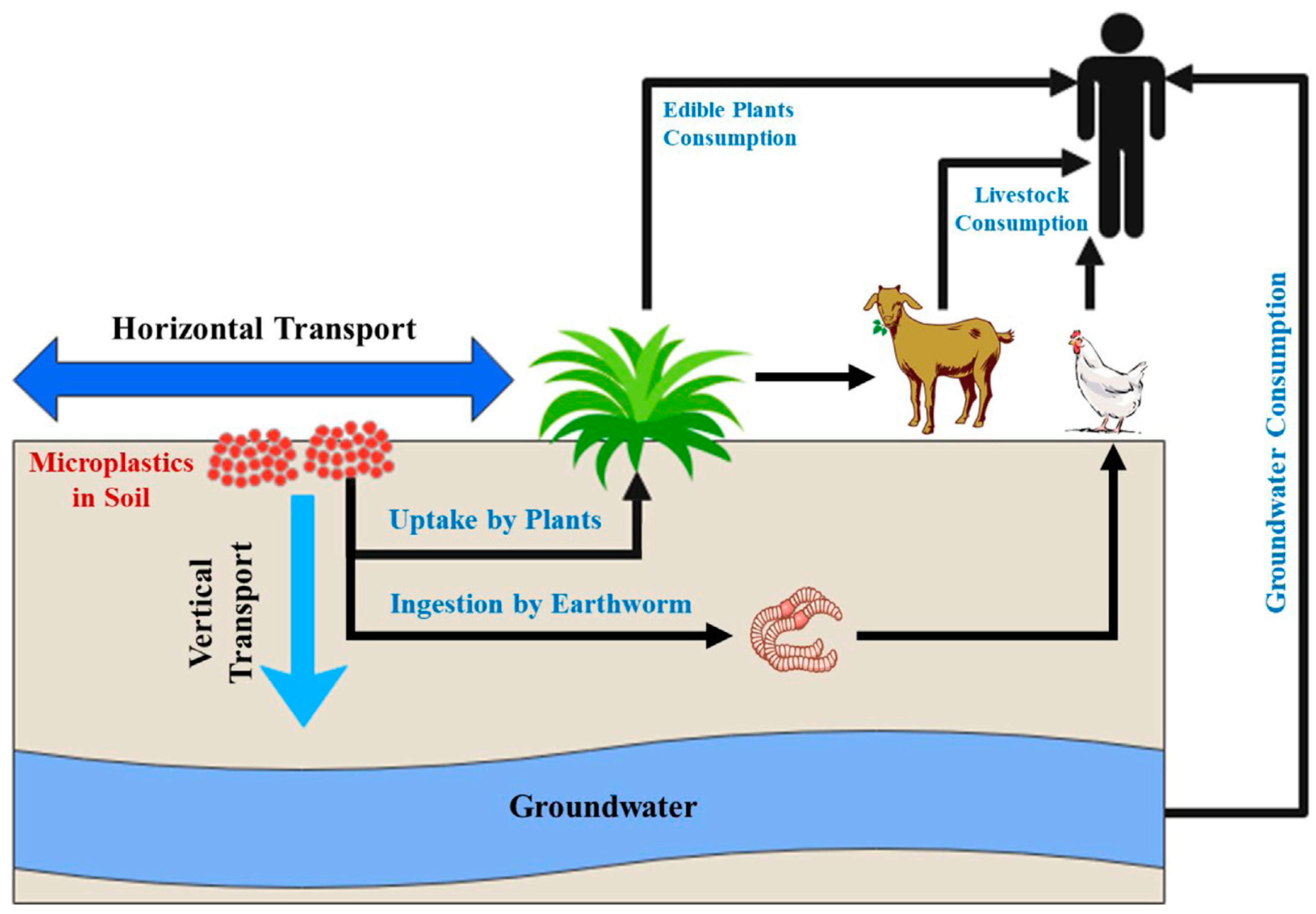
3.1. Microplastics
3.2. Sources of Microplastics
3.3. Chemical Effects of Plastics on Organisms
3.4. Dangers and Diseases Caused by Microplastics
3.5. Animal Health Affected by Microplastics
| Microplastic Type | Animal | Physiological Damage | Source |
|---|---|---|---|
| PS | Nematode (Caenorhabditis elegans) | Intestinal injury, oxidative stress | [104] |
| PA, PE, PP, PVC | Zebra fish (Dania rerio) | Intestinal damage | [105] |
| PS | Ascidian ciona Intestinalis | Decreased growth and food uptake | [106] |
| PET, PA | Sardinella gibbose (Fish) | Feeding habit, decreased body weight | [107] |
| PE | Emys orbicularis (Pond turtle) | Liver and kidney disease | [108] |
| PS | Crepidula onyx (Mollusca) | Decrease growth | [48] |
| PS | Rattus (Rat) | Alteration of the serum triglyceride and cholesterol concentrations | [109] |
| MP | Mus musculus (Mouse) | Increase in SOD (superoxide dismutase) and MDA (malondialdehyde) contents | [110] |
| MPs and NPs | Poultry | NPs could be transported into the embryo and ac- cumulate in the yolk sac, leading to alterations in nutrient absorption | [111] |
| BPA | Female mouse | Impaired cytoskeletal dynamics in the oocyte, induction of oxidative stress, increased DNA damage and epigenetic alterations in oocytes | [112] |
| BPA | Cattle | Increased apoptotic gene expression in bovine oocytes | [113] |
| BPA | Cattle | Decreased likelihood of mature oocytes | [114] |
| BPA | Chickens | Increased embryo mortality and the malformation of reproductive organs | [115] |
| monoethylhexyl phthalate | Cattle | Inhibits the meiotic maturation of oocytes | [116] |
| BPA | Mice, pigs, cattle | Abnormalities in meiosis, spindle fibers and congenital defects in mice, pigs, cattle and humans | [117,118] |
3.6. Consumption of Microplastics by Marine Organisms
3.7. The Effect of Microplastics on Marine Organisms
3.8. Physical Effects
3.9. Chemical Effects
3.10. Effects of Microplastics on Farmed Animals—Poultry and Ruminants
3.11. Transmission of Microplastics to Humans and Their Possible Effects
3.12. Legal Framework
3.13. Solutions to Deal with Microplastics
3.14. Strategies to Reduce Microplastic Freight in Farmed Animals
3.15. Technological Solutions against MPs and NPs
3.16. Behavioral Aspects
3.16.1. Specific Policy Recommendations
