1. Introduction
The main purpose of hand sanitizers is to kill or inhibit microorganism growth. Among the different forms of hand sanitizers (i.e., spray, liquid or foam), antibacterial gels are the most widely used [
1]. Their formulation is usually light and watery, resulting in the ability to penetrate the skin and kill bacteria [
2,
3]. With the aim of mitigating the burden of the virus, the use of antibacterial gels significantly increased during the COVID-19 pandemic in public facilities, transportation, hospitals, nursing and ordinary homes. A study reported that 71% of the adults in the research used hand sanitizer more frequently during the pandemic than before it [
4].
Antibacterial gel formulations contain gelling agents, such as the most common and widely used, cross-linked polyacrylic acids. Poly(acrylic acids) (PAAs) are high-molecular-weight (from 10
3 to 10
6 g/mol) compounds belonging to the class of superabsorbent polymers due to their ability to absorb a huge amount of water in their network [
5] and their swelling in water to up to 1000 times their original volume [
6]. PAAs are used in several applications, such as in diapers, surgical pads, incontinence, feminine napkins, paints, detergents, dispersants and flocculants [
7]. PAAs are relatively resistant to microbial attack under environmental conditions, with low degradability in soil due to adsorption phenomena on soil particles. Degradation rates are therefore expected to be no more than 10% per year and depend on their structure and composition [
5].
Carbopol
® is a cross-linked polyacrylic acid copolymer widely used on the market, and it is applied in beauty, health and homecare products due to its versatility. Carbopol
® is extensively used in the development of various gel-based systems for drug delivery through different routes, such as buccal, vaginal and rectal [
8,
9]. Carbopol
® is also exploited for health products since it has interesting characteristics such as highly effective controlled-release properties, bio-adhesion, taste-masking and good binding characteristics. It is also able to improve the flow properties in a broad range of product types, as well as in surfactant-containing applications. When used in pharmaceutical products, Carbopol
® polymers are exploited as rheology modifiers, tablet binders, suspension stabilizers, extended-release polymers, mucoadhesive aids and bioavailability enhancers. When used in clear gels, such as hand sanitizers, Carbopol
® polymer provides thickening and clarity at the same time. There are different polymers in Carbopol
® family, depending on their chemical crosslinking [
10]. Carbopol
® Ultrez 21 (INCI name: Acrylates/C10-30 alkyl acrylate cross-polymer), a crosslinked copolymer of polyacrylic acid polymerized in a toxicologically preferred co-solvent system, is the polymer employed in the typical, widely used, hand sanitizer gels formulations present in the Italian market such as Amuchina
® Gel X-GERM or Gel Aloe. It is an extremely efficient rheology modifier capable of providing high viscosity, and it forms sparkling clear gels or hydro-alcoholic gels and creams.
In the literature, several examples of the use of Carbopol
® in drug formulation are reported. This polymer is exploited for topical delivery, such as dermal gel containing the anti-fungal drug clotrimazole [
11], in ophthalmic gels with the prolonged effect of metoprolol tartrate [
12], in biogel containing doxycycline hyclate for the treatment of acne [
13], in systemic delivery, such as the Carbopol
®-based mucoadhesive tablets for the buccal delivery of hydrocortisone [
14], or for controlled release, such as the use of the Carbopol
®-crospovidone interpolymer complex for the pH-dependent release of desloratadine [
15].
Carbopol
® polymers family are widely used in the pharmaceutical and cosmetics fields, although in their pristine physical forms they fall under the definition of microplastics reported in the ECHA ANNEX XV restriction report [
16].
The term microplastics was introduced by Thompson et al. [
17] in 2004 to report on small plastic fragments in the marine environment. An upper size limit of 5 mm for microplastics was proposed by Arthur et al. [
18] in 2009. Currently, plastic particles and fibers in the size range 1 μm to 1 mm are defined as microplastics [
19,
20,
21,
22]. Fragments in the size range 1–5 mm can be referred as large microplastics [
20,
21]. In what follows, the abbreviations MPs will be used for microplastics.
