Microplastic Volatile Organic Compounds Found within Chrysaora chesapeakei in the Patuxent River, Maryland
Abstract
1. Introduction
C. chesapeakei
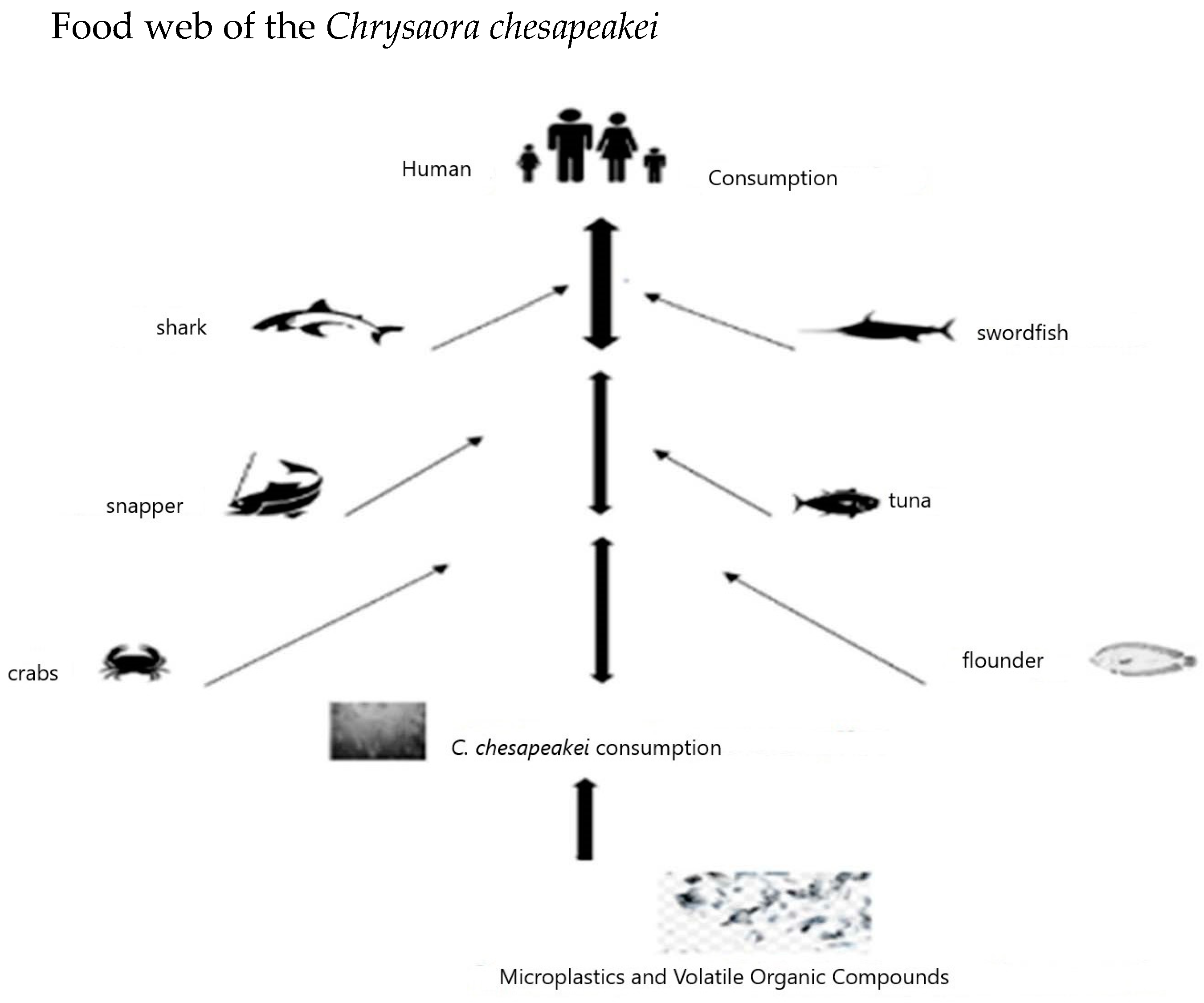
2. Microplastic Movements and C. chesapeakei
3. Material and Methods
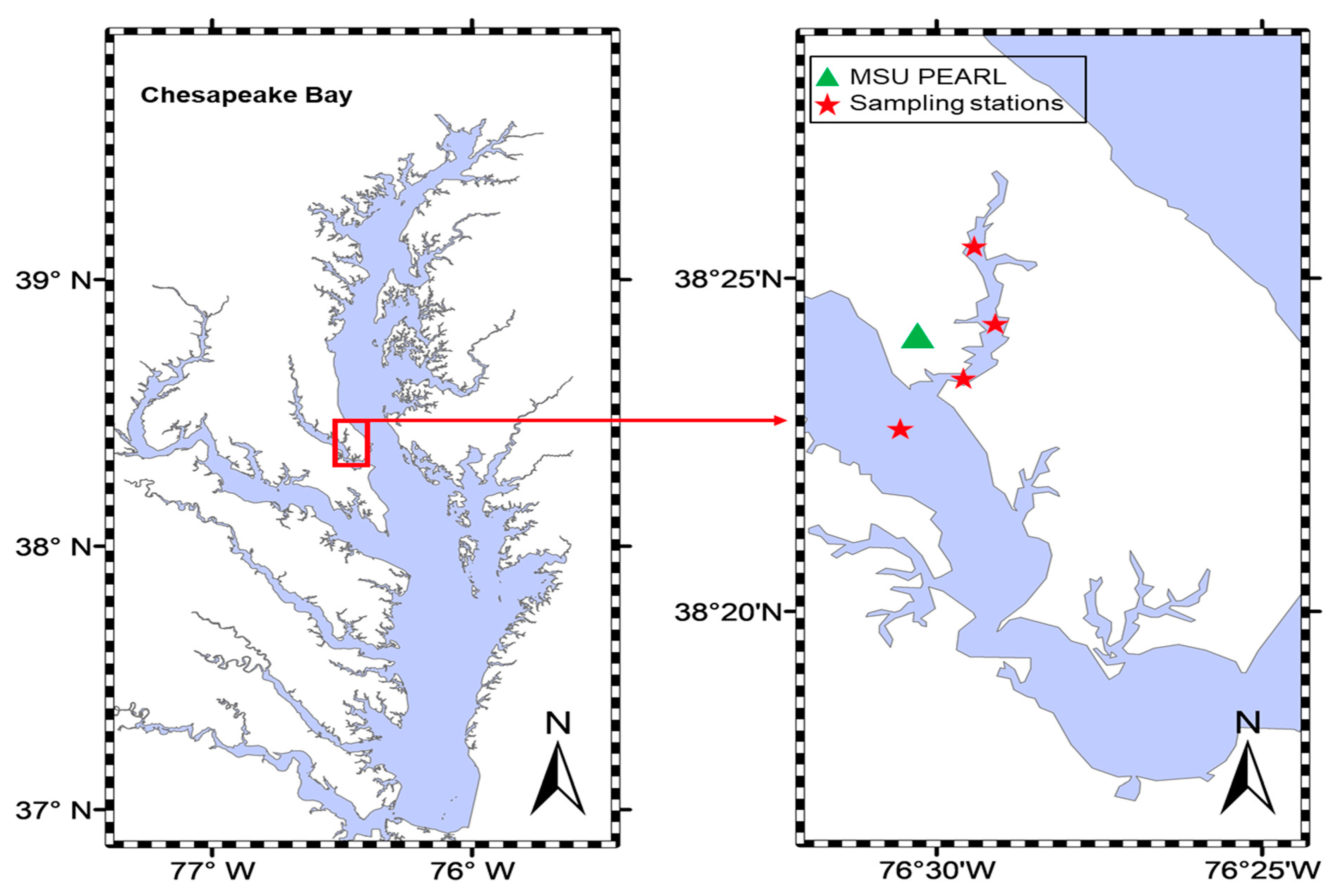
3.1. Sample Collections
- 38°23.104 N, 076°30.025 W;
- 38°23.470 N, 076°29.584 W;
- 38°26.170 N, 076°29.386 W;
- 38°25.481 N, 076°29.372 W.
3.2. Contamination Prevention
4. Procedures
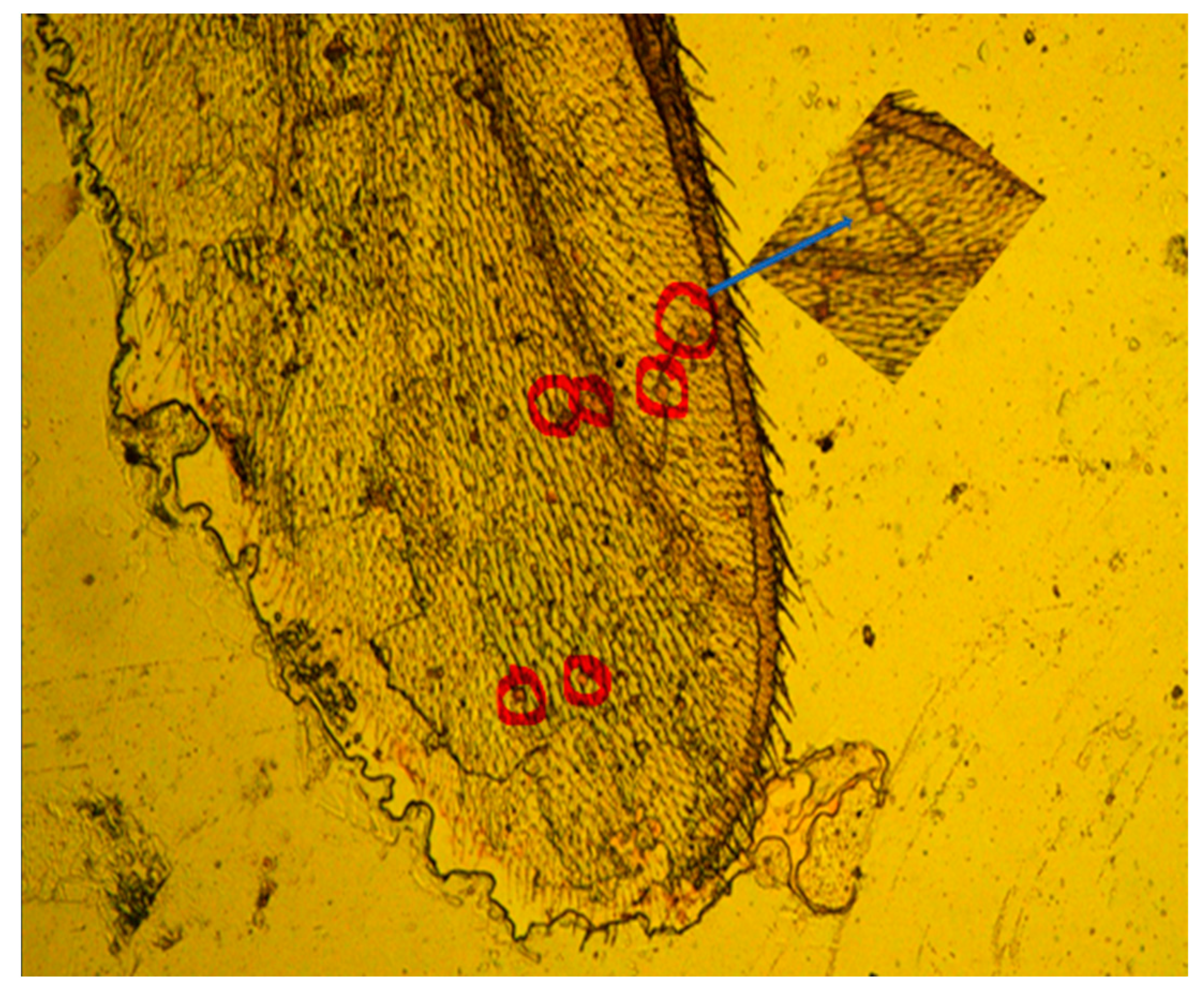
4.1. Identification of Native Microplastic in Jellyfish (Before GC-MS)
Rhodamine B Staining
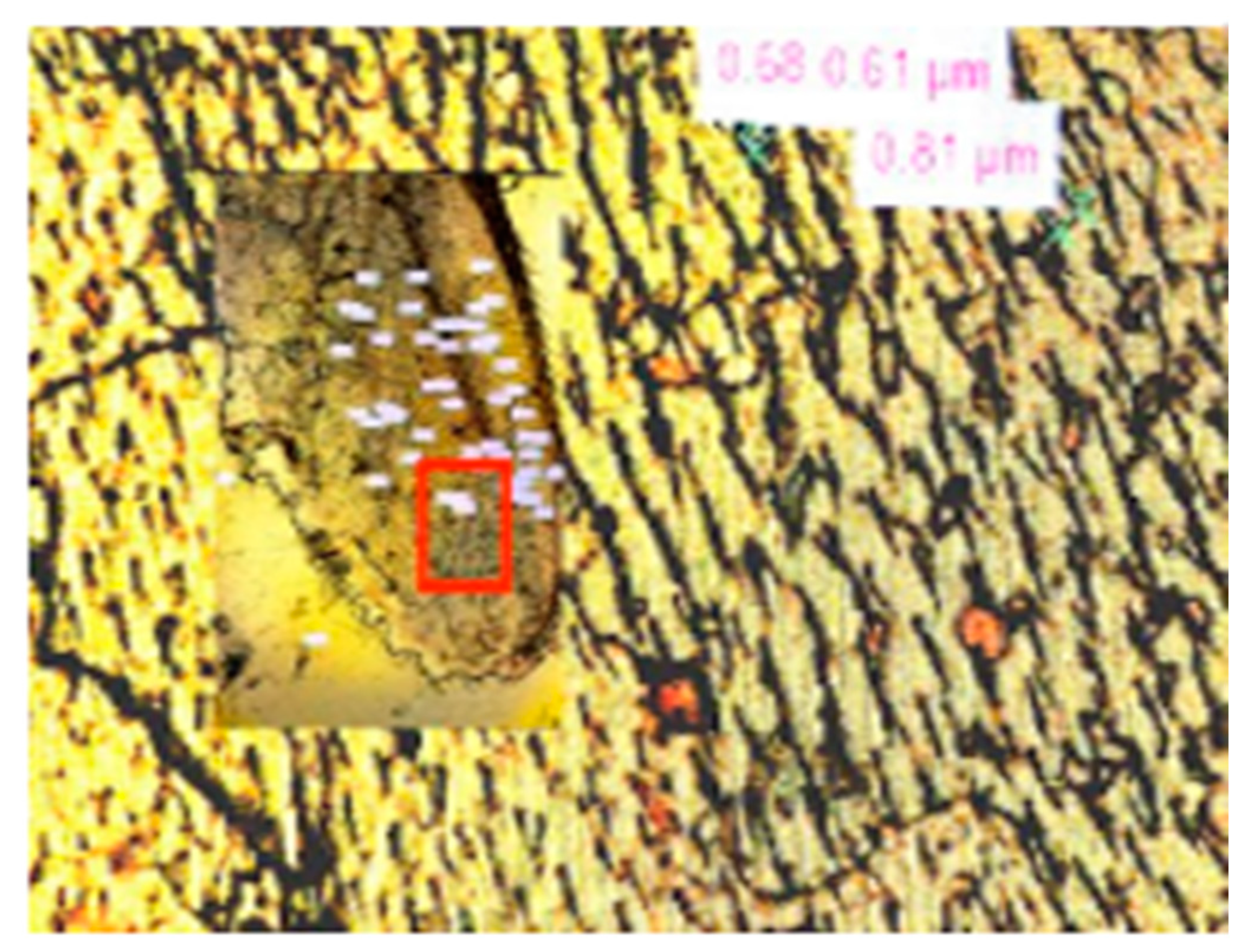
4.2. Microplastic Identification Imaging
4.3. Gas Chromatography Studies for Volatile Organic Compounds
4.4. GC-MS Techniques
4.4.1. Headspace-SPME-GC-MS Method
4.4.2. Solvent Extraction Method
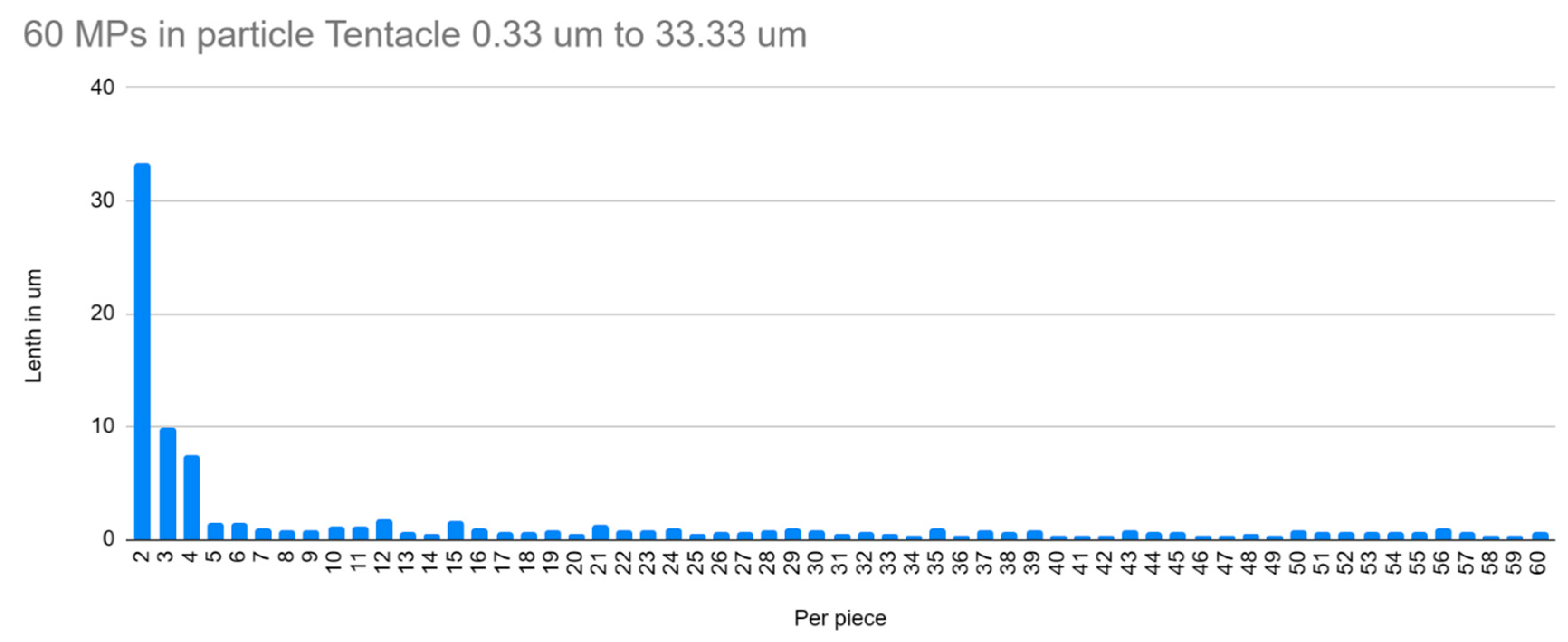
5. Results
6. Discussion
6.1. How Does the Chrysaora chesapeakei Microplastic Content Reach the Human Population from the Chesapeake Bay?
6.2. C. chesapeakei Tentacle from the Field
