Adsorption of PAHs and PCDD/Fs in Microplastics: A Review
Abstract
:1. Introduction
2. Search and Review Procedure
3. MPs as Input Vector of PAHs and POPs in the Food Chain
4. Kinetics of the Adsorption and Desorption Process
5. Effect of Aging
6. Main Findings Related to Different Aspects of Microplastics or Pollutants
6.1. Modifications of Microplastics
6.2. Chemical Modifications of the Adsorbate
6.3. The Different Plastic Polymers
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laist, D.W. Overview of the Biological Effects of Lost and Discarded Plastic Debris in the Marine Environment. Mar. Pollut. Bull. 1987, 18, 319–326. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S. A Growing Problem Sources, Distribution, Composition, and Impacts. Mar. Pollut. New Res. 2008, 2, 53–100. [Google Scholar]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris; NOAA: Washington, DC, USA, 2009.
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Nacimba-Aguirre, D.; Conesa, J.A.; Fullana, A. Presence of Microplastics in Commercial Canned Tuna. Food Chem. 2022, 385, 132721. [Google Scholar] [CrossRef] [PubMed]
- Takada, H. Call for Pellets! International Pellet Watch Global Monitoring of POPs Using Beached Plastic Resin Pellets. Mar. Pollut. Bull. 2006, 52, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Marine Debris Occurrence and Treatment: A Review. Renew. Sustain. Energy Rev. 2016, 64, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Fisner, M.; Taniguchi, S.; Moreira, F.; Bícego, M.C.; Turra, A. Polycyclic Aromatic Hydrocarbons (PAHs) in Plastic Pellets: Variability in the Concentration and Composition at Different Sediment Depths in a Sandy Beach. Mar. Pollut. Bull. 2013, 70, 219–226. [Google Scholar] [CrossRef]
- Krüger, O.; Kalbe, U.; Meißner, K.; Sobottka, S. Sorption Effects Interfering with the Analysis of Polycyclic Aromatic Hydrocarbons (PAH) in Aqueous Samples. Talanta 2014, 122, 151–156. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.H.J.-H. Sorption Capacity of Plastic Debris for Hydrophobic Organic Chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.M.; Hoh, E. Polystyrene Plastic: A Source and Sink for Polycyclic Aromatic Hydrocarbons in the Marine Environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreisz, S.; Hunsinger, H.; Vogg, H. Technical Plastics as PCDD/F Absorbers. Chemosphere 1997, 34, 1045–1052. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Ohiagu, F.O.; Verla, A.W.; Wang, Q.; Shafea, L.; Verla, E.N.; Isiuku, B.O.; Chowdhury, T.; Ibe, F.C.; Chowdhury, M.A.H. “Plasti-Remediation”: Advances in the Potential Use of Environmental Plastics for Pollutant Removal. Environ. Technol. Innov. 2021, 23, 101791. [Google Scholar] [CrossRef]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of Polybrominated Diphenyl Ethers from Microplastics by the Marine Amphipod, Allorchestes Compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarf, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 287. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to Microplastic and Their Bioavailability and Toxicity to Marine Copepods under Co-Exposure Conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, P.; Sun, H.; Ma, J.; Li, B. The Structure of Agricultural Microplastics (PT, PU and UF) and Their Sorption Capacities for PAHs and PHE Derivates under Various Salinity and Oxidation Treatments. Environ. Pollut. 2019, 257, 113525. [Google Scholar] [CrossRef]
- Conesa, J.A.; Nuñez, S.S.; Ortuño, N.; Moltó, J. PAH and POP Presence in Plastic Waste and Recyclates: State of the Art. Energies 2021, 14, 3451. [Google Scholar] [CrossRef]
- Núñez, S.S.; Moltó, J.; Conesa, J.A.; Fullana, A. Heavy Metals, PAHs and POPs in Recycled Polyethylene Samples of Agricultural, Post-Commercial, Post-Industrial and Post-Consumer Origin. Waste Manag. 2022, 144, 113–121. [Google Scholar] [CrossRef]
