Progress in Research on Microplastic Prevalence in Tropical Coastal Environments: A Case Study of the Johor and Singapore Straits
Abstract
1. Introduction
2. Methodology
3. Microplastics from Tropical Coastal Environments
3.1. Microplastics from Sediments
| Sampled Matrix | Location | Date Sampled | Total Abundance | Microplastic Type | Size | Color | Reference |
|---|---|---|---|---|---|---|---|
| Beach sediment | |||||||
| Pasir Ris | Johor Strait | 2004 | 0–9 particles/kg | Polystyrene | NA | NA | [24] |
| Changi | 0 particles/kg | NA | |||||
| Sembawang | |||||||
| East Coast | Singapore Strait | 0–6 particles/kg | Polyvinyl alcohol, Polypropylene | ||||
| Sentosa Island | 0 particles/kg | NA | |||||
| Sembawang Park (S1b) | Johor Strait | 2018 | 31.1 particles/kg | Foam (66.4%), Fragment (28.0%), Pellet (3.4%), Fiber (1.1%), Film (1.1%) | NA | NA | [17] |
| Lazarus Island (LI1) | Singapore Strait | 9.2 particles/kg | Fragment (35.7%), Foam (31.0%), Pellet (23.8%), Fiber (7.2%), Film (2.3%) | ||||
| Changi Jetty (S7b) | Johor Strait | 59.9 particles/kg | Foam (54.1%), Fragment (39.6%), Fiber (4.2%), Pellet (1.4%), Film (0.7%) | ||||
| Bedok Jetty | Singapore Strait | 2022–2023 | 71.9 particles/m2 | Foam (44%), Fragment (23%), Fiber (17%) | NA | NA | [35] |
| Benthic sediment | |||||||
| Sembawang | Johor Strait | 2020–2021 | 303.3 particles/kg. d.w.s | Fragment (32%), Fiber (29%), Film (28%), Foam (8%), Others (3%) | <500 µm (70%), 500–1000 µm (10%), 1–5 mm (20%) | NA | [34] |
| East Coast | Singapore Strait | 470.8 particles/kg. d.w.s | <500 µm (60%), 500–1000 µm (30%), 1–5 mm (10%) | ||||
3.2. Microplastics from Water Column
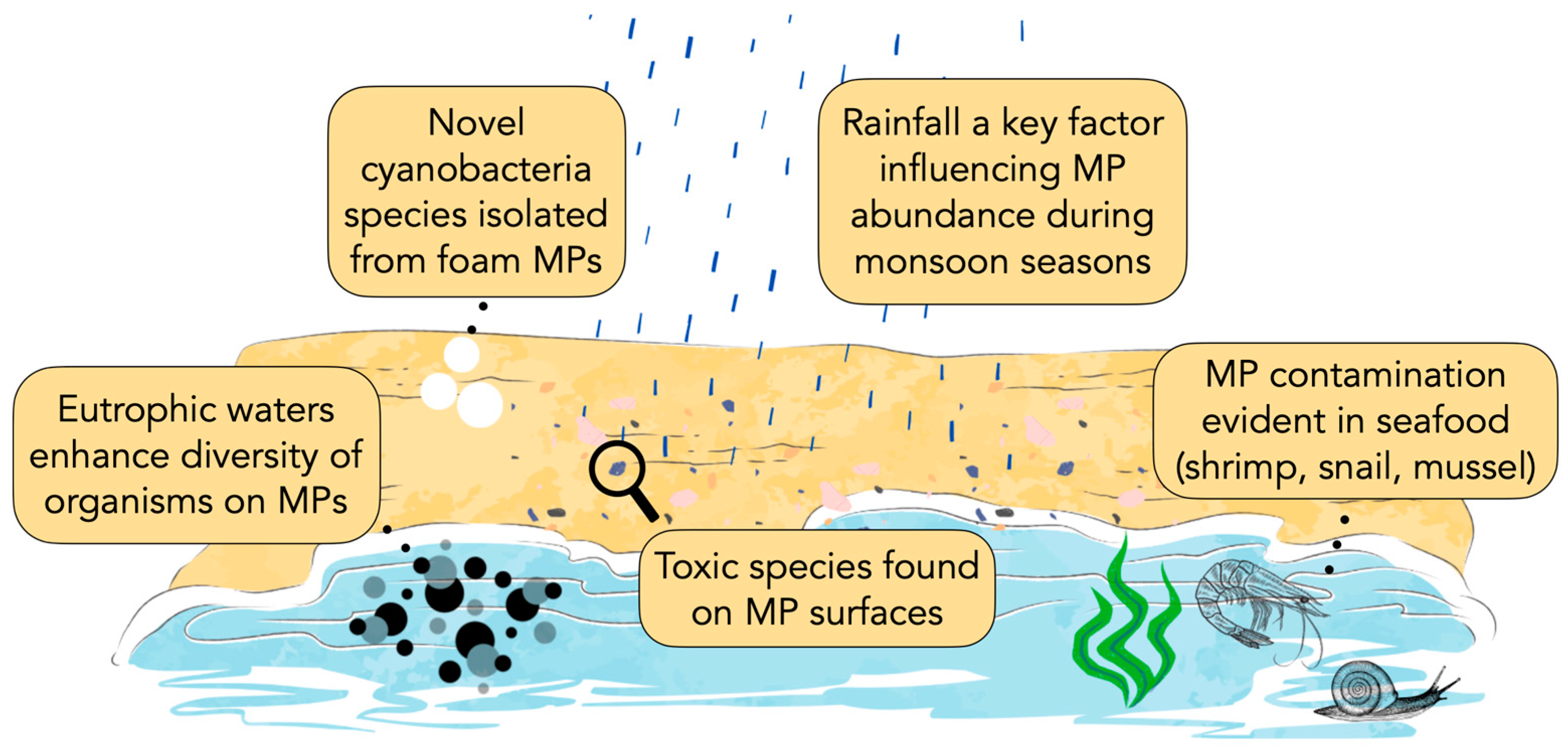
3.3. Spatiotemporal Variation in Microplastics
3.4. Diversity of Organisms on the Plastisphere
3.5. Microplastics Ingested by Marine Organisms
| Sampled Matrix | Location | Date Sampled | Total Abundance | Microplastic Type | Size | Color | Reference |
|---|---|---|---|---|---|---|---|
| Shrimp | |||||||
| Fenneropenaeus indicus | Indonesia, Eastern Indian Ocean, FAO57 | January 2020 | 5570 ± 100 pieces/g.w.w. | Sphere (61.6%), film (25.4%), fragment (10.8%), fiber (2.2%) | NA | NA | [9] |
| Lithopenaeus vannamei | Malaysia | 21 ± 4 pieces/g.w.w. | Film (98%), fragment (0.8%), fiber (0.6%), sphere (0.6%) | ||||
| Ecuador | 13 ± 1 pieces/g.w.w. | Film (93%), fragment (4.7%), fiber (2%), sphere (0.3%) | |||||
| Pleoticus muelleri | Argentina, Southwest Atlantic, FAO41 | 7050 ± 418 pieces/g.w.w. | Sphere (69.6%), fragment (21.5%), film (7.4%), fibers (1.5%) | ||||
| Snail | |||||||
| Laevistrombus turturella | Singapore | 2022 | 273 pieces/individual | Fiber (35%), fragment (34%), film (31%) | <500 µm (74%), 500–1000 µm (17%), 1–5 mm (9%) | Transparent (47%), black (38%), blue (9%), pink (3%), green (3%) | [36] |
| Mussel | |||||||
| Perna viridis | Johor Strait | 2021 | 29–60 pieces/kg. d.w. | Fragment (79%), fiber (18%), beads (3%) | NA | Black (32%), red (32%), transparent (25%), blue (9%), white (1%) | [65] |