6.3. Volatile Compound Adverse Effects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomonaco, T.; Manco, E.; Corti, A.; La Nasa, J.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Modugno, F.; Ceccarini, A.; Fuoco, R.; et al. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: A neglected source of environmental pollution. J. Hazard. Mater. 2020, 394, 122596. [Google Scholar] [CrossRef]
- Chary, N.S.; Fernandez-Alba, A.R. Determination of volatile organic compounds in drinking and environmental waters. TrAC Trends Anal. Chem. 2012, 32, 60–75. [Google Scholar] [CrossRef]
- Wright, S.; Ulke, J.; Font, A.; Chan, K.; Kelly, F. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2019, 136, 105411. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Lebreton, L.-M.; Greer, S.; Borrero, J. Numerical modelling of floating debris in the world’s oceans. Mar. Pollut. Bull. 2012, 64, 653–661. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef]
- Ceccarini, A.; Corti, A.; Erba, F.; Modugno, F.; La Nasa, J.; Bianchi, S.; Castelvetro, V. The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments. Environ. Sci. Technol. 2018, 52, 5634–5643. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Bianchi, S.; Ceccarini, A.; Manariti, A.; Vinciguerra, V. Quantification of poly(ethylene terephthalate) micro- and nanoparticle contaminants in marine sediments and other environmental matrices. J. Hazard. Mater. 