3.16.2. Recommendations for Future Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hidayaturrahman, H.; Lee, T.G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Microplastics in the marine environment: Sources, consequences and solutions. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 185–200. [Google Scholar]
- Karuna Society, The Plastic Cow Project–Karuna Society for Animals and Nature. 2012. Available online: https://karunasociety.org/the-plastic-cow-project (accessed on 14 July 2024).
- Egger, M.; Sulu-Gambari, F.; Lebreton, L. First evidence of plastic fallout from the North Pacifc Garbage Patch. Sci. Rep. 2020, 10, 7495. [Google Scholar] [CrossRef] [PubMed]
- Chang, C. Wasted Humans and Garbage Animals: Deadly Transcorporeality and Documentary Activism. In Ecodocumentaries; Alex, R.K., Deborah, S.S., Eds.; Palgrave Macmillan: London, UK, 2016. [Google Scholar] [CrossRef]
- Aitchison, J.; Aitchison, R.; Devas, F. Assessing the environmental impacts of wildlife television Programmes. People Nat. 2021, 3, 1138–1146. [Google Scholar] [CrossRef]
- Corte Pause, F.; Urli, S.; Crociati, M.; Stradaioli, G.; Baufeld, A. Connecting the Dots: Livestock Animals as Missing Links in the Chain of Microplastic Contamination and Human Health. Animals 2024, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.; Hardesty, B.D.; Law, K.L. Abundance of floating plastic particles is increasing in the Western North Atlantic Ocean. Environ. Sci. Technol. 2019, 54, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Reisser, J.; Cunsolo, S.; Kwadijk, C.; Kotterman, M.; Proietti, M.; Koelmans, A.A. Pollutants in plastics within the north Pacific subtropical gyre. Environ. Sci. Technol. 2018, 52, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.O.; Anani, O.A. Plastic-Eating Microorganisms: Recent Biotechnological Techniques for Recycling of Plastic. In Microbial Rejuvenation of Polluted Environment. Microorganisms for Sustainability; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; Volume 25. [Google Scholar] [CrossRef]
- WWF. Plastics: The Costs to Society, the Environment and the Economy. 2021. Available online: https://media.wwf.no/assets/attachments/Plastics-the-cost-to-society-the-environment-and-the-economy-WWF-report.pdf (accessed on 27 April 2024).