| Seagrass | |||||||
| Cymodocea rotundata | Singapore Strait | 2018 | 0.051 pieces/cm2 | Fiber (97.3%), fragment (2.7%) | <500 µm (2.7%), 500–1000 µm (34.3%), >1 mm (63%) | NA | [68] |
| Cymodocea serrulata | 0.060 pieces/cm2 | ||||||
| Thalassia hemprichii | 0.036 pieces/cm2 | ||||||
| Macroalgae | |||||||
| Padina sp. | 0.012 pieces/cm2 | ||||||
| Sargassum ilicifolium | 0.007 pieces/cm2 |
4. Data Gaps and Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M. Glitters as a source of primary microplastics: An approach to environmental responsibility and ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Corcoran, P.L. Degradation of microplastics in the environment. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 531–542. [Google Scholar]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Lv, M.; Jiang, B.; Xing, Y.; Ya, H.; Zhang, T.; Wang, X. Recent advances in the breakdown of microplastics: Strategies and future prospectives. Environ. Sci. Pollut. Res. 2022, 29, 65887–65903. [Google Scholar] [CrossRef] [PubMed]
- Campelo, R.P.; Melo Júnior, M.; Santana, C.S.; Bezerra, L.E.; Newmann-Leitão, S. Morphological Abnormalities in Corycaeus speciosus Dana, 1849 (Copepoda, Cyclopoida) from the Area of an Equatorial Atlantic Island; Cahiers de Biologie Marine: Roscoff, France, 2018; Volume 59. [Google Scholar]
- Bai, Z.; Wang, N.; Wang, M. Effects of microplastics on marine copepods. Ecotoxicol. Environ. Saf. 2021, 217, 112243. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leaw, C.P.; Lim, P.T.; Leong, S.C. Evidence of marine microplastics in commercially harvested seafood. Front. Bioeng. Biotechnol. 2020, 8, 562760. [Google Scholar] [CrossRef]
- Curren, E.; Yu, D.C.Y.; Leong, S.C.Y. From the Seafloor to the Surface: A Global Review of Gastropods as Bioindicators of Marine Microplastics. Water Air Soil Pollut. 2024, 235, 45. [Google Scholar] [CrossRef]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.; et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Change Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Naik, R.K.; Naik, M.M.; D’Costa, P.M.; Shaikh, F. Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. Pollut. Bull. 2019, 149, 110525. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leong, S.C.Y. Plankton assemblages from microplastics of tropical coastal environments reveal high diversity and evidence of toxic species. Mar. Environ. Res. 2024, 193, 106251. [Google Scholar] [CrossRef]
- Aslani, H.; Pashmtab, P.; Shaghaghi, A.; Mohammadpoorasl, A.; Taghipour, H.; Zarei, M. Tendencies towards bottled drinking water consumption: Challenges ahead of polyethylene terephthalate (PET) waste management. Health Promot. Perspect. 2021, 11, 60. [Google Scholar] [CrossRef]
- GAIA. Discarded: Communities on the Frontlines of the Global Plastic Crisis. April 2019. Available online: https://www.no-burn.org/resources/discarded-communities-on-the-frontlines-of-the-global-plastic-crisis/. (accessed on 22 April 2024).