2019, 385, 121517. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ran, C.; Ding, Q.-W.; Liu, H.-L.; Xie, M.-X.; Yang, Y.-L.; Xie, Y.-D.; Gao, C.-C.; Zhang, H.-L.; Zhou, Z.-G. Ability of prebiotic polysaccharides to activate a HIF1α-antimicrobial peptide axis determines liver injury risk in zebrafish. Commun. Biol. 2019, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Bay Nettle (Chrysaora chesapeakei)—Jungle Dragon. (n.d.). Available online: https://www.jungledragon.com/specie/26324/bay_nettle.html (accessed on 10 March 2024).
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Chan, K.Y.K. Microplastics reduced posterior segment regeneration rate of the polychaete Perinereis aibuhitensis. Mar. Pollut. Bull. 2018, 129, 782–786. [Google Scholar] [CrossRef]
- Murphy, F.; Quinn, B. The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ. Pollut. 2018, 234, 487–494. [Google Scholar]
- Lo, H.K.A.; Chan, K.Y.K. Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ. Pollut. 2018, 233, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Dinasquet, J.; Titelman, J.; Møller, L.F.; Setälä, O.; Granhag, L.; Andersen, T.; Båmstedt, U.; Haraldsson, M.; Hosia, A.; Katajisto, T.; et al. Cascading effects of the ctenophore Mnemiopsis leidyi on the planktonic food web in a nutrient-limited estuarine system. Mar. Ecol. Prog. Ser. 2012, 460, 49–61. [Google Scholar] [CrossRef]
- McNamara, M.; Lonsdale, D.; Cerrato, R. Role of eutrophication in structuring planktonic communities in the presence of the ctenophore Mnemiopsis leidyi. Mar. Ecol. Prog. Ser. 2014, 510, 151–165. [Google Scholar] [CrossRef]
- Wright, R.M.; Le Quéré, C.; Buitenhuis, E.; Pitois, S.; Gibbons, M.J. Role of jellyfish in the plankton ecosystem revealed using a global ocean biogeochemical model. Biogeosciences 2021, 18, 1291–1320. [Google Scholar] [CrossRef]
- GESAMP (2016) Sources, Fate, and Effects of Microplastics in the Marine Environment: Part Two of a Global Assessment Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Rep. Stud. No. 93. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment|UNEP—UN Environment Programme. Available online: http://www.gesamp.org/site/assets/files/1275/sources-fate-and-effects-of-microplastics-in-the-marine-environment-part-2-of-a-global-assessment-en.pdf (accessed on 10 March 2024).