Recently, the European Commission took an important step in the protection of environment and human health regarding microplastics intentionally added to products by proposing restrictions in order to reduce and prevent the release into the environment of about half a million tons of microplastics [
23]. Since the main purpose of the aforementioned ECHA document is the reduction in MP pollution during the life cycle of MP-containing products, the potential release of Carbopol
® particles into the environment should be carefully evaluated. Due to the widespread use of these synthetic polymers (also called “liquid microplastics”) and the recent ban on cosmetic microbeads in many countries, the eco-toxicity, biodegradability and overall impact of PAA polymers should be considered. PAA-containing cosmetic products are usually washed into sewage and subsequently released into aquatic ecosystems via effluents from wastewater treatment plants [
24]. Details on PAAs behavior, physical state, fate and impact on the environment are limited since no experimental data on PAAs’ removal during wastewater treatment are published, especially for polymers with an MW of 2000–4000 kDa (i.e., the MW of the PAAs used in cosmetics). Moreover, the biodegradation rates of PAAs were demonstrated to be generally below 20% [
25,
26].
To predict PAAs’ fate after the use of the hand sanitizer gels, and to evaluate the impact on the environment, life-cycle assessment (LCA) studies should be performed. To date only a few holistic LCAs have been performed to determine the environmental consequences of the poor management of plastic wastes, and no LCA study was performed on polymers such as PAAs that do not dissolve in water but produce aqueous dispersions. The lack of data about the environmental impact of polymers such as Carbopol
® raises some questions regarding the fate of Carbopol
® in commercial products like sanitizer gels and their environmental sustainability. Microplastics pollution may lead to adverse effects to ecosystems and then on human health [
27,
28]. Therefore, the fate of the microplastics contained in widely used commercial products such as hand sanitizers should be carefully investigated in order to understand the potential exposure along the entire product life cycle, from manufacturing, to its proposed use, up to the rinsing of hands.
This work describes, for the first time, the experimental evaluation of the fate of the microplastic Carbopol® along its entire life cycle—from its pristine form as a microplastic to end-of-life. Amuchina® Gel X-GERM, a hand sanitizer gel largely present in the Italian market, was used in this study as a typical representative of its product category, characterized by a hydroalcoholic base containing 70% ethanol, perfume, excipients and an acrylate jelly agent (0.5–1% w/v). The activity first aimed to demonstrate the presence of solid particles of Carbopol® in the product and then to evaluate if the polymer retained its pristine form as a microplastic during the product’s typical mode of use (real-life usage conditions). Based on the obtained results, the Carbopol® polymer contained in Amuchina® Gel X-GERM does not retain its pristine particulate form under usage conditions.
2. Materials and Methods
2.1. Carbopol® Characterization
Microplastics are defined as plastic polymers with a size less than 5 mm, and optical microscopy and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) analysis were selected as the best analytical techniques for Carbopol® characterization to obtain information on both particle size and the chemical identity of the powder.
The Carbopol® type used in the Amuchina® Gel X-GERM product (Ultrez 21) is reported by the manufacturer Lubrizol (Lubrizol Advanced Materials, Inc., Cleveland, OH, USA) as a dried powder of microparticles between 2 µm and 7 µm in size. When the polymer is exposed to water, the size of the aggregates increases up to 10 times. To better characterize Carbopol® (Lubrizol Advanced Materials, Inc., Cleveland, OH, USA), optical microscopy (AXIO Scope A1, Carl Zeiss, Oberkochen, Germany) was used for measuring particle size and ATR-FTIR (Frontier FTIR, Perkin Elmer, Shelton, CT, USA) was carried out to determine chemical identity. ATR-FTIR was performed by collecting 4 scans in the infrared spectral range 4000–600 cm−1 with a 4 cm−1 spectral resolution. The collected IR spectrum was used as a reference and compared with particles detected in Amuchina® Gel X-GERM.
2.2. Detection and Identification of Carbopol® in the Finished Product Amuchina Gel X-GERM®
MPs were characterized using FTIR microscopy (µ-FTIR) since it represents the most widely used analytical technique to obtain information on particle size and chemical identification [
29]. FTIR provides the chemical bond information of compounds and the generation of peak types and specific spectrum rely on the bond structure. Chemical identification through comparing the spectra with standard libraries can distinguish MPs from other organic and inorganic substances. The composition of MPs can be determined if the matching degree of MPs detected by infrared spectroscopy reaches more than 80% with the standard library. µ-FTIR not only improved spatial resolution but also enabled the detection of smaller plastic particles [
30]. Similarly to Raman microscopy, µ-FTIR is also a reliable method for the correct identification and characterization of MPs in terms of size and composition [
31]. Based on these considerations, µ-FTIR can be considered the most appropriate technique for the aim of the present study.
Particles potentially present in a product should be separated from the matrix and analyzed by a proper technique in order to identify and characterize them from a chemical and particle size point of view. In the present study, different approaches were used to isolate and identify particles in the product.