- Curren, E.; Kuwahara, V.S.; Yoshida, T.; Leong, S.C.Y. Marine microplastics in the ASEAN region: A review of the current state of knowledge. Environ. Pollut. 2021, 288, 117776. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Cordova, M.R.; Ulumuddin, Y.I.; Purbonegoro, T.; Puspitasari, R.; Afianti, N.F.; Rositasari, R.; Yogaswara, D.; Hafizt, M.; Iswari, M.Y.; Fitriya, N.; et al. Seasonal heterogeneity and a link to precipitation in the release of microplastic during COVID-19 outbreak from the Greater Jakarta area to Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2022, 181, 113926. [Google Scholar] [CrossRef]
- Sterl, M.F.; Delandmeter, P.; van Sebille, E. Influence of barotropic tidal currents on transport and accumulation of floating microplastics in the global open ocean. J. Geophys. Res. Ocean. 2020, 125, e2019JC015583. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Obbard, J.P. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Ta, A.T.; Loan, N.T.; Sembiring, E.; Setiadi, T.; Sharp, A. Microplastics pollution in selected rivers from Southeast Asia. APN Sci. Bulletin. 2022, 12, 1741. [Google Scholar] [CrossRef]
- Van Maren, D.S.; Gerritsen, H. Residual flow and tidal asymmetry in the Singapore Strait, with implications for resuspension and residual transport of sediment. J. Geophys. Res. Ocean. 2012, 117, C4. [Google Scholar] [CrossRef]
- Li, X.; Meshgi, A.; Babovic, V. Spatio-temporal variation of wet and dry spell characteristics of tropical precipitation in Singapore and its association with ENSO. Int. J. Climatol. 2016, 36, 4831–4846. [Google Scholar] [CrossRef]
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Ibrahim, A.; Sipaut, C.S.; Foo, J.; Seay, J.; Taufiq-Yap, Y.H.; Janaun, J. Plastic waste and microplastic issues in Southeast Asia. Front. Environ. Sci. 2023, 11, 1142071. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Yuan, X.; Attanayake, C.P.; Wang, S.; You, S.; Tsang, D.C.; Nzihou, A.; Ok, Y.S. Sustainable management of plastic wastes in COVID-19 pandemic: The biochar solution. Environ. Res. 2022, 212, 113495. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H. COVID-19 pandemic and microplastic pollution. Nanomaterials 2022, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. PubMed and beyond: A survey of web tools for searching biomedical literature. Database 2011, 2011, baq036. [Google Scholar] [CrossRef]
- Younger, P. Using google scholar to conduct a literature search. Nurs. Stand. 2010, 24, 45. [Google Scholar] [CrossRef]
- Curren, E.; Leong, S.C.Y. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total Environ. 2019, 655, 313–320. [Google Scholar] [CrossRef]
- Jong, M.C.; Tong, X.; Li, J.; Xu, Z.; Chng, S.H.; He, Y.; Gin, K.Y. Microplastics in equatorial coasts: Pollution hotspots and spatiotemporal variations associated with tropical monsoons. J. Hazard. Mater. 2022, 424, 127626. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.C.Y.; Chan, T.J.R.; Chu, Q.E.; Gan, A.J.H.; Lee, A.E.; Yoshida, T.; Kuwahara, V.S.; Leong, S.C.Y. Profiling beach plastics and their hitchhiking phytoplankton. 2024; Unpublished data. [Google Scholar]