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.-A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2000, 35, 318–324. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Garrigós, M.; Garrigós, J. Plastic as a Vector of Dispersion for Marine Species With Invasive Potential. A Review. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Americus, B.; Lotan, T.; Bartholomew, J.L.; Atkinson, S.D. A comparison of the structure and function of nematocysts in free-living and parasitic cnidarians (Myxozoa). Int. J. Parasitol. 2020, 50, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Thorington, G.U.; Hessinger, D.A. Efferent Mechanisms of Discharging Cnidae: II. A Nematocyst Release Response in the Sea Anemone Tentacle. Biol. Bull. 1998, 195, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, K.J. Biphasic Feeding Response in a Sea Anemone: Control by Asparagine and Glutathione. Science 1971, 173, 333–334. [Google Scholar] [CrossRef]
- Wei, X.-F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef]
- NOAA. What Are Jellyfish Bodies Made from? What Are Jellyfish Made of? NOAA: Washington, DC, USA, 2023.
- Purcell, J.E.; Decker, M.B. Effects of climate on relative predation by scyphomedusae and ctenophores on copepods in Chesapeake Bay during 1987–2000. Limnol. Oceanogr. 2005, 50, 376–387. [Google Scholar] [CrossRef]
- Morabito, R.; Marino, A.; La Spada, G. Nematocytes’ activation in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms. J. Comp. Physiol. A 2012, 198, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ormond, R.F.; Caldwell, S. The effect of oil pollution on the reproduction and feeding behavior of the sea anemone Actinia equina. Mar. Pollut. Bull. 1982, 13, 118–122. [Google Scholar] [CrossRef]
- Available online: https://www.seatemperature.org/north-america/united-states/solomons (accessed on 10 March 2024).
- Smith, C.; Denaro, F.; Fan, C.; Pramanik, S. The Effective Use of the Inexpensive LED Microscope with Rhodamine Blue Staining to Identify Microplastics. Microsc. Today 2023, 31, 36–37. [Google Scholar] [CrossRef]
- Sauls-Smith, C.A.; Pramanik, S.; Drichko, N.; Fan, C. The Occurrence of Microplastics in Chrysaora Chesapeakei in the Patuxent River, Maryland; Science Symposium Morgan State University: Baltimore, MD, USA, 2022. [Google Scholar]
- Gemmell, B.J.; Du Clos, K.T.; Colin, S.P.; Sutherland, K.R.; Costello, J.H. The most efficient metazoan swimmer creates a ‘virtual wall’ to enhance performance. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202494. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2020, 28, 4209–4215. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.; Chen, G.; Wang, X.; Yuan, W.; Lv, Z. Optimization of a headspace solid-phase microextraction-gas chromatography–mass spectrometry procedure for odor compounds from polyolefin resin used in plastic food packaging. Packag. Technol. Sci. 2020, 33, 515–529. [Google Scholar] [CrossRef]
- Fabris, S.; Freire, M.T.d.A.; Wagner, R.; Reyes, F.G. A method to determine volatile contaminants in polyethylene terephthalate (PET) packages by HDC-GC-FID and its application to post-consumer materials. Food Sci. Technol. 2010, 30, 1046–1055. [Google Scholar] [CrossRef]
- Strangl, M.; Fell, T.; Schlummer, M.; Maeurer, A.; Buettner, A. Characterization of odorous contaminants in post-consumer plastic packaging waste using multidimensional gas chromatographic separation coupled with olfactometric resolution. J. Sep. Sci. 2017, 40, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Camacho, W.; Karlsson, S. Quality-determination of recycled plastic packaging waste by identification of contaminants by GC–MS after microwave assisted extraction (MAE). Polym. Degrad. Stab. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Lin, Q.-B.; Song, X.-C.; Chen, S.; Zhong, H.-N.; Nerin, C. Discrimination of Virgin and Recycled Polyethylene Based on Volatile Organic Compounds Using a Headspace GC-MS Coupled with Chemometrics Approach. Food Packag. Shelf Life 2020, 26, 100553. [Google Scholar] [CrossRef]
- He, Z.; Li, G.; Chen, J.; Huang, Y.; An, T.; Zhang, C. Pollution characteristics and health risk assessment of volatile organic compounds emitted from different plastic solid waste recycling workshops. Environ. Int. 2015, 77, 85–94. [Google Scholar] [CrossRef]
- Curran, K.; Strlič, M. Polymers and volatiles: Using VOC analysis for the conservation of plastic and rubber objects. Stud. Conserv. 2014, 60, 1–14. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, H.; Peng, X.; Wang, Z.; Wang, R.; Li, Z.; Fang, P. Aging behaviors of silicone rubber composite materials under outdoor environment. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 1289–1293. [Google Scholar] [CrossRef]
- Nerìn, C.; Albiñana, J.; Philo, M.R.; Castle, L.; Raffael, B.; Simoneau, C. Evaluation of some screening methods for the analysis of contaminants in recycled polyethylene terephthalate flakes. Food Addit. Contam. 2003, 20, 668–677. [Google Scholar] [CrossRef]
- Mihreteab, M.; Stubblefield, B.A.; Gilbert, E.S. Microbial bioconversion of thermally depolymerized polypropylene by Yarrowia lipolytica for fatty acid production. Appl. Microbiol. Biotechnol. 2019, 103, 7729–7740. [Google Scholar] [CrossRef]
- Oseji, O.F.; Fan, C.; Chigbu, P. Composition and Dynamics of Phytoplankton in the Coastal Bays of Maryland, USA, Revealed by Microscopic Counts and Diagnostic Pigments Analyses. Water 2019, 11, 368. [Google Scholar] [CrossRef]
- Hirose, E.; Sakai, D.; Iida, A.; Obayashi, Y.; Nishikawa, J. Exumbrellar Surface of Jellyfish: A Comparative Fine Structure Study with Remarks on Surface Reflectance. Zool. Sci. 2021, 38, 170–178. [Google Scholar] [CrossRef]
- Britannica, T. Editors of Encyclopedia (2009, April 1). epithelium. Encyclopedia Britannica. Available online: https://www.britannica.com/science/epithelium (accessed on 10 March 2024).