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2023, 20, 789. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded milks–Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Barboza LG, A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Zhao, X.; You, F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ. Sci. Technol. 2024, 58, 8709–8723. [Google Scholar] [CrossRef]
- Danso, I.K.; Woo, J.-H.; Lee, K. Pulmonary Toxicity of Polystyrene, Polypropylene, and Polyvinyl Chloride Microplastics in Mice. Molecules 2022, 27, 7926. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lai, K.P.; Stoeger, T.; Ji, S.; Lin, Z.; Lin, X.; Chan, T.F.; Fang, J.K.-H.; Lo, M.; Gao, L.; et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J. Hazard. Mater. 2021, 416, 126069. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, S.; Kim, O.-H.; Kim, C.-H.; Kim, J.; Kim, J.-W.; Hong, S.; Lee, H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef]
- Domenech, J.; Annangi, B.; Marcos, R.; Hernandez, A.; Catalan, J. Insights into the potential carcinogenicity of micro- and nano-plastics. Mutat. Res. Rev. Mutat. Res. 2023, 791, 108453. [Google Scholar] [CrossRef] [PubMed]
- Driver, W.E. Plastics Chemistry and Technology; Van Nostrand Reinhold Company: New York, NY, USA, 1979. [Google Scholar]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Norwegian University of Science and Technology. More Than 16,000 Chemicals Can Be Found in Plastic, and Many Are Harmful: Report. 2024. Available online: https://phys.org/news/2024-03-chemicals-plastic.html (accessed on 27 April 2024).
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Coombes, C.; Foster, L.C.; Galloway, T.S.; Godley, B.J.; Lindeque, P.K.; Witt, M.J. Marine anthropogenic litter on British beaches: A 10-year nationwide assessment using citizen science data. Sci. Total Environ. 2017, 579, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Rota, E.; Bergami, E.; Corsi, I.; Bargagli, R. Macro- and Microplastics in the Antarctic Environment: Ongoing Assessment and Perspectives. Environments 2022, 9, 93. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2015, PlasticsEurope, 2015. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2015/ (accessed on 10 April 2024).
- Oceancare, 2023. Available online: https://www.oceancare.org/en/stories_and_news/cigarette-butts-pollution/ (accessed on 4 April 2024).
- Suárez-Rodríguez, M.; López-Rull, I.; Garcia, C.M. Incorporation of cigarette butts into nests reduces nest ectoparasite load in urban birds: New ingredients for an old recipe? Biol. Lett. 2012, 9, 20120931. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.L.E.; Hall, N.; Critchell, K.; Chan, K.; Bennett, B.; Mortimer, M.; Lewis, P.J. Plastics. In Marine Pollution–Monitoring, Management and Mitigation. Springer Textbooks in Earth Sciences, Geography and Environment; Reichelt-Brushett, A., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A. Microplastic pollution in St. Lawrence river sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets 2017, 2, 395–409. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2017, 2, 301–314. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics; Springer: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.L.; Heubeck, M.; Jensen, J.K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Pilapitiya, P.G.C.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Valentini, F.; Pegoretti, A. End-of-life options of tyres. A review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef]
- Neupert, J.W.; Venghaus, D.; Barjenbruch, M. Measures to Reduce the Discharge of tire Wear into the Environment. Microplastics 2024, 3, 305–321. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Lau, P.; Stein, J.; Reinhold, L.; Barjenbruch, M.; Fuhrmann, T.; Urban, I.; Bauerfeld, K.; Holte, A. Reduction in the Input of Microplastics into the Aquatic Environment via Wastewater Treatment Plants in Germany. Microplastics 2024, 3, 276–292. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 123807. [Google Scholar] [CrossRef]
- Martin, P. Ingested microplastics: Do humans eat one credit card per week? J. Hazard. Mater. Lett. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Bai, C.L.; Liu, L.Y.; Hu, Y.B.; Zeng, E.Y.; Guo, Y. Microplastics: A review of analytical methods, occurrence and characteristics in food, and potential toxicities to biota. Sci. Total Environ. 2022, 806, 150263. [Google Scholar] [CrossRef]
- Gruber, E.S.; Stadlbauer, V.; Pichler, V.; Resch-Fauster, K.; Todorovic, A.; Meisel, T.C.; Trawoeger, S.; Holloczki, O.; Turner, S.D.; Wadsak, W.; et al. To Waste or not to waste: Questioning potential health risks of micro- and nanoplastics with a focus on their ingestion and potential carcinogenicity. Expo. Health 2022, 15, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Sundt, P.; Schulze, P.E.; Syversen, F. Sources of Microplastic-Pollution to the Marine Environment; Mepex for the Norwegian Environment Agency. 2014. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/M321/M321.pdf (accessed on 4 April 2024).