- Curren, E.; Leong, S.C.Y. Spatiotemporal characterisation of microplastics in the coastal regions of Singapore. Heliyon 2023, 9, E12961. [Google Scholar] [CrossRef]
- Senathirajah, K.; Kemp, A.; Saaristo, M.; Ishizuka, S.; Palanisami, T. Polymer prioritization framework: A novel multi-criteria framework for source mapping and characterizing the environmental risk of plastic polymers. J. Hazard. Mater. 2022, 429, 128330. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- La Daana, K.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar]
- Jiang, Y.; Zhao, Y.; Wang, X.; Yang, F.; Chen, M.; Wang, J. Characterization of microplastics in the surface seawater of the South Yellow Sea as affected by season. Sci. Total Environ. 2020, 724, 138375. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, M.; Song, L.; Yu, J.; Liu, R.; Wang, S.; Wang, Z.; Sokolova, I.M.; Huang, W.; Wang, Y. Coastal zone use influences the spatial distribution of microplastics in Hangzhou Bay, China. Environ. Pollut. 2020, 266, 115137. [Google Scholar] [CrossRef] [PubMed]
- Azman, S.; Chiang, B.C.; Ismail, R.; Jaafar, J.; Said, M.I. Effect of land use on coastal water and Perna viridis at Johor Straits, Malaysia. Int. J. Environ. Sci. Dev. 2012, 3, 237. [Google Scholar] [CrossRef]
- van Wijnen, J.; Ragas, A.M.; Kroeze, C. Modelling global river export of microplastics to the marine environment: Sources and future trends. Sci. Total Environ. 2019, 673, 392–401. [Google Scholar] [CrossRef]
- He, B.; Smith, M.; Egodawatta, P.; Ayoko, G.A.; Rintoul, L.; Goonetilleke, A. Dispersal and transport of microplastics in river sediments. Environ. Pollut. 2021, 279, 116884. [Google Scholar] [CrossRef]
- Gupta, P.; Saha, M.; Rathore, C.; Suneel, V.; Ray, D.; Naik, A.; Unnikrishnan, K.; Dhivya, M.; Daga, K. Spatial and seasonal variation of microplastics and possible sources in the estuarine system from central west coast of India. Environ. Pollut. 2021, 288, 117665. [Google Scholar] [CrossRef]
- Tang, C.N.; Kuwahara, V.S.; Leong, S.C.Y.; Moh, P.Y.; Yoshida, T. Effect of monsoon on microplastic bioavailability and ingestion by zooplankton in tropical coastal waters of Sabah. Mar. Pollut. Bull. 2023, 193, 115182. [Google Scholar] [CrossRef] [PubMed]
- Jiwarungrueangkul, T.; Phaksopa, J.; Sompongchaiyakul, P.; Tipmanee, D. Seasonal microplastic variations in estuarine sediments from urban canal on the west coast of Thailand: A case study in Phuket province. Mar. Pollut. Bull. 2021, 168, 112452. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Zaldívar, J.M.; Marinov, D.; Dachs, J. Fate of persistent organic pollutants in the water column: Does turbulent mixing matter? Mar. Pollut. Bull. 2007, 54, 441–451. [Google Scholar] [CrossRef]