- Sharma, M.D.; Elanjickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of Cancer Risk of Microplastics Enriched with Polycyclic Aromatic Hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive Sorption of Persistent Organic Pollutants onto Microplastics in the Marine Environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced Desorption of Persistent Organic Pollutants from Microplastics under Simulated Physiological Conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Bartonitz, A.; Anyanwu, I.N.; Geist, J.; Imhof, H.K.; Reichel, J.; Graßmann, J.; Drewes, J.E.; Beggel, S. Modulation of PAH Toxicity on the Freshwater Organism G. Roeseli by Microparticles. Environ. Pollut. 2020, 260, 113999. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Agboola, O.D.; Fred-Ahmadu, O.H.; De-la-Torre, G.E.; Oluwalana, A.; Williams, A.B. Micro(Nano)Plastics Prevalence, Food Web Interactions, and Toxicity Assessment in Aquatic Organisms: A Review. Front. Mar. Sci. 2022, 9, 291. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Kang, H.-M.; Byeon, E.; Kim, M.-S.; Ha, S.Y.; Kim, M.; Jung, J.-H.; Lee, J.-S. Phenotypic and Transcriptomic Responses of the Rotifer Brachionus Koreanus by Single and Combined Exposures to Nano-Sized Microplastics and Water-Accommodated Fractions of Crude Oil. J. Hazard. Mater. 2021, 416, 125703. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic Pollutants in Marine Plastic Debris from Canary Islands Beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef]
- Černá, T.; Pražanová, K.; Beneš, H.; Titov, I.; Klubalová, K.; Filipová, A.; Klusoň, P.; Cajthaml, T. Polycyclic Aromatic Hydrocarbon Accumulation in Aged and Unaged Polyurethane Microplastics in Contaminated Soil. Sci. Total Environ. 2021, 770, 145254. [Google Scholar] [CrossRef]
- Chaomeng, D.; Si, L.; Yanping, D.; Shuguang, L.; Yaoren, T. Advances in Research on Effects of Microplastics on Migration, Transformation and Bioavailability of Organic Pollutants in Water. Mater. Rep. 2020, 34, 21033–21037. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.E.; Luo, Y.; Yang, Y.; Shi, H.; Hollert, H. Marine Microplastics Bound Dioxin-like Chemicals: Model Explanation and Risk Assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef]
- Cormier, B.; Borchet, F.; Kärrman, A.; Szot, M.; Yeung, L.W.Y.; Keiter, S.H. Sorption and Desorption Kinetics of PFOS to Pristine Microplastic. Environ. Sci. Pollut. Res. 2022, 29, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Salt Intended for Human Consumption: A Systematic Review and Meta-Analysis. SN Appl. Sci. 2020, 2, 1950. [Google Scholar] [CrossRef]
- Ding, T.; Wei, L.; Hou, Z.; Li, J.; Zhang, C.; Lin, D. Microplastics Altered Contaminant Behavior and Toxicity in Natural Waters. J. Hazard. Mater. 2022, 425, 127908. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High Temperature Depended on the Ageing Mechanism of Microplastics under Different Environmental Conditions and Its Effect on the Distribution of Organic Pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef]
- Eder, M.L.; Oliva-Teles, L.; Pinto, R.; Carvalho, A.P.; Almeida, C.M.R.; Hornek-Gausterer, R.; Guimarães, L. Microplastics as a Vehicle of Exposure to Chemical Contamination in Freshwater Systems: Current Research Status and Way Forward. J. Hazard. Mater. 2021, 417, 125980. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Akhdhar, A.; Elwakeel, K.Z. Microplastics Prevalence, Interactions, and Remediation in the Aquatic Environment: A Critical Review. J. Environ. Chem. Eng. 2021, 9, 106224. [Google Scholar] [CrossRef]