Before proceeding with sample preparation procedure, reagents and solutions were filtered using a glass vacuum filtration system equipped with silver membranes (3 µm pore size, 25 mm diameter, Sterlitech, Auburn, WA, USA). To avoid any environmental contamination, reagent filtration and sample preparation operations were carried out in an ISO 7 clean room using glass labware, while operators wore cotton lab coats throughout the experiments. Before use, glass labware was washed twice with soapy tap water and the surface scratched with a natural fiber pipe cleaner to remove any possible particles or residues. Glassware was then rinsed at least 4 times with pre-filtered ultrapure water and the operation was repeated in the clean room, performing at least 4 rinses with pre-filtered ultrapure water.
Filtration was performed either directly, by filtering about 0.5 g of Amuchina® Gel X-GERM under vacuum through a silver membrane (3 µm pore size, 25 mm diameter, Sterlitech, Auburn, WA, USA) or after fluidification. The product was diluted 2× and 10× with a pre-filtered solution of ethanol (puriss. p.a., absolute ≥ 99.8%, Merck KGaA, Darmstadt, Germany) and ultrapure water (Zeneer Power III water purification system, Human Corporation, Seoul, Republic of Korea) at a volume ratio of 80:20 or added to a pre-filtered supersaturated solution of sodium chloride (NaCl). The latter was prepared as follows: about 10 g of Amuchina® Gel X-GERM undiluted and diluted 2× or 10×, as reported above, were weighted in a tube and the supersaturated saline solution was added to reach a final volume of 50 mL. The obtained suspension was filtered through a silver membrane (3 µm pore size, 25 mm diameter, Sterlitech, Auburn, WA, USA).
An alternative approach was also adopted with the aim of avoiding any possible artifacts or sample alterations. The product was directly spread on an optical microscope slide to form a thin layer for analysis with optical microscope (Axio Scope A.1, Carl Zeiss, Oberkochen, Germany) and µ-FTIR (Spotlight 400, Perkin Elmer, Shelton, CT, USA) analysis. In order to evaluate the water contribution, thin layers were analyzed immediately after preparation and after drying at 70 °C overnight in an oven (Excellent UNE 500, Memmert GmbH, Schwabach, Germany).
2.3. Detection and Characterization of Carbopol® and MPs after Amuchina® Gel X-GERM Application
Direct filtration, fluidization and gel collapse were not suitable for isolating particles in the hand sanitizer due to the formation of artifacts. Therefore, Carbopol® microplastic analysis was performed after the direct application of the product on an inert sample support suitable for µ-FTIR analysis. To quantify Carbopol® and MPs in the product, two samples of 0.4 g of Amuchina® Gel X-GERM were poured on silver membranes (3 µm pore size, 25 mm diameter, Sterlitech, Auburn, WA, USA) and the gel was spread on the filter surface until a thin film was obtained. One sample was analyzed immediately after deposition, while the other was observed after drying overnight at 70 °C in an oven (Excellent UNE 500, Memmert). Filters were analyzed by collecting the IR spectrum of each particle and recording 4 scans in the infrared spectral range 4000–650 cm−1 with a 4 cm−1 spectral resolution. The filter area was fully scanned collecting bright-field images of the observed particles and their IR spectrum was acquired using a Spotlight 400 (Perkin Elmer, Shelton, CT, USA) instrumentation. The chemical identity of each particle was determined by evaluating the correspondence of the peaks detected in the IR spectra with those available in the instrumental libraries with a match score higher than 80%.
