Microfibers: Environmental Problems and Textile Solutions
Abstract
1. Introduction
2. Measuring Environmental Concentrations
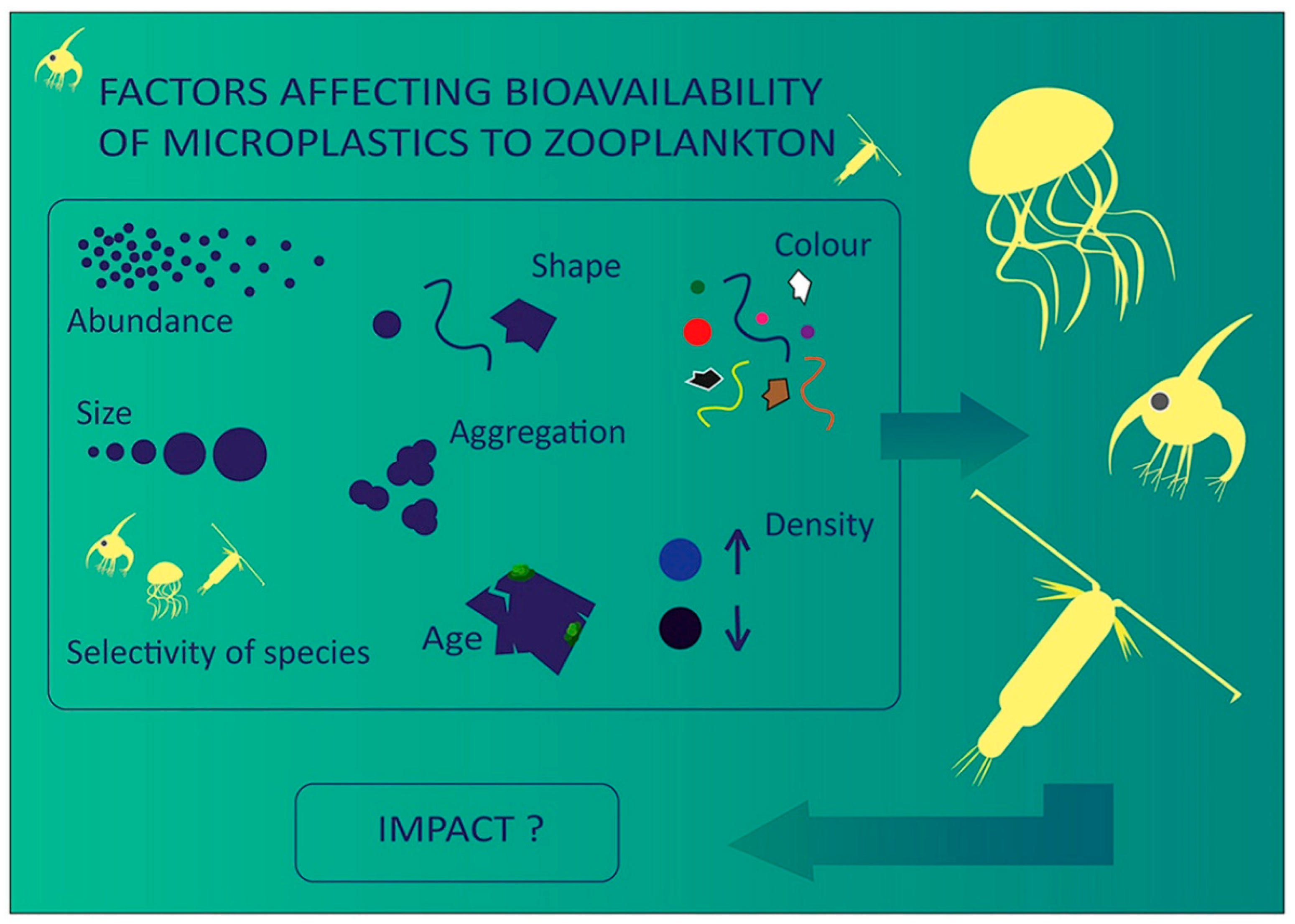
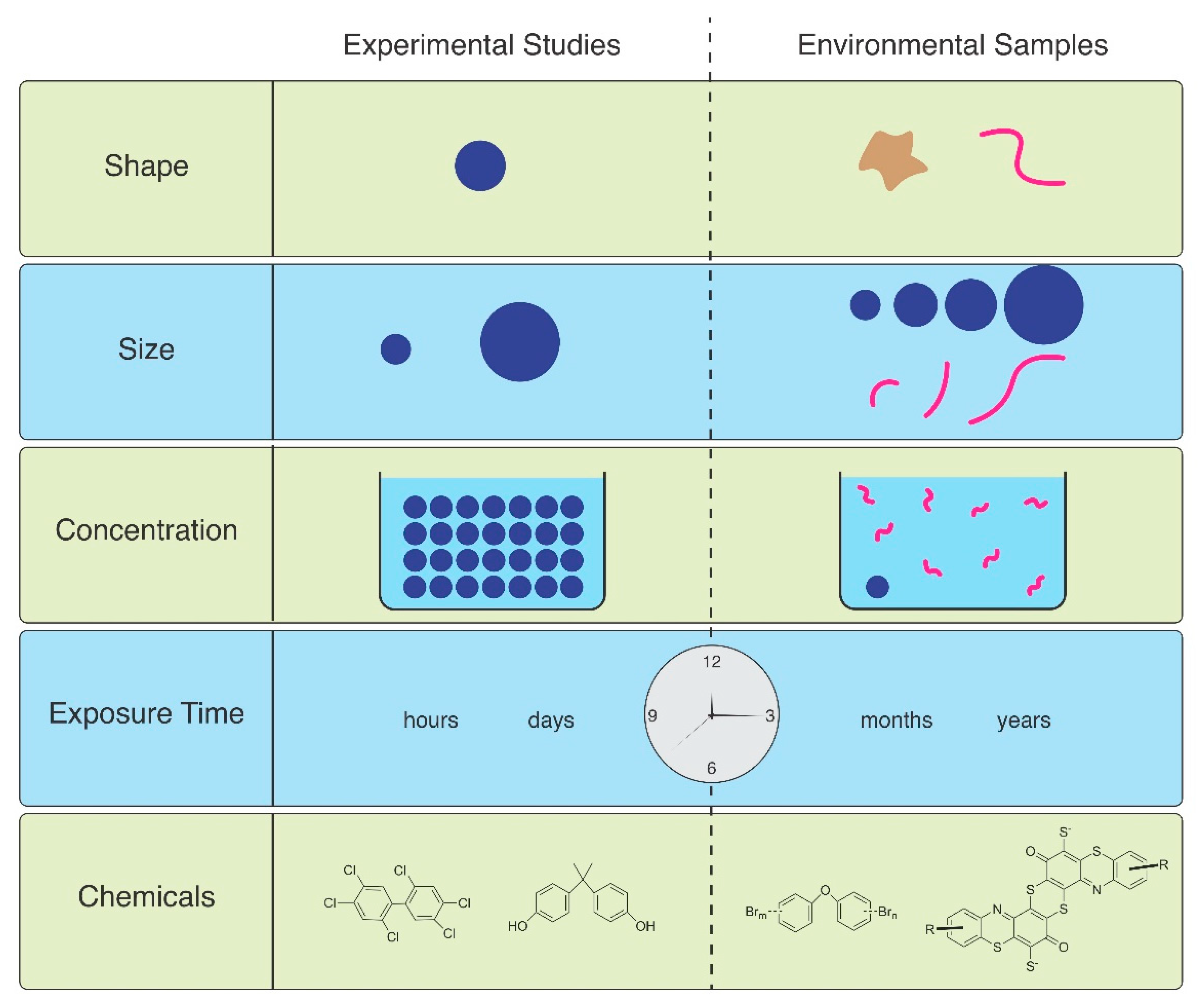
3. Ingestion and Egestion
4. Effects
5. Associated Chemicals
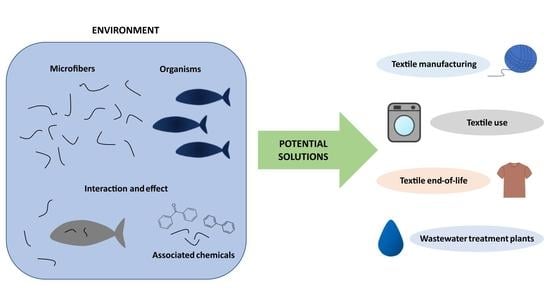
6. Potential Solutions
7. Textile Manufacturing
8. Textile Use
9. Textile End of Life
10. Wastewater Treatment Plants
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vidal, A.; Thompson, R.; Canals, M.; De Haan, W.P. The imprint of microfibres in Southern European deep seas. PLoS ONE 2018, 13, e0207033. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A. A critical overview of te analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Green, D.S.; Kregting, L.; Boots, B.; Blockley, D.J.; Brickle, P.; da Costa, M.; Crowley, Q. A comparison of sampling methods for seawater microplastics and a first report of the microplastic litter in coastal waters of Ascension and Falkland Islands. Mar. Pollut. Bull. 2018, 137, 695–701. [Google Scholar] [CrossRef]
- Burns, E.; Boxall, A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef]
- Carr, S.A. Sources and dispersive modes of microfibers in the environment. Integr. Environ. Assess. Manag. 2017, 13, 466–469. [Google Scholar] [CrossRef]
- Constant, M.; Kerhervé, P.; Mino-Vercellio-Verollet, M.; Dumontier, M.; Vidal, A.S.; Canals, M.; Heussner, S. Beached microplastics in the northwestern Mediterranean Sea. Mar. Pollut. Bull. 2019, 142, 263–273. [Google Scholar] [CrossRef]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Colom, E.; Cartes, J.E.; Constenia, M.; Welden, N.A.; Soler-Membrives, A.; Carrasson, M. An affordable method for monitoring plastic fibre ingestion in Nephrops norvegicus (Linnaeus, 1758) and implementation on wide temporal and geographical scale comparison. Sci. Total Environ. 