- Fraser, M.A.; Chen, L.; Ashar, M.; Huang, W.; Zeng, J.N.; Zhang, C.F.; Zhang, D.D. Occurrence and Distribution of Microplastics and Polychlorinated Biphenyls in Sediments from the Qiantang River and Hangzhou Bay, China. Ecotoxicol. Environ. Saf. 2020, 196, 110536. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Sobral, P.; Ferreira, A.M. Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the Capability and Characteristics of Heavy Metals Enriched on Microplastics in Marine Environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef]
- Gao, L.; Su, Y.; Yang, L.; Li, J.; Bao, R.; Peng, L. Sorption Behaviors of Petroleum on Micro-Sized Polyethylene Aging for Different Time in Seawater. Sci. Total Environ. 2022, 808, 152070. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Oysaed, K.B.; Fabi, G. First Occurrence and Composition Assessment of Microplastics in Native Mussels Collected from Coastal and Offshore Areas of the Northern and Central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Xu, X.; Zhang, S.; Wang, Y.; Li, C.; Zhang, D.; Su, L.; Zhao, Y. Prediction of Organic Compounds Adsorbed by Polyethylene and Chlorinated Polyethylene Microplastics in Freshwater Using QSAR. Environ. Res. 2021, 197, 111001. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, L.; Seiwert, B.; Huppertsberg, S.; Knepper, T.P.; Reemtsma, T.; Braunbeck, T. Biomarker Responses in Zebrafish (Danio Rerio) Following Long-Term Exposure to Microplastic-Associated Chlorpyrifos and Benzo(k)Fluoranthene. Aquat. Toxicol. 2022, 245, 106120. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Skrzypek, G.; Hernández-Sánchez, C.; Ortega-Zamora, C.; González-Sálamo, J.; González-Curbelo, M.Á.; Hernández-Borges, J. Microplastic-Adsorbed Organic Contaminants: Analytical Methods and Occurrence. Trends Anal. Chem. 2021, 136, 116186. [Google Scholar] [CrossRef]
- Jose, S.; Jordao, L.; José, S.; Jordao, L.; Jose, S.; Jordao, L.; José, S.; Jordao, L.; Jose, S.; Jordao, L. Exploring the Interaction between Microplastics, Polycyclic Aromatic Hydrocarbons and Biofilms in Freshwater. Polycycl. Aromat. Compd. 2020, 1–12. [Google Scholar] [CrossRef]
- Kedzierski, M.; D’Almeida, M.; Magueresse, A.; Le Grand, A.; Duval, H.; César, G.; Sire, O.; Bruzaud, S.; Le Tilly, V. Threat of Plastic Ageing in Marine Environment. Adsorption/Desorption of Micropollutants. Mar. Pollut. Bull. 2018, 127, 684–694. [Google Scholar] [CrossRef]
- Lai, C.; He, H.; Xie, W.; Fan, S.; Huang, H.; Wang, Y.; Huang, B.; Pan, X. Adsorption and Photochemical Capacity on 17α-Ethinylestradiol by Char Produced in the Thermo Treatment Process of Plastic Waste. J. Hazard. Mater. 2022, 423, 127066. [Google Scholar] [CrossRef]
- Li, R.L.; Tan, H.D.; Zhang, L.L.; Wang, S.P.; Wang, Y.H.; Yu, K.F. The Implications of Water Extractable Organic Matter (WEOM) on the Sorption of Typical Parent, Alkyl and N/O/S-Containing Polycyclic Aromatic Hydrocarbons (PAHs) by Microplastics. Ecotoxicol. Environ. Saf. 2018, 156, 176–182. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Wu, J.; Wei, S.; Yin, L.; Xiao, X.; Hu, S.; Shen, Y.; Ouyang, G. Sorption Properties of Hydrophobic Organic Chemicals to Micro-Sized Polystyrene Particles. Sci. Total Environ. 2019, 690, 565–572. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y. Microplastics as Both a Sink and a Source of Bisphenol A in the Marine Environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The Joint Toxicity of Polyethylene Microplastic and Phenanthrene to Wheat Seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Fokkink, R.; Koelmans, A.A. Sorption of Polycyclic Aromatic Hydrocarbons to Polystyrene Nanoplastic. Environ. Toxicol. Chem. 2016, 35, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Liu, R.; Chen, Y.; Fu, J.; Ou, H. Modifications of Ultraviolet Irradiation and Chlorination on Microplastics: Effect of Sterilization Pattern. Sci. Total Environ. 2022, 812, 152541. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Pan, S.; Dai, J.; Zhang, Z.; Guo, X. Photochlorination-Induced Degradation of Microplastics and Interaction with Cr(VI) and Amlodipine. Sci. Total Environ. 2022, 835, 155499. [Google Scholar] [CrossRef]