2.4. Carbopol® and MPs’ Detection and Characterization after Application of the Sanitizing Gel to the Hands of 10 Volunteers
To assess the potential release of Carbopol
® and MPs from the product into the environment, Amuchina
® Gel X-GERM was applied to the hands of 10 volunteers with the aim of simulating real-life use conditions. The activity was performed in an ISO 7 clean room and the ultrapure water used for rinsing volunteers’ hands was pre-filtered to limit any environmental contamination. The initial amount and identity of the particles on volunteers’ hands was evaluated through running blank samples before the application of the hand sanitizer. Volunteers lathered their hands by rubbing them together with soap under tap water. They rinsed their hands by first using deionized water and then scrubbing them with 400 mL of pre-filtered ultrapure water. The entire volume of pre-filtered ultrapure water was collected in a glass container, vacuum-filtered on a silver membrane (3 µm pore size, 25 mm diameter, Sterlitech, Auburn, WA, USA) and used as a blank. Immediately after producing blank samples, volunteers applied the hand sanitizer to their hands following the product instructions reported on the label. An amount of product sufficient to cover the entire surface of the hands (about 0.5–1 g) was poured and volunteers rubbed their hands for 30 s. After the product dried completely, volunteers rinsed their hands by using a procedure inspired by U.S. Department of Health & Human Services [
29]. In particular, volunteers rinsed their hands by rubbing with 400 mL of pre-filtered ultrapure water, and the liquid was collected in a glass container. After carefully mixing the liquid, a volume of 50 mL was vacuum-filtered on a 3 µm pore size silver membrane (25 mm diameter, Sterlitech, Auburn, WA, USA) with the aim of retaining all particles larger than that pore size and therefore detectable by µ-FTIR. All filters were dried at 70 °C for 30 min and analyzed directly by µ-FTIR (Spotlight 400, Perkin Elmer, Shelton, CT, USA). The filter area was fully scanned, collecting bright-field images of the observed particles and acquiring their IR spectrum. Each IR spectrum was collected, recording 4 scans in the infrared spectral range 4000–650 cm
−1 with a 4 cm