- Atwood, E.C.; Falcieri, F.M.; Piehl, S.; Bochow, M.; Matthies, M.; Franke, J.; Carniel, S.; Sclavo, M.; Laforsch, C.; Siegert, F. Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: Comparing remote sensing and hydrodynamic modelling with in situ sample collections. Mar. Pollut. Bull. 2019, 138, 561–574. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric microplastics in the Northwestern Pacific Ocean: Distribution, source, and deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.E.; Ockelford, A.; O’Brien, P.; Neuman, C.M. Preferential transport of microplastics by wind. Atmos. Environ. 2021, 245, 118038. [Google Scholar] [CrossRef]
- Ng, C.S.; Ong, L.J.; Chou, L.M. Lyngbya majuscula Blooms in an Enclosed Marine Environment. EnvironmentAsia 2012, 5, 93–98. [Google Scholar]
- Curren, E.; Leong, S.C.Y. New records of benthic coastal cyanobacteria observed from the Southern Islands of Singapore. Algol. Stud. 2018, 154, 93–112. [Google Scholar] [CrossRef]
- Lee, A.E.; Chan, T.J.R.; Gan, A.J.H.; Yu, D.C.Y.; Yoshida, T.; Kuwahara, V.S.; Leong, S.C.Y. Colonization of plastics by microorganisms. 2024; Unpublished data. [Google Scholar]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Kedzierski, M.; Palazot, M.; Soccalingame, L.; Pedrotti, M.L.; Bruzaud, S. Microplastic fouling: A gap in knowledge and a research imperative to improve their study by infrared characterization spectroscopy. Mar. Pollut. Bull. 2022, 185, 114306. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Melchiorsen, J.; Ciacotich, N.; Gram, L.; Sonnenschein, E.C. Effect of polymer type on the colonization of plastic pellets by marine bacteria. FEMS Microbiol. Lett. 2021, 368, fnab026. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; Boyd, C.E. Eutrophication. In Water Quality: An Introduction; Springer: Berlin/Heidelberg, Germany, 2020; pp. 311–322. [Google Scholar]
- Kautz, R.S. Effects of eutrophication on the fish communities of Florida lakes. In Proceedings of the Annual Conference-Southeastern Association of Fish and Wildlife Agencies (USA), Miami, FL, USA, 1 March 1980; 1980; 34, pp. 67–80. [Google Scholar]
- Ansari, A.A.; Khan, F.A.; Gill, S.S.; Varshney, J. Aquatic plant diversity in eutrophic ecosystems. In Eutrophication: Causes, Consequences and Control; Springer: Berlin/Heidelberg, Germany, 2011; pp. 247–263. [Google Scholar]
- Kok, J.W.; Leong, S.C.Y. Nutrient conditions and the occurrence of a Karenia mikimotoi (Kareniaceae) bloom within East Johor Straits, Singapore. Reg. Stud. Mar. Sci. 2019, 27, 100514. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wu, N.; Zhao, Z.; Xu, W.A.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ. Sci. Technol. 2019, 53, 10763–10773. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chung, N.; Bae, M.J.; Kwon, Y.S.; Kwon, T.S.; Park, Y.S. Temperature change and macroinvertebrate biodiversity: Assessments of organism vulnerability and potential distributions. Clim. Change 2013, 119, 421–434. [Google Scholar] [CrossRef]