- Llorca, M.; Ábalos, M.; Vega-Herrera, A.; Adrados, M.A.; Abad, E.; Farré, M. Adsorption and Desorption Behaviour of Polychlorinated Biphenyls onto Microplastics’ Surfaces in Water/Sediment Systems. Toxics 2020, 8, 59. [Google Scholar] [CrossRef]
- Lončarsk, M.; Tubic, A.; Isakovski, M.K.; Jovic, B.; Apostolovic, T.; Nikic, J.; Agbaba, J. Modelling of the Adsorption of Chlorinated Phenols on Polyethylene and Polyethylene Terephthalate Microplastic. J. Serb. Chem. Soc. 2020, 85, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Loncarski, M.; Gvoic, V.; Prica, M.; Cveticanin, L.; Agbaba, J.; Tubic, A.; Lončarski, M.; Gvoić, V.; Prica, M.; Cveticanin, L.; et al. Sorption Behavior of Polycyclic Aromatic Hydrocarbons on Biodegradable Polylactic Acid and Various Nondegradable Microplastics: Model Fitting and Mechanism Analysis. Sci. Total Environ. 2021, 785, 147289. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Chatterjee, A.; Pramanick, K. Co-Occurrence of Co-Contaminants: Cyanotoxins and Microplastics, in Soil System and Their Health Impacts on Plant—A Comprehensive Review. Sci. Total Environ. 2021, 794, 148752. [Google Scholar] [CrossRef]
- Martins, J.; Sobral, P. Plastic Marine Debris on the Portuguese Coastline: A Matter of Size? Mar. Pollut. Bull. 2011, 62, 2649–2653. [Google Scholar] [CrossRef]
- Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; de Pedro, Z.M.; Casas, J.A. Adsorption of Micropollutants onto Realistic Microplastics: Role of Microplastic Nature, Size, Age, and NOM Fouling. Chemosphere 2021, 283, 131085. [Google Scholar] [CrossRef] [PubMed]
- Näkki, P.; Eronen-Rasimus, E.; Kaartokallio, H.; Kankaanpää, H.; Setälä, O.; Vahtera, E.; Lehtiniemi, M. Polycyclic Aromatic Hydrocarbon Sorption and Bacterial Community Composition of Biodegradable and Conventional Plastics Incubated in Coastal Sediments. Sci. Total Environ. 2021, 755, 143088. [Google Scholar] [CrossRef] [PubMed]
- Noro, K.; Yabuki, Y. Photolysis of Polycyclic Aromatic Hydrocarbons Adsorbed on Polyethylene Microplastics. Mar. Pollut. Bull. 2021, 169, 112561. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, X.; Zhang, M.; Li, Z.; Cao, C.; Shi, H.; Yang, Y.; Zhao, Y. Sorption and Leaching Behaviors between Aged MPs and BPA in Water: The Role of BPA Binding Modes within Plastic Matrix. Water Res. 2021, 195, 116956. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Cwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of Pharmaceuticals on the Surface of Microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.H. Adsorption of Environmental Contaminants on Micro- and Nano-Scale Plastic Polymers and the Influence of Weathering Processes on Their Adsorptive Attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.E.; Zucker, I. Interactions of Microplastics and Organic Compounds in Aquatic Environments: A Case Study of Augmented Joint Toxicity. Chemosphere 2022, 289, 133212. [Google Scholar] [CrossRef]
- Scott, J.W.; Gunderson, K.G.; Green, L.A.; Rediske, R.R.; Steinman, A.D. Perfluoroalkylated Substances (Pfas) Associated with Microplastics in a Lake Environment. Toxics 2021, 9, 106. [Google Scholar] [CrossRef]
- Shan, J.; Wang, J.; Zhan, J.; Liu, L.; Wu, F.; Wang, X. Sorption Behaviors of Crude Oil on Polyethylene Microplastics in Seawater and Digestive Tract under Simulated Real-World Conditions. Chemosphere 2020, 257, 127225. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory Simulation of Microplastics Weathering and Its Adsorption Behaviors in an Aqueous Environment: A Systematic Review. Environ. Pollut. 2020, 265, 114864. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A Review of Human and Animals Exposure to Polycyclic Aromatic Hydrocarbons: Health Risk and Adverse Effects, Photo-Induced Toxicity and Regulating Effect of Microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef]