- Hydrophobic Interactions. (2020, August 15). Available online: https://chem.libretexts.org/@go/page/1506 (accessed on 10 March 2024).
- Gall, S.; Thompson, R. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Wu, L.; Yang, Y.; Yu, X.; Liu, Q.; Liu, X.; Li, Y.; Wang, X. Vertical distribution and river-sea transport of microplastics with tidal fluctuation in a subtropical estuary, China. Sci. Total. Environ. 2022, 822, 153603. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Ray, S.; Cooney, R.P. Thermal Degradation of Polymer and Polymer Composites. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gardette, M.; Perthue, A.; Gardette, J.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stabil. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- McMullen, K.; Vargas, F.H.; Calle, P.; Alavarado-Cadena, O.; Pakhomov, E.A.; Alava, J.J. Modelling microplastic bioaccumulation and biomagnification potential in the Galápagos penguin ecosystem using Ecopath and Ecosim (EwE) with Ecotracer. PLoS ONE 2024, 19, e0296788. [Google Scholar] [CrossRef]
- Kang, P.; Wu, P.; Jin, Y.; Shi, S.; Gao, D.; Chen, G.; Li, Q. Formation and Emissions of Volatile Organic Compounds from Homo-PP and Co-PP Resins during Manufacturing Process and Accelerated Photoaging Degradation. Molecules 2020, 25, 2761. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Lucas, A.; Mallenco-Fornies, R.; Briones-Rizo, M.; Calvo, E.; Pelejero, C. Abiotic plastic leaching contributes to ocean acidification. Sci. Total Environ. 2023, 854, 158683. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nationalgeographic.com/science/article/plastic-breaks-down-in-ocean-after-all-and-fast (accessed on 10 March 2024).
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Landmark study links microplastics to serious health problems. Nature 2024. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Braun, A.; Seitz, H. Uptake and Cellular Effects of Polymethylmethacrylate on Human Cell Lines. Microplastics 2024, 3, 205–216. [Google Scholar] [CrossRef]
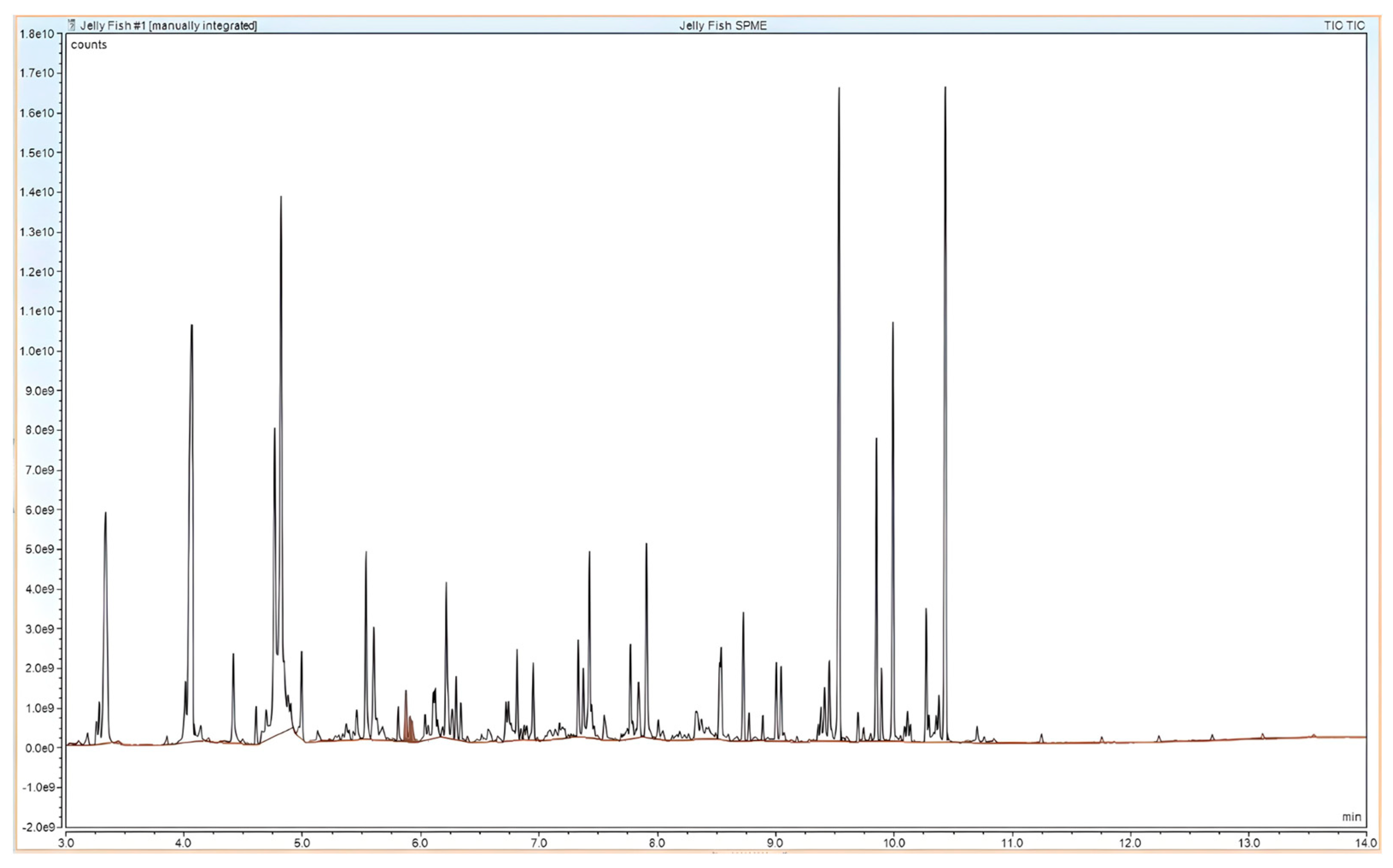
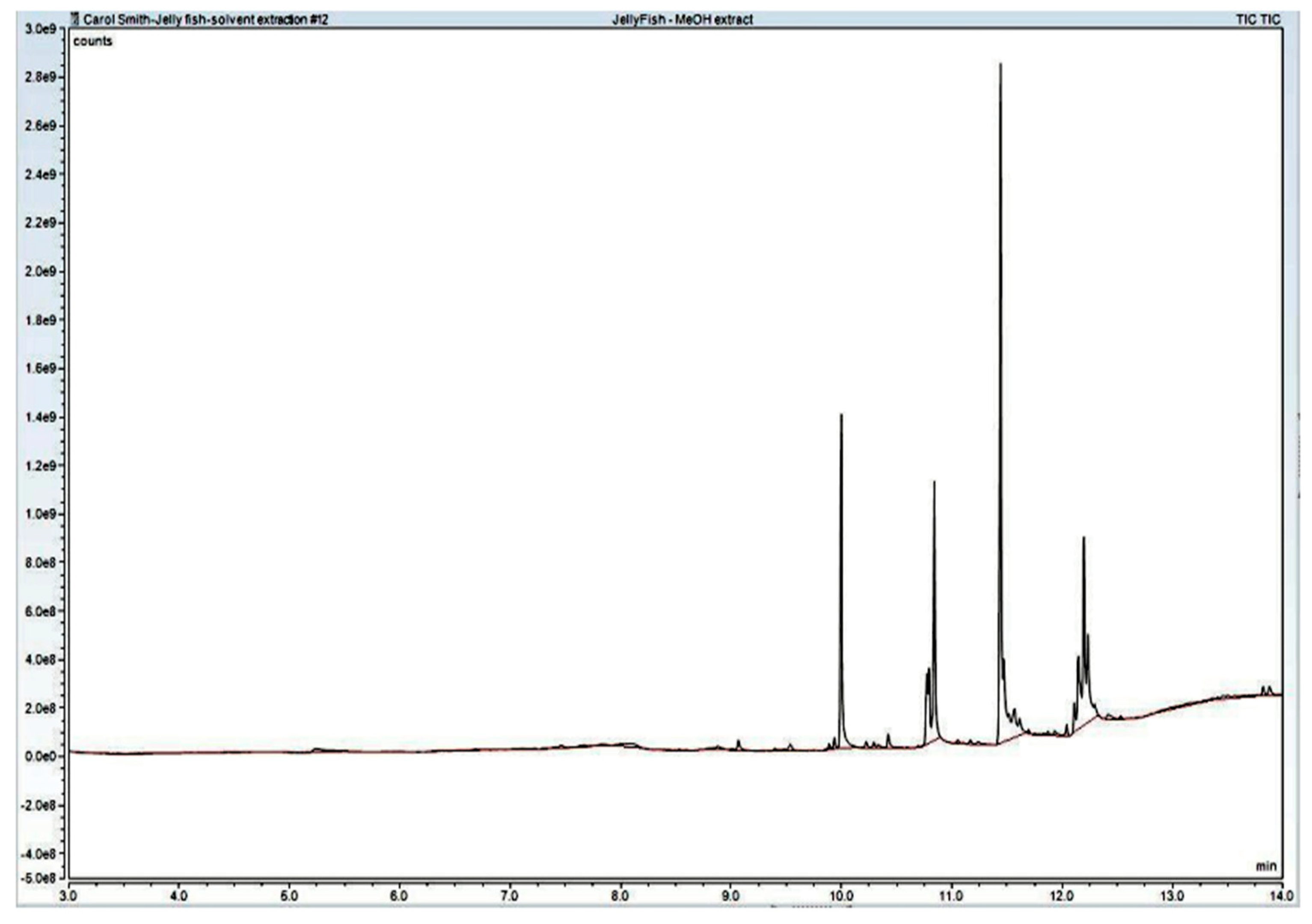