2022, 810, 152264. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Cowie, P. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Saborowski, R.; Paulischkis, E.; Gutow, L. How to get rid of ingested microplastic fibers? A straightforward approach of the Atlantic ditch shrimp Palaemon varians. Environ. Pollut. 2019, 254, 113068. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.S.; Palmquist, K.H. Reality check: Experimental studies on microplastics lack realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Horn, D.A.; Granek, E.F.; Steele, C.L. Effects of environmentally relevant concentrations of microplastic fibers on Pacific mole crab (Emerita analoga) mortality and reproduction. Limnol. Oceanogr. Lett. 2020, 5, 74–83. [Google Scholar] [CrossRef]
- Au, S.; Bruce, T.F.; Bridges, W.; Klaine, S. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Blarer, P.; Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res. 2016, 23, 23522–23532. [Google Scholar] [CrossRef]
- Mendrik, F.M.; Henry, T.B.; Burdett, H.; Hackney, C.R.; Waller, C.; Parsons, D.R.; Hennige, S. Species-specific impact of microplastics on coral physiology. Environ. Pollut. 2021, 269, 116238. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Sigwart, J.D. Environmentally accurate microplastic levels and their absence from exposure studies. Integr. Comp. Biol. 2019, 59, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.; Milošević, D.; Jovanović, B.; Savić-Zdravković, D.; Petrović, A.; Raković, M.; Stanković, N.; Stojković Piperac, M. In situ effects of a microplastic mixture on the community structure of benthic macroinvertebrates in a freshwater pond. Environ. Toxicol. Chem. 2021, 41, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Rist, S.; Bodin, J.; Jensen, L.; Schmidt, S.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef]