- Pelamatti, T.; Rios-Mendoza, L.M.; Hoyos-Padilla, E.M.; Galván-Magaña, F.; De Camillis, R.; Marmolejo-Rodríguez, A.J.; González-Armas, R. Contamination Knows No Borders: Toxic Organic Compounds Pollute Plastics in the Biodiversity Hotspot of Revillagigedo Archipelago National Park, Mexico. Mar. Pollut. Bull. 2021, 170, 112623. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and Associated PAHs in Surface Water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Tubić, A.; Lončarski, M.; Maletić, S.; Jazić, J.M.; Watson, M.; Tričković, J.; Agbaba, J. Significance of Chlorinated Phenols Adsorption on Plastics and Bioplastics during Water Treatment. Water 2019, 11, 2358. [Google Scholar] [CrossRef] [Green Version]
- Vedolin, M.C.; Teophilo, C.Y.S.; Turra, A.; Figueira, R.C.L. Spatial Variability in the Concentrations of Metals in Beached Microplastics. Mar. Pollut. Bull. 2018, 129, 487–493. [Google Scholar] [CrossRef]
- Vo, H.C.; Pham, M.H. Ecotoxicological Effects of Microplastics on Aquatic Organisms: A Review. Environ. Sci. Pollut. Res. 2021, 28, 44716–44725. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Qin, L.; Yin, D. Evaluating the Effect of Different Modified Microplastics on the Availability of Polycyclic Aromatic Hydrocarbons. Water Res. 2020, 170, 115290. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative Evaluation of Sorption Kinetics and Isotherms of Pyrene onto Microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J. Different Partition of Polycyclic Aromatic Hydrocarbon on Environmental Particulates in Freshwater: Microplastics in Comparison to Natural Sediment. Ecotoxicol. Environ. Saf. 2018, 147, 648–655. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Zhang, L.; Wang, K.; Yu, X.; Zheng, Z.; Zheng, R. Sorption Behaviors of Phenanthrene on the Microplastics Identified in a Mariculture Farm in Xiangshan Bay, Southeastern China. Sci. Total Environ. 2018, 628–629, 1617–1626. [Google Scholar] [CrossRef]
- Abaroa-Pérez, B.; Ortiz-Montosa, S.; Hernández-Brito, J.J.; Vega-Moreno, D. Yellowing, Weathering and Degradation of Marine Pellets and Their Influence on the Adsorption of Chemical Pollutants. Polymers 2022, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, D.; Wang, H. In Situ Fe3O4 Nanoparticles Coating of Polymers for Separating Hazardous PVC from Microplastic Mixtures. Chem. Eng. J. 2021, 407, 127170. [Google Scholar] [CrossRef]
- Wang, L.-C.; Lin, J.C.-T.; Dong, C.-D.; Chen, C.-W.; Liu, T.-K. The Sorption of Persistent Organic Pollutants in Microplastics from the Coastal Environment. J. Hazard. Mater. 2021, 420, 126658. [Google Scholar] [CrossRef] [PubMed]
- Wiśniowska, E.; Włodarczyk-Makuła, M.; Wisniowska, E.; Wlodarczyk-Makula, M. Adsorption of Selected 3-and 4-Ring Polycyclic Aromatic Hydrocarbons on Polyester Microfibers—Preliminary Studies. Desalin. Water Treat. 2021, 232, 414–420. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of Pharmaceuticals and Personal Care Products to Polyethylene Debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Cao, W.; Qu, R.; Zhou, D.; Sun, C.; Wang, Z. Photochemical Transformation of Decachlorobiphenyl (PCB-209) on the Surface of Microplastics in Aqueous Solution. Chem. Eng. J. 2021, 420, 129813. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Hu, J.; Zhang, Z.; Li, L.; Wu, Q. Co-Exposure to Different Sized Polystyrene Microplastics and Benzo[a]Pyrene Affected Inflammation in Zebrafish and Bronchial-Associated Cells. Kexue Tongbao/Chinese Sci. Bull. 2020, 65, 4281–4290. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Chen, J.; Wang, F.; Chen, X.; Huang, B.; He, Y.; Cai, Z. Exploring the Adsorption Behavior of Benzotriazoles and Benzothiazoles on Polyvinyl Chloride Microplastics in the Water Environment. Sci. Total Environ. 2022, 821, 153471. [Google Scholar] [CrossRef]