−1 spectral resolution. The chemical identity of each particle was determined by evaluating the correspondence of the peaks detected in the IR spectra with those available in the instrumental libraries. The minimum threshold used for automatic identification was set at an 80% match with the results proposed by the instrument software. In case of a lower match score, peaks were identified and assigned manually.
3. Results
Amuchina® Gel X-GERM, like most hand sanitizing gels present on the Italian market, is a Carbopol®-containing product that is used as hand sanitizer, especially during the pandemic. In its pristine form, Carbopol® can be classified as a microplastic since the powder particle size is in the micron range, and its intentional addition to products could raise some concerns about its end-of-life once released into the environment. Therefore, the detection and characterization of Carbopol®-based microparticles in commercial products and along its life cycle were addressed in this research activity.
3.1. Carbopol® Characterization
Before starting the present research activity, Carbopol
® particle size was determined by using optical microscopy. The particle size distribution of the used Carbopol
®, expressed as equivalent diameter, is reported in
Table 1.
Despite the nominal particle size declared by the supplier (Lubrizol Advanced Materials, Inc., Cleveland, OH, USA) corresponding to 2–7 µm, the pristine material is in agglomerated/aggregated form with the particle size reported above.
Carbopol
® spectroscopic characterization was verified by ATR-FTIR.
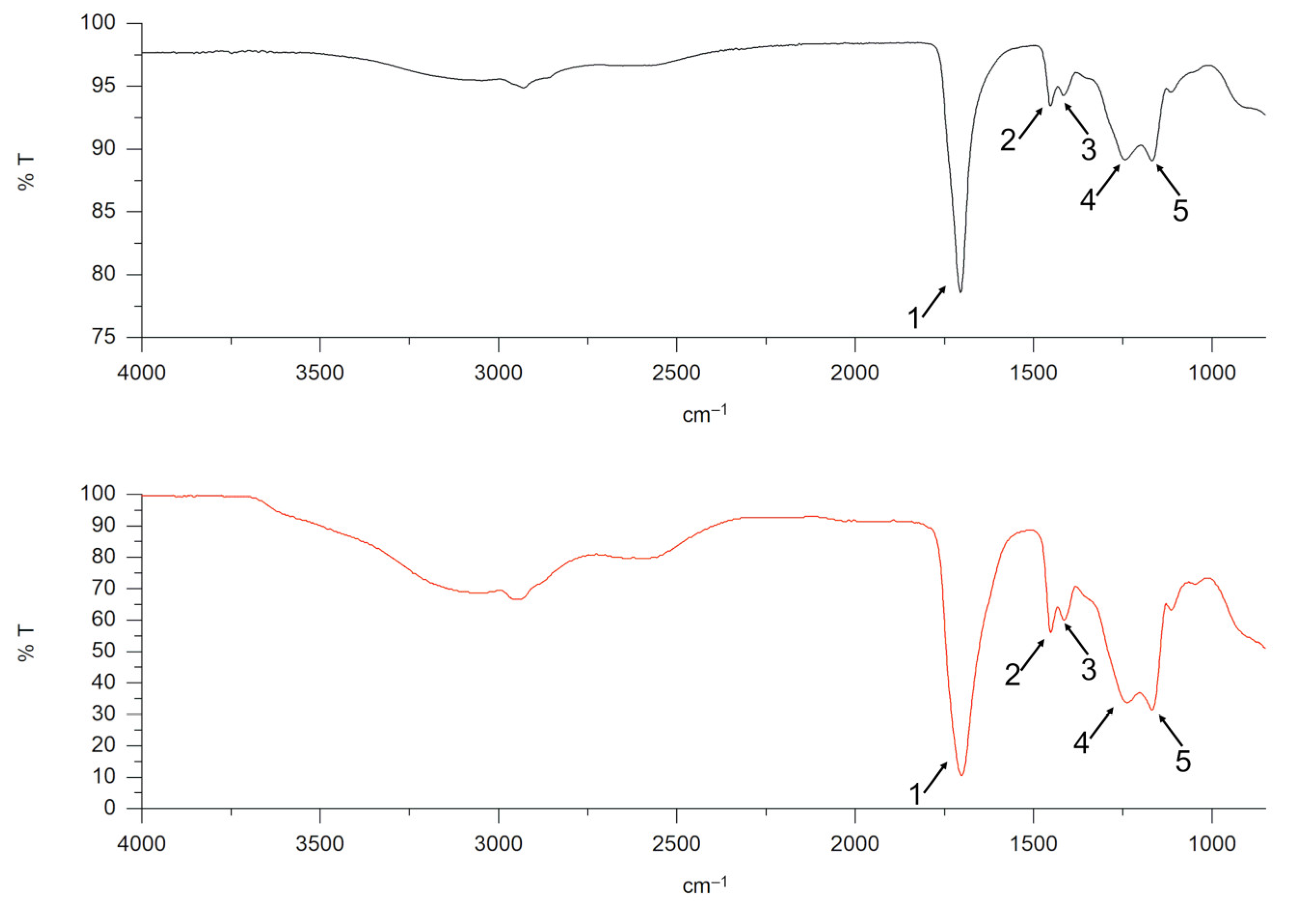
Figure 1 reports the comparison between the FTIR spectrum of the analyzed polymer and the reference spectrum of polyacrylic acid. Both spectra show bands at (i) ~1700 cm
−1 typical of COO
– stretching; (ii) bands at ~1450 cm
−1 due to the scissors vibration of CH
2; and (iii) bands at ~1400 cm
−1 that are related to the bending of CHCO group [
30,
31]. Other characteristic bands are displayed at ~1240 cm
−1, associated with the OH bending of the neighboring carboxyl group, and ~1170 cm
−1, related to C=O stretching.
Table 2 shows the characteristic bands of polyacrylic acid and the corresponding peaks identified in the Carbopol
® spectrum. The match score between Carbopol