- Curren, E.; Kuwahara, V.S.; Yoshida, T.; Leong, S.C. Draft genome of novel cyanobacteria Sphaerothrix gracilis isolated from coastal microplastics reveal insights to chemical ecology, bloom and plastic-utilization potential. Funct. Integr. Genom. 2024, 24, 46. [Google Scholar] [CrossRef]
- Zin, M.M.; Azman, S.; Anaziah, S.H.; Khalid, N.; Jumali, S.; Yusof, N.U. Microplastic contamination in the sediment of the Johor Strait Estuary, Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1263, 012039. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.; Sun, C.; Hossain, M.S.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef]
- Seng, N.; Lai, S.; Fong, J.; Saleh, M.F.; Cheng, C.; Cheok, Z.Y.; Todd, P.A. Early evidence of microplastics on seagrass and macroalgae. Mar. Freshw. Res. 2020, 71, 922–928. [Google Scholar] [CrossRef]
- Fong, J.M.; Lai, S.; Yaakub, S.M.; Ow, Y.X.; Todd, P.A. The diet and feeding rates of gastropod grazers in Singapore’s seagrass meadows. Bot. Mar. 2018, 61, 181–192. [Google Scholar] [CrossRef]
- Ramsperger, A.F.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano-and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. EBioMedicine 2022, 82. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2021, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G.; Shruti, V.C. Coverage of microplastic data underreporting and progress toward standardization. Sci. Total Environ. 2022, 829, 154727. [Google Scholar] [CrossRef]
- Nyadjro, E.S.; Webster, J.A.; Boyer, T.P.; Cebrian, J.; Collazo, L.; Kaltenberger, G.; Larsen, K.; Lau, Y.H.; Mickle, P.; Toft, T.; et al. The NOAA NCEI marine microplastics database. Sci. Data 2023, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Lipizer, M.; Molina Jack, M.E.; Holdsworth, N.; Jensen, H.M.; Buga, L.; Sarbu, G.; Iona, A.; Gatti, J.; Larsen, M.; et al. Aggregated and validated datasets for the european seas: The contribution of EMODnet chemistry. Front. Mar. Sci. 2020, 7, 583657. [Google Scholar] [CrossRef]
- Čerkasova, N.; Enders, K.; Lenz, R.; Oberbeckmann, S.; Brandt, J.; Fischer, D.; Fischer, F.; Labrenz, M.; Schernewski, G. A public database for microplastics in the environment. Microplastics 2023, 2, 132–146. [Google Scholar] [CrossRef]
- Singapore Environment Council. Consumer Plastic and Plastic Resource Ecosystem in Singapore; Singapore Environment Council: Singapore, 2018. [Google Scholar]
- The Straits Times. Plastic Bag Use Falls by Up to 80% at Some Supermarkets in First Month of Bag Charge. 9 January 2024. Available online: https://www.straitstimes.com/singapore/environment/supermarkets-gave-out-fewer-plastic-bags-in-first-month-of-bag-charge (accessed on 22 April 2024).
- The Straits Times. Domestic Recycling Rate in Singapore in 2022 Lowest in More Than a Decade. 4 May 2023. Available online: https://www.straitstimes.com/singapore/domestic-recycling-rate-in-singapore-lowest-in-over-a-decade (accessed on 22 April 2024).