- da Costa, J.P.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. TrAC Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. Peer J. 2022, 10, e13503. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Hua, J.; Kelpsiene, E.; Lundqvist, M.; Cedervall, T. Environmental Impact of Nanoplastics from Fragmentized Consumer Plastics, REPORT 7054|OCTOBER 2022. Available online: https://www.naturvardsverket.se/4a9f96/globalassets/media/publikationer-pdf/7000/978-91-620-7054-0.pdf (accessed on 4 April 2024).
- Huang, J.; Chen, H.; Zheng, Y.; Yang, Y.; Zhang, Y.; Gao, B. Microplastic pollution in soils and groundwater: Characteristics, analytical methods and impacts. Chem. Eng. J. 2021, 425, 131870. [Google Scholar] [CrossRef]
- Mendes, L.A.; Beiras, R.; Domínguez, J. Earthworm (Eisenia andrei)-Mediated Degradation of Commercial Compostable Bags and Potential Toxic Effects. Microplastics 2024, 3, 322–338. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Niu, S.; Yu, J. Microplastics as vectors of organic pollutants in aquatic environment: A review on mechanisms, numerical models, and influencing factors. Sci. Total Environ. 2023, 887, 164008. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Said, N.S.M.; Imron, M.F.; Abdullah, S.R.S. Microplastic pollution in the environment: Insights into emerging sources and potential threats. Environ. Technol. Innov. 2021, 23, 101790. [Google Scholar] [CrossRef]
- Cruz Salas, A.A.; Perez, M.V.; Laura, A.; Bobadilla, A.L.T.; Morillas, A.V.; Valdemar, R.M.E. Chapter 15: Human Toxicity of Nano- and Microplastics. In Toxic Effects of Micro- and Nanoplastics: Environment, Food and Human Health; Inamuddin, T.A., Fernandes, V.C., Eds.; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Liang, J.; Ji, F.; Abdullah, A.L.B.; Qin, W.; Zhu, T.; Tay, Y.J.; Li, Y.; Han, M. Micro/nano-plastics impacts in cardiovascular systems across species. Sci. Total Environ. 2024, 942, 173770. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, S.K.; Rao, A.; KS, A.; Jodalli, P.S.; Shenoy Kudpi, R. Microplastic content of over-the-counter toothpastes—A systematic review. F1000Research 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Qi, Z.; Ouyang, Z.; Liu, P.; Guo, X. Interactions between microplastics and microorganisms in the environment: Modes of action and influencing factors. Gondwana Res. 2022, 108, 102–119. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the environment: Intake through the food web, human exposure and toxicological effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef]
- Zhou, T.; Guo, T.; Wang, Y.; Wang, A.; Zhang, M. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 2023, 314, 137723. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Hasselerharm, P.E.; Rico, A.; Lwanga, E.H.; van Gestel, C.A.; Koelmans, A.A. Source-specific probabilistic risk assessment of microplastics in soils applying quality criteria and data alignment methods. J. Hazard. Mater. 2024, 467, 133732. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Sang, M.K.; El-Naggar, A.; Shi, L.; Chang, S.X.; Sung, J.; Zhang, W.; Ok, Y.S. Low-density polyethylene microplastics alter chemical properties and microbial communities in agricultural soil. Sci. Rep. 2023, 13, 16276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Peng, C.; Li, H.; Zhang, P.; Liu, X. The impact of microplastic-microbe interactions on animal health and biogeochemical cycles: A mini-review. Sci. Total Environ. 2021, 773, 145697. [Google Scholar] [CrossRef]
- Gong, M.; Yang, G.; Zhuang, L.; Zeng, E.Y. Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms. Environ. Pollut. 2019, 252, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of seafood intended for human consumption: A systematic review and meta-analysis. Environ. Health Perspect. 2020, 128, 126002-1–126002-32. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi J de los, A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the Food Chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, J.; Zhao, L.; Mu, X.; Wang, C.; Wang, M.; Xue, X.; Qi, S.; Wu, L. Gut microbiota protects honey bees (Apis mellifera L.) against polystyrene microplastics exposure risks. J. Hazard. Mater. 2021, 402, 123828. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Messinetti, S.; Mercurio, S.; Scarì, G.; Pennati, A.; Pennati, R. Ingested microscopic plastics translocate from the gut cavity of juveniles of the ascidian Ciona intestinalis. Eur. Zool. J. 2019, 86, 189–195. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sobhan, F.; Uddin, M.N.; Sharifuzzaman, S.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastics in fishes from the Northern Bay of Bengal. Sci. Total Environ. 2019, 690, 821–830. [Google Scholar] [CrossRef]