® polymer and the reference polyacrylic acid was higher than 97%.
3.2. Detection and Identification of Carbopol® in the Finished Product Amuchina Gel X-GERM®
To perform the morphological and spectroscopical characterization, microparticles should first be collected on a suitable filter surface. To perform this step, particles need to be isolated from the matrix by applying a proper sample preparation procedure, and then collected on the filter via a filtration step. To avoid the clogging effects observed when diluting Amuchina
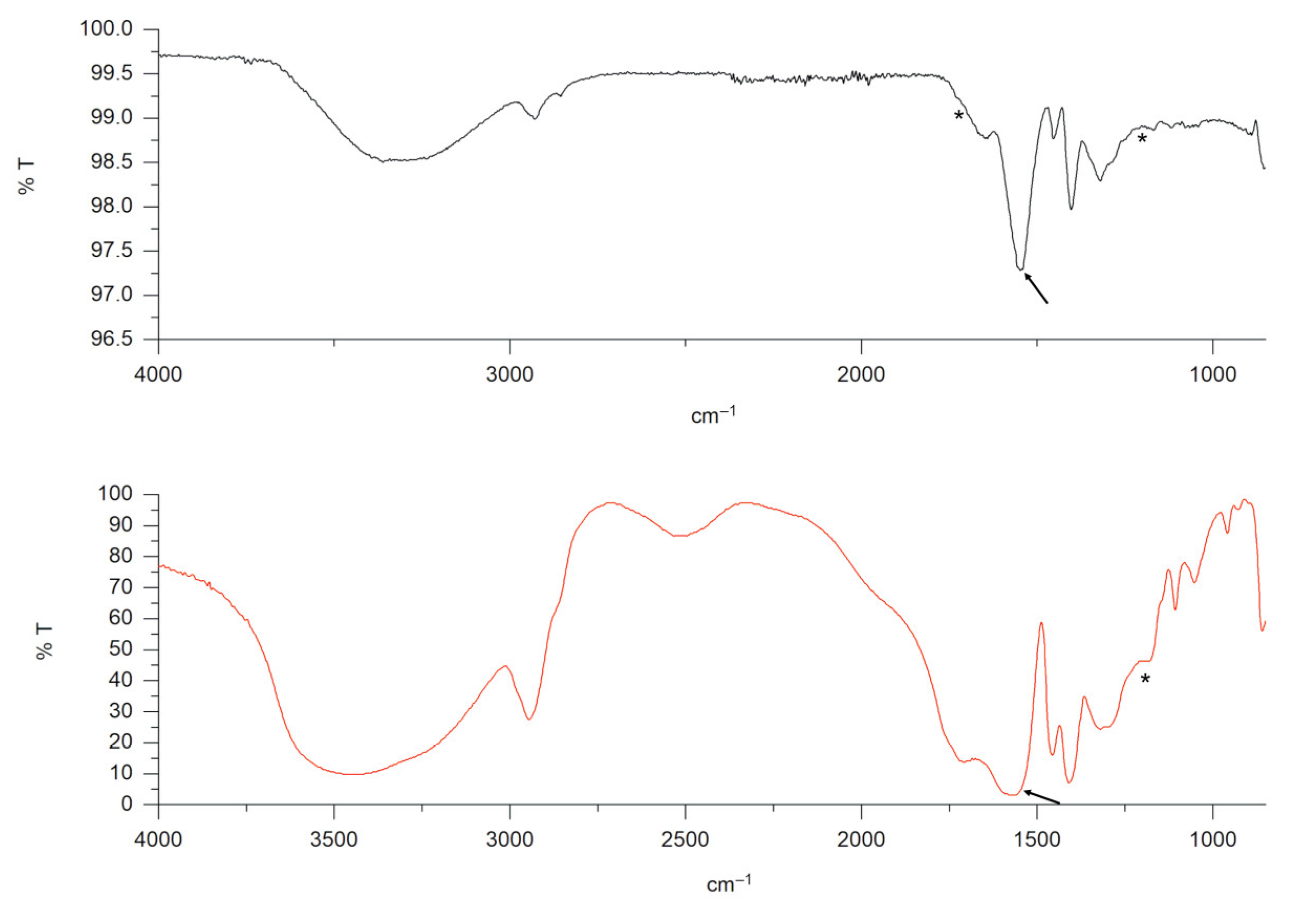
® Gel X-GERM in ethanol/water (80:20) due to the high viscosity of the polymeric-based gel, the product was fluidified by adding a supersaturated saline solution. The addition of a supersaturated saline solution induced collapse of the gel matrix, with the following phase separation: (i) a liquid part able to pass the filter and (ii) a solid flocculate retained on the filter surface. The FTIR spectrum of the flocculate particles retained on the filter is reported in
Figure 2 (black line). The spectrum refers to polyacrylic acid sodium salt, confirming the Carbopol
® composition of flocculate particles.
Although the fluidization process greatly facilitated the filtration of the product, the changes introduced by a supersaturated salt solution leading to a dispersion with a milky pellet from a viscous, transparent gel represent a non-real exposure condition that could affect subsequent characterization steps.
To avoid any potential artifact or product alteration, an alternative approach was applied. A thin layer of the product was prepared on an optical microscope slide, dried overnight and observed with optical microscope for a preliminary screening. The thin layer appears as a continuous layer with some clusters, confirming that no artifacts or alterations in product behavior were introduced (
Figure 3).
This approach proved to be the most promising for the analysis of Carbopol®-derived microplastics in the product, as it preserves the product while allowing for the visualization and identification of particles, if any are present. Therefore, it was applied for the quantitative detection and characterization of microplastics in Amuchina® Gel X-GERM after its application.