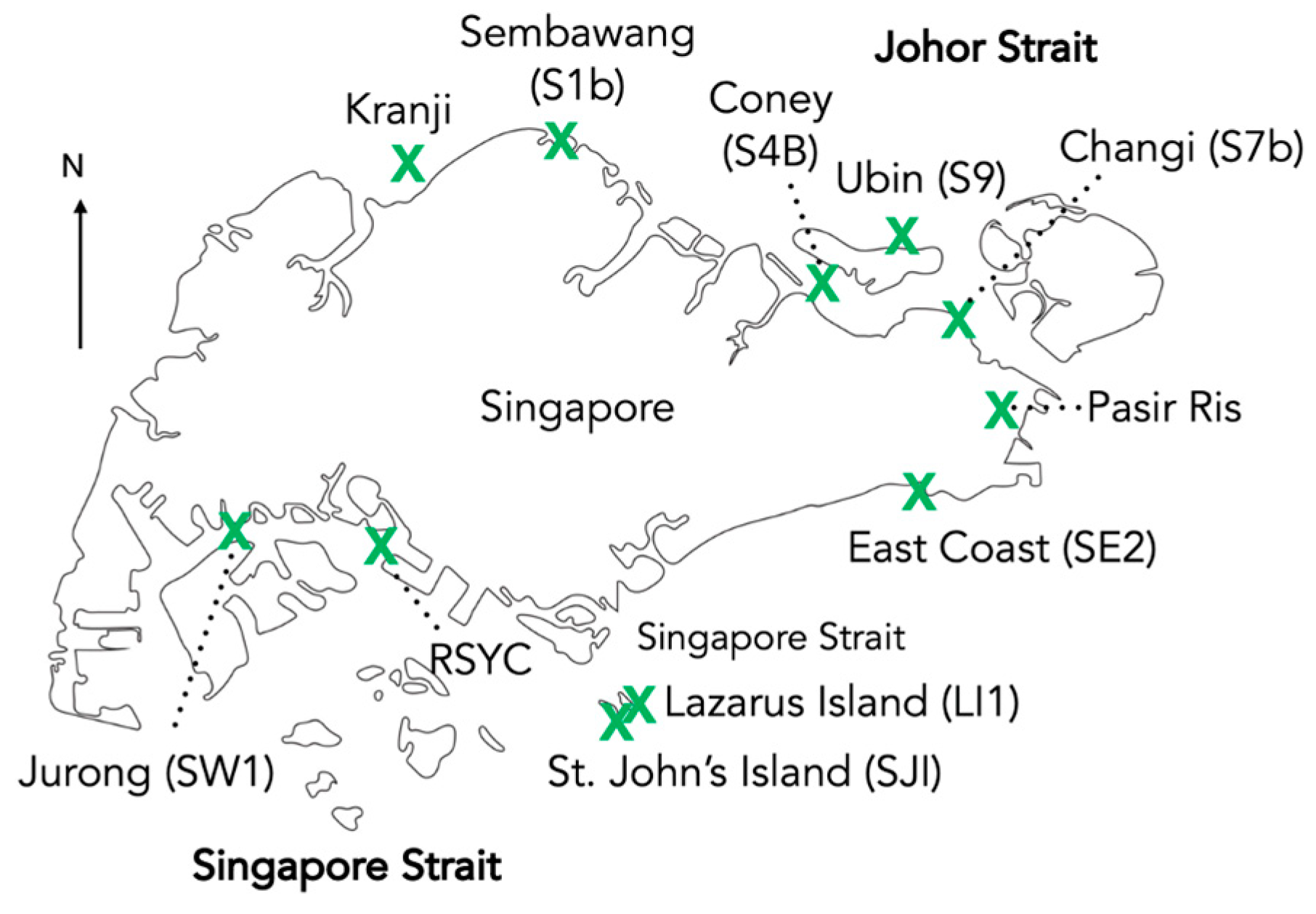
| Sampled Matrix | Location | Date Sampled | Total Abundance | Microplastic Type | Size | Color | Reference |
|---|---|---|---|---|---|---|---|
| RSYC (0 m) | Singapore Strait | 2004 | 0–1 particles/L | Polyethylene, Polystyrene | NA | NA | [24] |
| RSYC (1 m) | Polyethylene | ||||||
| Kranji (0 m) | Johor Strait | 0–1 particles/L | Polyethylene, Polystyrene | ||||
| Kranji (1 m) | 0–0.5 particles/L | Polypropylene | |||||
| Sembawang (0 m) | Johor Strait | 2021–2022 | 95.5 pieces/mL | Fragment (69.2%), Film (22.3%), Fiber (8.5%) | <500 µm (96.3%), 500–1000 µm (2.8%), 1–5 mm (0.9%) | Black (90.3%), Purple (5.2%), Transparent (3.85%), Red (0.5%) | [36] |
| Sembawang (5 m) | 10.3 pieces/mL | Fragment (56.8%), Film (31.2%), Fiber (12.0%) | <500 µm (96.6%), 500–1000 µm (3.4%) | Black (84.8%), Blue (8.3%), Transparent (3.5%), Purple (1.7%), Red (1.7%) | |||
| Coney S4B (0 m) | 71.4 pieces/mL | Fragment (64.8%), Film (33.8%), Fiber (1.4%) | <500 µm (98.5%), 500–1000 µm (1.5%) | Black (91.7%), Transparent (7.5%), Purple (0.8%) | |||
| Coney S4B (5 m) | 70 pieces/mL | Fragment (71.1%), Film (28.0%), Fiber (0.9%) | <500 µm (99.4%), 500–1000 µm (0.6%) | Black (77.3%), Blue (1.0%), Transparent (11.1%), Purple (9.9%), Red (0.7%) | |||
| Ubin S9 (0 m) | 119 pieces/mL | Fragment (70.4%), Film (24.2%), Fiber (5.4%) | <500 µm (99.5%), 500–1000 µm (0.5%) | Black (56.0%), Blue (17.0%), Transparent (12.0%), Purple (12.0%), Red (3.0%) | |||
| Ubin S9 (5 m) | 120 pieces/mL | Fragment (70.4%), Film (24.2%), Fiber (5.4%) | <500 µm (100%) | Black (60.5%), Blue (3.2%), Transparent (7.4%), Purple (23.5%), Red (5.4%) | |||
| Jurong SW1 (0 m) | Singapore Strait | 76.2 pieces/mL | Fragment (54.1%), Film (44.8%), Fiber (1.1%) | <500 µm (100%) | Black (58.4%), Blue (26.9%), Transparent (11.0%), Purple (2.0%), Red (1.7%) | ||