- Banaee, M.; Gholamhosseini, A.; Sureda, A.; Soltanian, S.; Fereidouni, M.S.; Ibrahim, A.T.A. Effects of microplastic exposure on the blood biochemical parameters in the pond turtle (Emys orbicularis). Environ. Sci. Pollut. Res. 2020, 28, 9221–9234. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, Z.; Shen, R.; Huang, Y.; Ren, H.; Zhang, Y. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J. Hazard. Mater. 2021, 406, 124644. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Ding, Z.M.; Jiao, X.F.; Wu, D.; Zhang, J.Y.; Chen, F.; Wang, Y.S.; Huang, C.J.; Zhang, S.X.; Li, X.; Huo, L.J. Bisphenol F negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Biol. Interact. 2017, 278, 222–229. [Google Scholar] [CrossRef]
- Saleh, A.; Favetta, L. 159 Effect of bisphenol A and bisphenol S on AMH and AMHR mRNA expression during in vitro bovine oocyte maturation and early embryo development. Reprod. Fertil. Dev. 2019, 31, 204. [Google Scholar] [CrossRef]
- Fujimoto, V.Y.; Kim, D.; Vom Saal, F.S.; Lamb, J.D.; Taylor, J.A.; Bloom, M.S. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil. Steril. 2011, 95, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Talpade, J.; Shrman, K.; Sharma, R.K.; Gutham, V.; Singh, R.P.; Meena, N.S. Bisphenol a: An Endocrine Disruptor. J. Entomol. Zool. Stud. 2018, 6, 394–397. [Google Scholar]
- Grossman, D.; Kalo, D.; Gendelman, M.; Roth, Z. Effect of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate on in vitro developmental competence of bovine oocytes. Cell. Biol. Toxicol. 2012, 28, 383–396. [Google Scholar]
- Nandinee, D.; Mishra, G.; Shukla, A.; Soni, N.L.; Sharma, P. Bisphenol A and cattle fertility. Pharma Innov. 2021, 10, 524–528. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.; Duan, X.; Xiong, B.; Cui, X.S.; Kim, N.H.; Liu, H.L.; Sun, S.C. The Toxic Effects and Possible Mechanisms of Bisphenol A on Oocyte Maturation of Porcine in Vitro. Oncotarget 2016, 7, 32554–32565. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A. (Eds.) Characterization and Analysis of Microplastics. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75. [Google Scholar]
- Immerschitt, I.; Martens, A. Ejection, ingestion and fragmentation of mesoplastic fibres to microplastics by Anax imperator larvae (Odonata: Aeshnidae). Odonatologica 2021, 49, 57–66. [Google Scholar] [CrossRef]
- Christaki, U.; Dolan, J.R.; Pelegri, S.; Rassoulzadegan, F. Consumption of picoplankton-size particles by marine ciliates: Effects of physiological state of the ciliate and particle quality. Limnol. Oceanogr. 1998, 43, 458–464. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef]
- Wilson, D.S. Food size selection among copepods. Ecology 1973, 54, 909–914. [Google Scholar] [CrossRef]
- Bolton, T.F.; Havenhand, J.N. Physiological versus viscosity-induced effects of an acute reduction in water temperature on microsphere ingestion by trochophore larvae of the serpulid polychaete Galeolaria caespitosa. J. Plankton Res. 1998, 20, 2153–2164. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Welden, N.A.; Cowie, P.R. Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 214, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef]
- Ward, J.E.; Targett, N.M. Influence of marine microalgal metabolites on the feeding behavior of the blue mussel Mytilus edulis. Mar. Biol. 1989, 101, 313–321. [Google Scholar] [CrossRef]
- Davidson, K.; Dudas, S.E. Microplastic Ingestion by Wild and Cultured Manila Clams (Venerupis philippinarum) from Baynes Sound, British Columbia. Arch. Environ. Contam. Toxicol. 2016, 71, 147–156. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; Fernández, C.G.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Tourinho, P.S.; do Sul, J.A.I.; Fillmann, G. Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Mar. Pollut. Bull. 2010, 60, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Foekema, E.M.; Van Franeker, J.A.; Leopold, M.F.; Kühn, S.; Rebolledo, E.B.; Heße, E.; Mielke, L.J.I.J.; IJzer, J.; Kamminga, P.; et al. Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015, 95, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ. Pollut. 2018, 232, 154–163. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from NorthEast Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2019, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Carpenter EJAnderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonte, E.; Soares, M.E.; Carvalho, F.; Guilhermino, L. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: Gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 2016, 170, 89–103. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vendel, A.L.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef]
- IFFO. Changing Demands to Global Fishmeal Use. 2023. Available online: https://www.iffo.com/changing-demands-global-fishmeal-use (accessed on 10 April 2024).