3.3. Detection and Characterization of Carbopol® and MPs after Amuchina® Gel X-GERM Application and Carbopol® Chemical Characterization
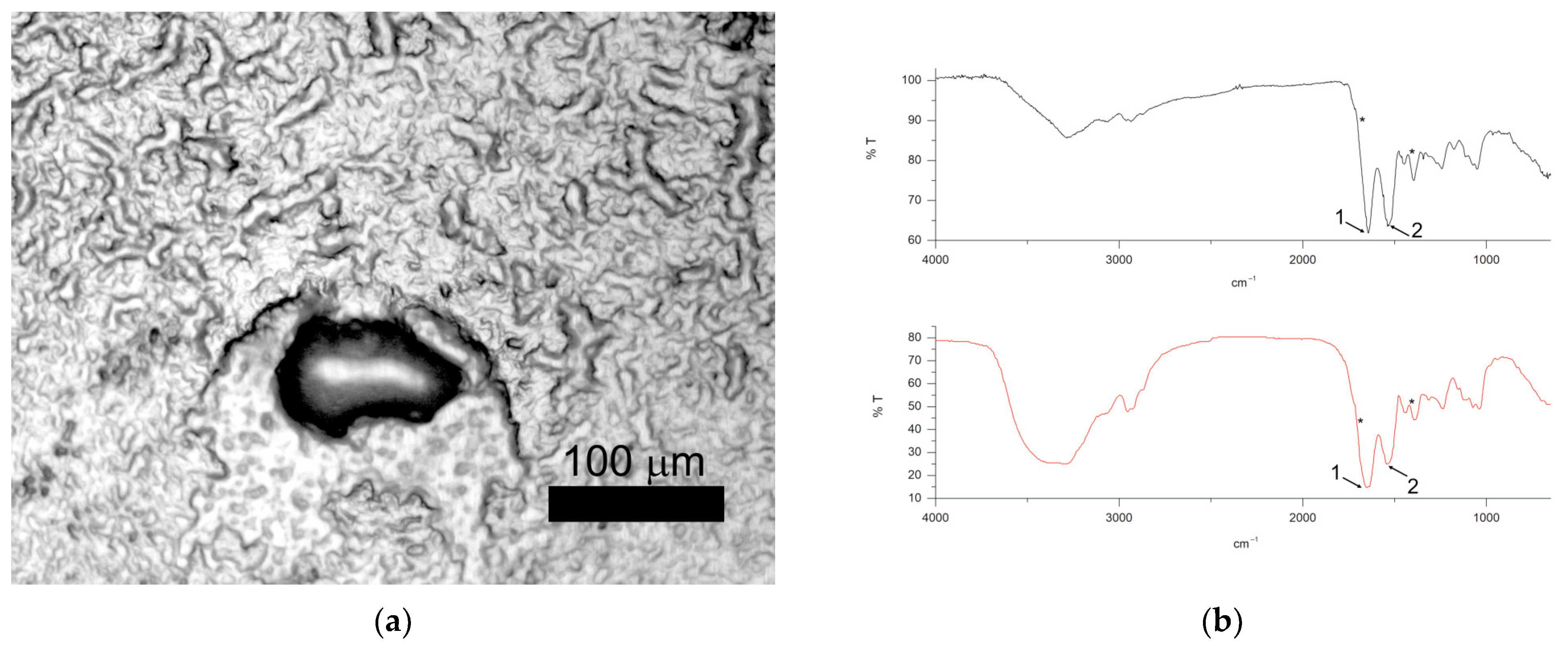
A thin layer of the product was produced by applying about 0.5 g of the product to the microscope slides and immediately observed with both optical and µ-FTIR microscopes. To assess the potential of the approach, random areas of the thin layer of the product were inspected. While optical microscopy permitted the detection of a few particles in the thin layer, chemical identification by µ-FTIR indicated those particles were composed of proteins, cellulose or silicate. Specifically, the IR spectrum was collected for each particle and compared with the IR spectra in the instrumental libraries, with the aim of associating particles with a well-defined category on the basis of a match score of above 80%. In the case of lower match, a score identification was assigned manually.
Figure 4a,b show an example of a particle assigned to cellulose. The list of characteristic bands in the reference and particle spectrum is given in
Table 3.
In order to avoid any water contribution to the analysis, thin layer samples were newly prepared as previously described and dried overnight at 70 °C. In this case, few particles, mainly composed of proteins and cellulose, were detected (
Figure 5a,b).
Table 4 reports the list of characteristic protein peaks identified in the FTIR spectrum that derived from the particle in
Figure 5. No Carbopol
®-derived particles were detected in all tested conditions.
The applied approach also makes it possible to study microplastics that are unintentionally present in the sample. Therefore, a quantitative analysis to identify microplastics within Amuchina
® Gel X-GERM was carried out with µ-FTIR. Two thin layers of the product were placed on two silver filters. While a filter was analyzed immediately after deposition, the second filter was observed after drying overnight at 70 °C. Representative particles, along with their related FTIR spectra, are reported in
Figure 6.
The particle numbers and typology obtained by µ-FTIR analyses are reported in
Table 5. Both samples are characterized by the absence of MPs as well as Carbopol
®-based particles. Few particles other than plastics were detected: (i) 64 particles were detected in the samples before drying and (ii) 42 particles were detected in the samples after drying. The detected particles are mainly composed of proteins or residues of components of the product (i.e., imidazolidinyl urea residues and polyethylene glycol).