- Beckingham, B.; Ghosh, U. Differential bioavailability of polychlorinated biphenyls associated with environmental particles: Microplastic in comparison to wood, coal and biochar. Environ. Pollut. 2017, 220, 150–158. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.M.; Martí, A.S.; Sureda, A.; Deudero, S. Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’Aniello, S.; Godley, B.J.; Pazdro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. Pollut. 2021, 269, 115744. [Google Scholar] [CrossRef]
- Sucharitakul, P.; Pitt, K.A.; Welsh, D.T. Trophic transfer of microbeads to jellyfish and the importance of aging microbeads for microplastic experiments. Mar. Pollut. Bull. 2021, 172, 112867. [Google Scholar] [CrossRef]
- Athira, N.; Jaya, D.S. The use of fish biomarkers for assessing textile effluent contamination of aquatic ecosystems: A Review. Nat. Environ. Pollut. Technol. 2018, 17, 25–34. [Google Scholar]
- Selvaraj, D.; Leena, R.; Kamal, D. Toxicological and histopathological impacts of textile dyeing industry effluent on a selected teleost fish Poecilia reticulata. Asian J. Pharmacol. Toxicol. 2015, 3, 26–30. [Google Scholar]
- Almroth, B.C.; Cartine, J.; Jönander, C.; Karlsson, M.; Langlois, J.; Lindström, M.; Lundin, J.; Melander, N.; Pesqueda, A.; Rahmqvist, I.; et al. Assessing the effects of textile leachates in fish using multiple testing methods: From gene expression to behavior. Ecotoxicol. Environ. Saf. 2021, 207, 111523. [Google Scholar] [CrossRef] [PubMed]
- Sait, S.T.L.; Sørensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Worldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- The Microfibre Consortium Website. Available online: https://www.microfibreconsortium.com/ (accessed on 21 June 2022).