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Charlton-Howard, H.S.; Bond, A.L.; Rivers-Auty, J.; Lavers, J.L. ‘Plasticosis’: Characterising macro- and microplastic-associated fibrosis in seabird tissues. J. Hazard. Mater. 2023, 450, 131090. [Google Scholar] [CrossRef]
- Lozano, R.L.; Mouat, J. Marine Litter in the North-East Atlantic Region: Assessment and Priorities for Response; KIMO Int.: Lerwick, UK, 2009; ISBN 978-1-906840-26-6. [Google Scholar]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar. Pollut. Bull. 2019, 145, 254–259. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. The New Plastics Economy Rethinking the Future of Plastics. 2016. Available online: https://www.ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 29 April 2024).
- Fraissinet, S.; De Benedetto, G.E.; Malitesta, C.; Holzinger, R.; Materić, D. Microplastics and nanoplastics size distribution in farmed mussel tissues. Commun. Earth Environ. 2024, 5, 128. [Google Scholar] [CrossRef]
- de Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic ingestion by fish: Body size, condition factor and gut fullness are not related to the amount of plastics consumed. Mar. Pollut. Bull. 2020, 151, 110827. [Google Scholar] [CrossRef] [PubMed]
- Haberle, I.; Bavčević, L.; Klanjscek, T. Fish condition as an indicator of stock status: Insights from condition index in a food-limiting environment. Fish Fish. 2023, 24, 567–581. [Google Scholar] [CrossRef]
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, degradation, and effect of polymer-based materials in the environment. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2014; Volume 227, pp. 1–53. [Google Scholar]
- Gregory, M.R. Environmental implications of plastic debris in marine settings entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zuo, Z.; Chen, M.; Chen, Y.; Wang, C. Reproductive and transgenerational toxicities of phenanthrene on female marine medaka (Oryzias melastigma). Aquat. Toxicol. 2015, 162, 109–116. [Google Scholar] [CrossRef] [PubMed]
- da Costa Araújo, A.P.; de Andrade Vieira, J.E.; Malafaia, G. Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci. Total Environ. 2020, 742, 140217. [Google Scholar] [CrossRef]
- da Costa, J.; Rocha-Santos, T.; Duarte, A.C. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures, E. Parliament, Brussels, EU, 2020. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2020/658279/IPOL_STU(2020)658279_EN.pdf (accessed on 10 April 2024).
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An emerging contaminant of potential concern? Integr. Environ. Assess. Manag. Int. J. 2007, 3, 559–561. [Google Scholar]
- da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano) plastics in the environment–sources, fates and effects. Sci. Total Environ. 2016, 566, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; Vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Tunali, M.; Uzoefuna, E.N.; Tunali, M.M.; Yenigun, O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci. Total Environ. 2020, 743, 140479. [Google Scholar] [CrossRef]
- Prata, J.C.; Lavorante, B.R.; Montenegro, M.d.C.B.; Guilhermino, L. Influence of microplastics on the toxicity of the pharmaceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquat. Toxicol. 2018, 197, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yin, K.; Zhang, Y.; Wang, D.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Polystyrene microplastics induced oxidative stress, inflammation and necroptosis via NF-κB and RIP1/RIP3/MLKL pathway in chicken kidney. Toxicology 2022, 478, 153296. [Google Scholar] [CrossRef]
- Hou, L.; Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Guo, T.; Li, J.; Zhao, H.; Xing, M. Polystyrene microplastics induce apoptosis in chicken testis via crosstalk between NF-κB and Nrf2 pathways. Comp. Biochem. Physiol. Part C 2022, 262, 109444. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Peng, H.; Qi, L.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Microplastic exposure induces muscle growth but reduces meat quality and muscle physiological function in chickens. Sci. Total Environ. 2023, 882, 163305. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lusher, A.; Nixon, M.; Wernery, U. The plight of camels eating plastic waste. J. Arid Environ. 2021, 185, 104374. [Google Scholar] [CrossRef]
- Mahadappa, P.; Krishnaswamy, N.; Karunanidhi, M.; Bhanuprakash, A.G.; Bindhuja, B.V.; Dey, S. Effect of plastic foreign body impaction on rumen function and heavy metal concentrations in various body fluids and tissues of buffaloes. Ecotoxicol. Environ. Saf. 2020, 189, 109972. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G. Effects of ingested plastic on seabird feeding: Evidence from chickens. Mar. Pollut. Bull. 1988, 19, 125–128. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.-A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in Central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef]