3.4. Carbopol® and MPs’ Detection and Characterization after Application of Amuchina® Gel X-GERM to the Hands of 10 Volunteers
To assess the potential release of Carbopol® microparticles into the environment during the product life cycle, a simulation of real-life use conditions was performed by applying the product to the hands of 10 volunteers. After the application of a suitable amount of Amuchina X-Germ according to the product instructions, reported on the label, which was left to completely dry on the hands, volunteers rinsed their hands with pre-filtered ultrapure water in an ISO 7 clean room. After rinsing, the collected water was filtered and analyzed by µ-FTIR. Also, in this case, chemical identification was performed by comparing the spectra with instrumental libraries with the aim of obtaining a match score higher than 80% and, in case of lower match score, identification was assigned manually.
Table 6 reports the results obtained for each volunteer; their corresponding blank is subtracted, as well as the average ± standard deviation for each polymer type. Although the data are characterized by high variability and standard deviation, proteins are the most abundant particles detected in the samples, with a content ranging from 53% to 100%. The high protein concentration, along with their high variability, are probably due to the different release rates of skin debris during handwashing procedures. Other particles detected in the samples were composed of polysaccharides (e.g., cellulose, cotton, etc.), minerals and silicates. Some microplastics were detected in the samples, although they were less than 10% of the total amount of particles (0–6%). Only for volunteer #6 did microplastics account for 11% of all particles detected in the sample. Four plastic polymer types were identified: (i) polyethylene terephthalate (PET), detected in 60% of the samples; (ii) polyethylene (PE) and polypropylene (PP), detected in 20% of the samples; and (iii) polyurethane (PU), detected in 10% of the samples.
No Carbopol® solid microparticles with size larger than 5 µm were detected in the rinsing water, indicating that the original form of Carbopol particles was not retrieved.
4. Discussion
Amuchina® Gel X-GERM is a typical antibacterial gel that is widely used as a hand sanitizer in the Italian market. It is a typical representative of its product category, characterized by a hydroalcoholic base containing 70% ethanol, perfume, excipients and an acrylate jelly agent. It is characterized by a typical good spreadability that allows it to easily penetrate the skin and kill most bacteria. These properties are due to the presence of Carbopol® (0.5–1% w/v), a polyacrylic acid powder whose particles swell, forming a gel that is widely used in the field of formulation of hand sanitizers. The pristine form of Carbopol® (i.e., powder) can be defined as microplastic since it is a synthetic polymeric solid compound with size well below 5 mm (typically within the micron range). The present activity aimed to evaluate if Carbopol® retained its microplastics form in the finished product. This evaluation is crucial for this kind of polymer, which does not dissolve in water but disperses, in light of the restriction on the intentional use of microplastics in certain products, such as wash-off cosmetics, which recently came into force in the European Union. The applied approach revealed the absence of Carbopol®-based particles or other types of microplastics in Amuchina® Gel X-GERM, indicating that Carbopol® does not retain its microplastics form in the product.
With the aim of assessing the fate of Carbopol® throughout its life cycle, 10 volunteers used Amuchina® Gel X-GERM to simulate the real-life conditions of use. After application, they rinsed their hands and the water used was collected for the subsequent investigation of the potential presence of solid Carbopol® particles. No solid Carbopol® microparticles larger than 5 µm were detected using µFTIR in the rinsing water, indicating that the original form of Carbopol particles was not retrieved throughout the product life cycle.
This study demonstrated that the original microplastic form of Carbopol® is definitely modified from the product preparation to real life use, meeting derogation 5b reported in the—ANNEX XV restriction report proposed by ECHA regarding the restriction on intentionally added microplastics regarding substances with physical properties that are permanently changed during the use of the mixture such that the polymers no longer fulfil the meaning of microplastics.
This work represents the first study in which the fate of a microplastic in a commercial product is assessed throughout its life cycle, from manufacturing throughout its use to disposal. The approach is useful to determine human exposure as well as the release of microplastics into the environment. Moreover, with a wider application and appropriate designs, this approach could be also applied in an easy and reproducible way to evaluate the potential release of chemical components deriving from the use of dermal products into the aquatic environment.
Further investigations would be useful to characterize the potential release of Carbopol® particles with a size smaller than 5 µm. This fraction is very difficult to identify and characterize due to instrumental limitations. However, it would represent a step further in the micro- and nano-plastics field.