- CIA Agreement. Available online: https://euratex.eu/cia/ (accessed on 21 June 2022).
- Ocean Clean Wash Website: Label & Benchmark. Available online: https://www.oceancleanwash.org/label-benchmark/ (accessed on 21 June 2022).
- De Falco, F.; Cocca, M.; Avella, M.; Thompson, R. Microfiber Release to Water, Via Laundering, and to Air, via Everyday Use: A Comparison between Polyester Clothing with Differing Textile Parameters. Environ. Sci. Technol. 2020, 54, 3288–3296. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Textile Exchange. Preferred Fiber & Materials Market Report 2021; Technical Report; Textile Exchange: Lamesa, TX, USA, 2021; pp. 1–118. [Google Scholar]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Palacios-Mateo, C.; van der Meer, Y.; Seide, G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 2021, 33, 2. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the aquatic biodegradability of cotton textile fibers and microfibers released on laundering clothes: Correlations between enzyme adsorption and activity and biodegradation rates. Mar. Pollut. Bull. 2021, 165, 112030. [Google Scholar] [CrossRef]
- Le Guen, C.; Suaria, G.; Sherley, R.B.; Ryan, P.G.; Aliani, S.; Boehme, L.; Brierley, A.S. Microplastic study reveals the presence of natural and synthetic fibres in the diet of King Penguins (Aptenodytes patagonicus) foraging from South Georgia. Environ. Int. 2020, 134, 105303. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef] [PubMed]
- Athey, S.N.; Erdle, L.M. Are We Underestimating Anthropogenic Microfiber Pollution? A Critical Review of Occurrence, Methods, and Reporting. Environ. Toxicol. Chem. 2021, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Lykaki, M.; Zhang, Y.; Markiewicz, M.; Brandt, S.; Kolbe, S.; Schrick, J.; Rabe, M.; Stolte, S. The influence of textile finishing agents on the biodegradability of shed fibres. Green Chem. 2021, 23, 5212–5221. [Google Scholar] [CrossRef]
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Microfiber Innovation Challenge. Available online: https://www.microfiberinnovation.org/home (accessed on 20 May 2022).
- Mango Materials. Available online: https://www.mangomaterials.com/ (accessed on 20 May 2022).
- Tandem Repeat Technologies. Available online: https://www.tandemrepeat.com/ (accessed on 20 May 2022).
- Natural Fiber Welding. 2022. Available online: https://www.naturalfiberwelding.com/technology (accessed on 20 May 2022).
- Werewool. Available online: https://www.werewool.bio/#anchor-technology (accessed on 20 May 2022).
- Microfibre Innovation Challenge. Available online: https://www.microfiberinnovation.org/innovation/pangaia-x-mtix (accessed on 20 May 2022).