- Hornek-Gausterer, R.; Oberacher, H.; Reinstadler, V.; Hartmann, C.; Liebmann, B.; Lomako, I.; Kübber-Heiss, A. A preliminary study on the detection of potential contaminants in the european brown hare (Lepus europaeus) by suspect and microplastics screening. Environ. Adv. 2021, 4, 100045. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Microscopic anthropogenic litter in terrestrial birds from Shanghai, China: Not only plastics but also natural fibers. Sci. Total Environ. 2016, 550, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, F.; Mohammadi, A.; Dobaradaran, S.; De-La-Torre, G.E.; Arfaeinia, H.; Ramavandi, B.; Saeedi, R.; Tekle-Röttering, A. Occurrence of microplastics in edible tissues of livestock (cow and sheep). Environ. Sci. Pollut. Res. 2024, 31, 22145–22157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Jiang, F.; Li, L.; Li, Y.; Zhang, M.; Qi, Z.; Ma, R.; Zhang, Y.; Fang, J.; et al. Detection of microplastics in domestic and fetal pigs’ lung tissue in natural environment: A preliminary study. Environ. Res. 2023, 216, 114623. [Google Scholar] [CrossRef] [PubMed]
- Rbaibi Zipak, S.; Muratoglu, K.; Buyukunal, S.K. Microplastics in raw milk samples from the Marmara region in Turkey. J. Consum. Prot. Food Saf. 2024, 19, 175–186. [Google Scholar] [CrossRef]
- Hannah, R. How Much of the World’s Food Production Is Dependent on Pollinators? 2021. Available online: https://ourworldindata.org/pollinator-dependence (accessed on 10 April 2024).
- Al Naggar, Y.; Brinkmann, M.; Sayes, C.M.; Al-Kahtani, S.N.; Dar, S.A.; El-Seedi, H.R.; Grünewald, B.; Giesy, J.P. Are Honey Bees at Risk from Microplastics? Toxics 2021, 15, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liebezeit, G.; Dubaish, F. Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012, 89, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.J.; Kim, S.K.; Kim, H.J. Global pattern of microplastics (MPs) in commercial food-grade salts: Sea salt as an indicator of seawater MP pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Schwabl, P.; Liebmann, B.; Köppel, S.; Königshofer, P.; Bucsics, T.; Reiberger, T. Assessment of microplastics concentrations in human stool– Preliminary results of a prospective study. UEG J. 2018, 6, a127. [Google Scholar]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- EFSA—European Food Safety Authority. Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J. 2014, 12, 3761. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low densitymicroplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef] [PubMed]
- Susanti, R.; Yuniastuti, A.; Fibriana, F. The evidence of microplastic contamination in central javanese local ducks from intensive animal husbandry. Water Air Soil Pollut. 2021, 232, 2–10. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- UNEP. 2024. Available online: https://www.unep.org/inc-plastic-pollution (accessed on 4 April 2024).
- The Conversion. 2024. Available online: https://theconversation.com/if-plastic-manufacturing-goes-up-10-plastic-pollution-goes-up-10-and-were-set-for-a-huge-surge-in-production-227365 (accessed on 4 April 2024).
- Scientists’ Coalition. The Scientists’ Coalition for an Effective Plastics Treaty. 2024. Available online: https://ikhapp.org/scientistscoalition/ (accessed on 10 April 2024).
- Calero, M.; Godoy, V.; Quesada, L.; Martín-Lara, M. Green strategies for microplastics reduction. Curr. Opin. Green Sustain. Chem. 2021, 28, 100442. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Singh, S.; Kalyanasundaram, M.; Diwan, V. Removal of microplastics from wastewater: Available techniques and way forward. Water Sci. Technol. 2021, 84, 3689–3704. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Schuhen, K. Beyond Microplastics: Implementation of a Two-Stage Removal Process for Microplastics and Chemical Oxygen Demand in Industrial Wastewater Streams. Water 2024, 16, 268. [Google Scholar] [CrossRef]
- Urli, S.; Corte Pause, F.; Crociati, M.; Baufeld, A.; Monaci, M.; Stradaioli, G. Impact of Microplastics and Nanoplastics on Livestock Health: An Emerging Risk for Reproductive Efficiency. Animals 2023, 13, 1132. [Google Scholar] [CrossRef]
- Nasir, M.S.; Tahir, I.; Ali, A.; Ayub, I.; Nasir, A.; Abbas, N.; Sajjad, U.; Hamid, K. Innovative technologies for removal of micro plastic: A review of recent advances. Heliyon 2024, 10, e25883. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M.; Mukherjee, A.; Koller, M. What Are “Bioplastics”? Defining Renewability, Biosynthesis, Biodegradability, and Biocompatibility. Polymers 2023, 15, 4695. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, A.; Hassan, S.; Meenatchi, R.; Bhat, M.A.; Hussain, N.; Arockiaraj, J.; Ngo, H.H.; Sharma, A.; Nguyen, H.; Pugazhendhi, A. Mitigating microplastic pollution: A critical review on the effects, remediation, and utilization strategies of microplastics. J. Environ. Manag. 2024, 351, 119988. [Google Scholar] [CrossRef] [PubMed]
- FAO. 2023. Available online: https://www.fao.org/sustainable-development-goals-helpdesk/transform/article-detail/breaking-the-plastic-cycle-in-agriculture/en (accessed on 10 April 2024).