| Volatile Organic Compounds from within the C. chesapeakei | Publications Verifying the MP VOC Distinction |
|---|---|
| Ketone (SPME) Heptanone | Cabanes et al., 2020 [48] |
| Aldehyde (SPME) | |
| Octadecanal | Cababes et al., 2020 [48], Han et al., 2020 [49] |
| 9-Octadecenal, (Z)- | Cababes et al., 2020, Han et al., 2020 |
| 13-Methyltetradecanal | Cababes et al., 2020, Han et al., 2020 |
| trans-2-Nonenal | Cababes et al., 2020, Han et al., 2020 |
| Nonanal | Cabanes et al., 2020, Han et al., 2020,Fabris et al. 2008 [50] |
| Benzeneacetaldehyde | (Cababes et al., 2020, Han et al., 2020) |
| Hexadecanal | Cababes et al., 2020 |
| Vanillin Phenolic | Cababes et al., 2020, Han et al., 2020, Stragel et al. 2017 [51] |
| Lilac aldehyde D | |
| Octanal | Cabanes et al., 2020, Han 2020, Fabris et al., 2008 |
| Alcohols (SPME) | |
| (R)-(-)-(Z)-14-Methyl-8-hexadecen-1-ol | Cabanes et al., 2020, Camacho and Karlsson 2000 [52] |
| 12-Methyl-E, E-2,13-octadecadien-1-ol | Camacho and Karlsson 2000 [53] |
| Ethanol, 2-(9-octadecenyloxy)-, (Z) | Cabanes et al., 2020, Han et al., 2020 |
| 1-Undecanol | Han et al., 2020 |
| 2-Decen-1-ol | Cabanes et al., 2020 |
| 1,3,5-Pentanetriol, 3-methyl | |
| trans-2-Ethyl-2-hexen-1-ol | Cabanes et al., 2020, Han et al., 2020 |
| trans-2-Dodecen-1-ol | Cabanes et al., 2020, Camacho and Karlsson 2000 |
| Alkanes (SPME) | |
| Decane, 2,3,5,8-tetramethyl | Chen et al. 2020 [54], Han et al., 2020 |
| 9-Oxabicyclo [6.1.0] nonane | Cabanes et al., 2020, Han et al., 2020 |
| Peroxide (SPME) | |
| 2,5-Dimethylhexane-2,5-dihydroperoxide | Han et al., 2020 |
| Alkene (SPME) | |
| 5-Ethyl-1-nonene | Cabanes et al., 2020 |
| Cyclohexene, 1,5,5-trimethyl-6-acetylmethyl | He et al., 2015 [55] |
| Siloxanes (SPME) | |
| Cyclononasiloxane, octadecamethyl | Curran and Strlic 2015 [56], Huang et al. 2016 [57] |
| Cyclohexasiloxane, dodecamethyl | Huang, Zhen, Lin, Peng et al. 2016 |
| Cyclotrisiloxane, hexamethyl | Huang, Zhen, Lin, Peng et al. 2016 |
| Ester (SPME and MeOH) | |
| 3-Trifluoroacetoxypentadecane | Nerin et al., 2001 [58] |
| 9,12,15-Octadecatrienoic acid, 2-(acetyloxy)-1-[(acetyloxy)methyl] ethyl ester, (Z, Z,Z) | Camacho and Karlsson 2000 |