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Almroth, B.M.C.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, M.; Park, M.-J.; Kim, J. Analysis of microplastics released from plain woven classified by yarn types during washing and drying. Polymers 2021, 13, 2988. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque-Ramos, J. Laundering and textile parameters influence fibres release in household washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef]
- Berruezo, M.; Bonet-Aracil, M.; Montava, I.; Bou-Belda, E.; Díaz-García, P.; Gisbert-Payá, J. Preliminary study of weave pattern influence on microplastics from fabric laundering. Text. Res. J. 2021, 91, 1037–1045. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibres from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, T.; Mitrano, D.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic study of microplastic fiber release from 12 different polyester textiles during washing. Environ. Sci. Technol. 2020, 54, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Mitrano, D.; Heuberger, M.; Hufenus, R.; Nowack, B. The origin of microplastic fiber in polyester textiles: The textile production process matters. J. Clean. Prod. 2020, 267, 121970. [Google Scholar] [CrossRef]
- Vassilenko, E.; Watkins, M.; Chastain, S.; Mertens, J.; Posacka, A.M.; Patankar, S.; Ross, P.S. Domestic laundry and microfiber pollution: Exploring fiber shedding from consumer apparel textiles. PLoS ONE 2021, 16, e0250346. [Google Scholar] [CrossRef]
- Dalla Fontana, G.; Mossotti, R.; Montarsolo, A. Influence of Sewing on Microplastic Release from Textiles During Washing. Water Air Soil Pollut. 2021, 232, 50. [Google Scholar] [CrossRef]
- De Falco, F.; Gentile, G.; Avolio, R.; Errico, M.E.; Di Pace, E.; Ambrogi, V.; Avella, M.; Cocca, M. Pectin based finishing to mitigate the impact of microplastics released by polyamide fabrics. Carbohydr. Polym. 2018, 198, 175–180. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.; Lee, B.; Ahn, J.; Kim, S. Impact of chitosan pretreatment to reduce microfibers released from synthetic garments during laundering. Water 2021, 13, 2480. [Google Scholar] [CrossRef]
- De Falco, F.; Cocca, M.; Guarino, V.; Gentile, G.; Ambrogi, V.; Ambrosio, L.; Avella, M. Novel finishing treatments of polyamide fabrics by electrofluidodynamic porcess to reduce microplastic release during washings. Polym. Degrad. Stab. 2019, 165, 110–116. [Google Scholar] [CrossRef]
- Pinlova, B.; Hufenus, R.; Nowack, B. Systematic study of the presence of microplastic fibers during polyester yarn production. J. Clean. Prod. 2022, 363, 132247. [Google Scholar] [CrossRef]
- Xu, X.; Hou, Q.; Xue, Y.; Jian, Y.; Wang, L. Pollution characteristics and fate of microfibers in the wastewater from textile dyeing wastewater treatment plant. Water Sci. Technol. 2018, 78, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef] [PubMed]
- Diekel, F.; Mikosch, N.; Bach, V.; Finkbeiner, M. Life Cycle Based Comparison of Textile Ecolabels. Sustainability 2021, 13, 1751. [Google Scholar] [CrossRef]
- Eunomia. Investigating Options for Reducing Releases in the Aquatic Environment of Microplastics Emitted by (but not Intentionally Added in) Products–Final Report; 2018, UK. Available online: https://www.eunomia.co.uk/reports-tools/investigating-options-for-reducing-releases-in-the-aquatic-environment-of-microplastics-emitted-by-products/ (accessed on 21 June 2022).
- SAPEA. Science Advice for Policy by European Academies. In Biodegradability of Plastics in the Open Environment; SAPEA: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Lant, N.J.; Hayward, A.S.; Peththawadu, M.M.D.; Sheridan, K.J.; Dean, J.R. Microfiber release from real soiled consumer laundry and the impact of fabric care products and washing conditions. PLoS ONE 2020, 15, e0233332. [Google Scholar] [CrossRef] [PubMed]
- Rathinamoorthy, R.; Raja Balasaraswathi, S. Investigations on the impact of handwash and laundry softener on microfiber shedding from polyester textiles. J. Text. Inst. 2021, 113, 1428–1437. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Evaluation of microfiber release from jeans: The impact of different washing conditions. Environ. Sci. Pollut. Res. 2021, 28, 58570–58582. [Google Scholar] [CrossRef]
- Hartline, N.; Bruce, N.; Karba, S.; Ruff, E.; Sonar, S.; Holden, P. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, F.; Lei, K.; Li, H.; Kang, Y.; Cui, S.; An, L. Microfiber release from different fabrics during washing. Environ. Pollut. 2019, 249, 136–143. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef]
- Kelly, M.; Lant, N.J.; Kurr, M.; Burgess, J.G. Importance of Water-Volume on the Release of Microplastic Fibers from Laundry. Environ. Sci. Technol. 2019, 53, 11735–11744. [Google Scholar] [CrossRef]
- Volgare, M.; De Falco, F.; Avolio, R.; Castaldo, R.; Errico, M.E.; Gentile, G.; Ambrogi, V.; Cocca, M. Washing load influences the microplastic release from polyester fabrics by affecting wettability and mechanical stress. Sci. Rep. 2021, 11, 19479. [Google Scholar] [CrossRef]
- Cora Ball Website. Available online: https://coraball.com/ (accessed on 21 June 2022).