| Jurong SW1 (5 m) | 66.5 pieces/mL | Fragment (65.5%), Film (28.7%), Fiber (5.8%) | <500 µm (98.8%), 500–1000 µm (1.2%) | Black (87.0%), Blue (2.3%), Transparent (3.9%), Purple (4.5%), Red (2.3%) | |||
| St. John’s Island (0 m) | 101 pieces/mL | Film (50.2%), Fragment (44.0%), Fiber (5.8%) | <500 µm (100%) | Black (50.0%), Transparent (37.5%), Purple (12.5%) | |||
| St. John’s Island (5 m) | 62.5 pieces/mL | Fragment (100%) | <500 µm (100%) | Black (100.0%) | |||
| East Coast (0 m) | 107 pieces/mL | Fragment (80.4%), Film (14.2%), Fiber (5.4%) | <500 µm (98.4%), 500–1000 µm (1.6%) | Black (84.9%), Transparent (7.5%), Red (0.9%), Blue (0.6%) | |||
| East Coast (5 m) | 88.5 pieces/mL | Film (54.3%), Fragment (43.0%), Fiber (2.7%) | <500 µm (100%) | Black (68.5%), Blue (12.3%), Transparent (17.3%), Purple (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curren, E.; Lee, A.E.; Yu, D.C.Y.; Leong, S.C.Y. Progress in Research on Microplastic Prevalence in Tropical Coastal Environments: A Case Study of the Johor and Singapore Straits. Microplastics 2024, 3, 373-389. https://doi.org/10.3390/microplastics3030023
Curren E, Lee AE, Yu DCY, Leong SCY. Progress in Research on Microplastic Prevalence in Tropical Coastal Environments: A Case Study of the Johor and Singapore Straits. Microplastics. 2024; 3(3):373-389. https://doi.org/10.3390/microplastics3030023
Chicago/Turabian StyleCurren, Emily, Audrey Ern Lee, Denise Ching Yi Yu, and Sandric Chee Yew Leong. 2024. "Progress in Research on Microplastic Prevalence in Tropical Coastal Environments: A Case Study of the Johor and Singapore Straits" Microplastics 3, no. 3: 373-389. https://doi.org/10.3390/microplastics3030023
APA StyleCurren, E., Lee, A. E., Yu, D. C. Y., & Leong, S. C. Y. (2024). Progress in Research on Microplastic Prevalence in Tropical Coastal Environments: A Case Study of the Johor and Singapore Straits. Microplastics, 3(3), 373-389. https://doi.org/10.3390/microplastics3030023