| Aromatic (SPME) | |
| 1,1-Biphenyl, 3,4-diethyl | Camacho and Karlsson 2000 |
| Acids (MeOH) | |
| Acetic acid | Cabanes et al., 2020 |
| Tetradecanoic acid | Cabanes et al., 2020 |
| 9-Hexadecenoic acid | Cabanes et al., 2020, Camacho and Karlsson 2000 |
| Z-8-Methyl-9-tetradecenoic acid | Cabanes et al., 2020 |
| n-Hexadecanoic acid | Cabanes et al., 2020, Camacho and Karlsson 2000 |
| Dodecanoic acid, 3-hydroxy | Cabanes et al., 2020, Camacho and Karlsson 2000 |
| n-Hexadecanoic acid | Cabanes et al., 2020, Camacho and Karlsson 2000 |
| Oleic Acid | Mihreteab et al. 2019 [59] |
| Octadecanoic acid MeOH | Cabanes et al., 2020, Fabris et al., 2008, Camacho Karlsson 2000 |
| Ether (SPME) | |
| Furan, 2-pentyl Cyclic | Cabanes et al., 2020 |
| Chemical Groups | SPME | MeOH |
|---|---|---|
| Ketone | 0.25 | |
| Aldehydes | 25.81 | |
| Alcohols | 6.63 | |
| Alkanes | 7.57 | |
| Siloxanes | 4.8 | |
| Esters | 0.3 | |
| Alkene | 0.6 | 3.71 |
| Aromatic | 0.08 | |
| Acid | 3.06 | 30.71 |
| Ether | 0.11 | |
| Other | 50.79 | 65.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, C.A.; Mandal, S.; Fan, C.; Pramanik, S. Microplastic Volatile Organic Compounds Found within Chrysaora chesapeakei in the Patuxent River, Maryland. Microplastics 2024, 3, 250-263. https://doi.org/10.3390/microplastics3020015
Smith CA, Mandal S, Fan C, Pramanik S. Microplastic Volatile Organic Compounds Found within Chrysaora chesapeakei in the Patuxent River, Maryland. Microplastics. 2024; 3(2):250-263. https://doi.org/10.3390/microplastics3020015
Chicago/Turabian StyleSmith, Carol A., Santosh Mandal, Chunlei Fan, and Saroj Pramanik. 2024. "Microplastic Volatile Organic Compounds Found within Chrysaora chesapeakei in the Patuxent River, Maryland" Microplastics 3, no. 2: 250-263. https://doi.org/10.3390/microplastics3020015
APA StyleSmith, C. A., Mandal, S., Fan, C., & Pramanik, S. (2024). Microplastic Volatile Organic Compounds Found within Chrysaora chesapeakei in the Patuxent River, Maryland. Microplastics, 3(2), 250-263. https://doi.org/10.3390/microplastics3020015