- Guppyfriend Washing Bag Website. Available online: https://en.guppyfriend.com/ (accessed on 21 June 2022).
- Environmental Enhancements Website. Available online: https://environmentalenhancements.com/store/ (accessed on 21 June 2022).
- PlanetCare Microfiber Filter. Available online: https://planetcare.org/products/microfiber-filter (accessed on 21 June 2022).
- Filtrol Website. Available online: https://filtrol.net/ (accessed on 21 June 2022).
- McIlwraith, H.K.; Lin, J.; Erdle, L.M.; Mallos, N.; Diamond, M.L.; Rochman, C.M. Capturing microfibres-marketed technologies reduce microfibre emissions from washing machines. Mar. Pollut. Bull. 2019, 139, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.; Barrett, A.; Thompson, R. The efficiency of devices intended to reduce microfibre release during clothes washing. Sci. Total Environ. 2020, 738, 140412. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Avella, M.; Gentile, G.; Errico, M.E.; Krzan, A.; ElKhiar, H.; Zupan, M.; Cocca, M. Development and Performance Evaluation of a Filtration System for Washing Machines to Reduce Microfiber Release in Wastewater. Water Air Soil Pollut. 2021, 232, 406. [Google Scholar] [CrossRef]
- Xeros Technologies Website. Available online: https://www.xerostech.com/filtration/ (accessed on 21 June 2022).
- Samsung News Website. Available online: https://news.samsung.com/global/samsung-collaborates-with-patagonia-to-keep-microplastics-out-of-our-oceans (accessed on 21 June 2022).
- PlanetCare Website. Available online: https://blog.planetcare.org/france-microfibre-filters-washing-machines/ (accessed on 21 June 2022).
- Ocean Clean Wash Website. Available online: https://www.oceancleanwash.org/2021/03/australia-and-california-join-the-fight-against-microfibers-from-clothes/ (accessed on 21 June 2022).
- MCS Website. Available online: https://www.mcsuk.org/news/pushing-appg-for-change-on-microplastics/ (accessed on 21 June 2022).
- Erdle, L.M.; Parto, D.N.; Sweetnam, D.; Rochman, C.M. Washing machine filters reduce microfiber emissions: Evidence from a community-scale pilot in Parry Sound, Ontario. Front. Mar. Sci. 2021, 8, 777865. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. 2019, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Approaching the environmental problem of microplastics: Importance of WWTP treatments. Sci. Total Environ. 2020, 740, 140016. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.T.; Huang, Q.S.; Ni, B.J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Res. 2019, 163, 114881. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Belzagui, F.; Gutiérrez-Bouzán, C.; Álvarez-Sánchez, A.; Vilaseca, M. Textile microfibers reaching aquatic environments: A new estimation approach. Environ. Pollut. 2020, 265, 114889. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dagnew, M.; Ray, M.B. Effect of coagulation on microfibers in laundry wastewater. Environ. Res. 2022, 212, 113401. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; Scb, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef]
- Hayany, B.; Rumpel, C.; Hafidi, M.; El Fels, L. Occurrence, analysis of microplastics in sewage sludge and their fate during composting: A literature review. J. Environ. Manag. 2022, 317, 115364. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, J.S.; De Falco, F. Microfibers: Environmental Problems and Textile Solutions. Microplastics 2022, 1, 626-639. https://doi.org/10.3390/microplastics1040043
Weis JS, De Falco F. Microfibers: Environmental Problems and Textile Solutions. Microplastics. 2022; 1(4):626-639. https://doi.org/10.3390/microplastics1040043
Chicago/Turabian StyleWeis, Judith S., and Francesca De Falco. 2022. "Microfibers: Environmental Problems and Textile Solutions" Microplastics 1, no. 4: 626-639. https://doi.org/10.3390/microplastics1040043
APA StyleWeis, J. S., & De Falco, F. (2022). Microfibers: Environmental Problems and Textile Solutions. Microplastics, 1(4), 626-639. https://doi.org/10.3390/microplastics1040043