- Hofmann, T.; Ghoshal, S.; Tufenkji, N.; Adamowski, J.F.; Bayen, S.; Chen, Q.; Demokritou, P.; Flury, M.; Hüffer, T.; Ivleva, N.P.; et al. Plastics can be used more sustainably in agriculture. Commun. Earth Environ. 2023, 4, 332. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.-J.; Liu, L.-Y.; Li, Z.; Zeng, E.Y. Drinking Boiled Tap Water Reduces Human Intake of Nanoplastics and Microplastics. Environ. Sci. Technol. Lett. 2024, 11, 273–279. [Google Scholar] [CrossRef]


| Animal | Plastics | Organ | Effect | Source |
|---|---|---|---|---|
| Chicken | PS | Kidney | Induced damage to kidney tissue, Mitochondria impairment, disturbance or disorderly arrangement of the blood–urinary barrier. | [180] |
| Chicken | PS | Heart | No notable impact on body weight or myocardial weight in chicken was observed; caused oxidative stress and inflammation in the chicken myocardium, mitochondrial impairment, and weakened or dysfunctional energy metabolism. | [93] |
| Chicken | PS | Testicular tissue and blood samples | The effect on the exposed chickens were impaired testicular tissue, inflammatory infiltration in the testicular tissue, and affected BTB-related proteins. | [181] |
| Chicken | PS | Heart | Caused “myocardial dysplasia” and myocardial endoplasmic reticulum (ER) stress in the exposed chickens. | [93] |
| Chicken | PE | Blood, intestine, liver, kidney, and spleen | Loss of body weight, significant levels of ALT and AST, induced liver inflammation, and renal glomerular hypoplasia, disruption of the intestinal villi, decline in gut microbiota composition and richness. | [29] |
| Chicken | PS | Leg and breast muscles, liver, intestine | Increase in breast and leg muscles of chickens, disruption of physiological function of chicken liver and skeletal muscle was observed; PS exposure affected chicken meat quality. | [182] |
| Camels | PE, PP | Intestines | Intestinal tract damage, pathogenic (bacterial) infection, organ failure. | [183] |
| Buffaloes | MP | Body fluids and tissues | Ruminal impaction, higher concentration of heavy metals in rumen, blood, liver, muscles and kidney. | [184] |
| Chicken | PE | Gizzards | Increased volume of gizzards, lowered feeding volume and fitness. | [185] |
| Aquatic birds | MP, PE | Stomach, adipose tissue | Biomagnification of toxic chemical. | [186] |
| Aquatic birds | PE | Stomachs and GI tracts | Decreased feeding capacity, lowered reproductive capacity, mortality. | [187] |
| Wild and indoor hares | PU, PA, PET, PS, PE, PP | Intestines | Intestinal inflammation, changes to intestinal mucosa. | [188] |
| Terrestrial birds | Microplastics (polyester) | Stomachs and GI tracts | Inflammation, GI blockage, cellular necrosis. | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lackner, M.; Branka, M. Microplastics in Farmed Animals—A Review. Microplastics 2024, 3, 559-588. https://doi.org/10.3390/microplastics3040035
Lackner M, Branka M. Microplastics in Farmed Animals—A Review. Microplastics. 2024; 3(4):559-588. https://doi.org/10.3390/microplastics3040035
Chicago/Turabian StyleLackner, Maximilian, and Manuela Branka. 2024. "Microplastics in Farmed Animals—A Review" Microplastics 3, no. 4: 559-588. https://doi.org/10.3390/microplastics3040035
APA StyleLackner, M., & Branka, M. (2024). Microplastics in Farmed Animals—A Review. Microplastics, 3(4), 559-588. https://doi.org/10.3390/microplastics3040035