- Yang, C.; Wu, W.; Zhou, X.; Hao, Q.; Li, T.; Liu, Y. Comparing the Sorption of Pyrene and Its Derivatives onto Polystyrene Microplastics: Insights from Experimental and Computational Studies. Mar. Pollut. Bull. 2021, 173, 113086. [Google Scholar] [CrossRef]
- Yu, H.D.; Yang, B.; Waigi, M.G.; Peng, F.; Li, Z.K.; Hu, X.J. The Effects of Functional Groups on the Sorption of Naphthalene on Microplastics. Chemosphere 2020, 261, 127592. [Google Scholar] [CrossRef]
- Bao, Z.-Z.Z.Z.; Chen, Z.-F.Z.F.; Zhong, Y.; Wang, G.; Qi, Z.; Cai, Z. Adsorption of Phenanthrene and Its Monohydroxy Derivatives on Polyvinyl Chloride Microplastics in Aqueous Solution: Model Fitting and Mechanism Analysis. Sci. Total Environ. 2021, 764, 142889. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Moore, F.; Keshavarzi, B. PET-Microplastics as a Vector for Polycyclic Aromatic Hydrocarbons in a Simulated Plant Rhizosphere Zone. Environ. Technol. Innov. 2021, 21, 101370. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, Y.; Wang, H.; Chen, Y.; Huang, S.; Yu, B.; Wang, J.; Tong, Y.; Wen, D.; Zhou, B.; et al. Interaction of Microplastics and Organic Pollutants: Quantification, Environmental Fates, and Ecological Consequences. Handb. Environ. Chem. 2020, 95, 161–184. [Google Scholar]
- Zhang, C.; Lei, Y.; Qian, J.; Qiao, Y.; Liu, J.; Li, S.; Dai, L.; Sun, K.; Guo, H.; Sui, G.; et al. Sorption of Organochlorine Pesticides on Polyethylene Microplastics in Soil Suspension. Ecotoxicol. Environ. Saf. 2021, 223, 112591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Li, J. The Removal of Microplastics in the Wastewater Treatment Process and Their Potential Impact on Anaerobic Digestion Due to Pollutants Association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; He, H.; Cheng, X.; Ma, T.; Hu, J.; Yang, S.; Li, S.; Zhang, L. Adsorption Behavior and Mechanism of 9-Nitroanthracene on Typical Microplastics in Aqueous Solutions. Chemosphere 2020, 245, 125628. [Google Scholar] [CrossRef]
- Zhao, L.F.; Rong, L.L.; Xu, J.P.; Lian, J.P.; Wang, L.; Sun, H.W. Sorption of Five Organic Compounds by Polar and Nonpolar Microplastics. Chemosphere 2020, 257, 127206. [Google Scholar] [CrossRef]
- Zhou, W.Z.; Chen, Y.; Deng, C.; Qi, H.Y.; Zhang, H. Salt Crust-Assisted Thermal Decomposition Method for Direct and Simultaneous Quantification of Polypropylene Microplastics and Organic Contaminants in High Organic Matter Soils. Anal. Chim. Acta 2022, 1194, 338801. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Shen, Y.; Wang, J.; Wang, H.; Zhan, X. Microplastics Lag the Leaching of Phenanthrene in Soil and Reduce Its Bioavailability to Wheat. Environ. Pollut. 2022, 292, 118472. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of Three Bivalent Metals by Four Chemical Distinct Microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef]
- Rai, P.K.; Kumar, V.; Sonne, C.; Lee, S.S.; Brown, R.J.C.; Kim, K.H. Progress, Prospects, and Challenges in Standardization of Sampling and Analysis of Micro- and Nano-Plastics in the Environment. J. Clean. Prod. 2021, 325, 129321. [Google Scholar] [CrossRef]
- Schuhen, K.; Defu, H.; Yongming, L. Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ahmad, M.; Li, J.L.; Wang, P.D.; Hozzein, W.N.; Li, W.J. Environmental Perspectives of Microplastic Pollution in the Aquatic Environment: A Review. Mar. Life Sci. Technol. 2020, 2, 414–430. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Jarvis, P.; Zhou, W.; Zhang, J.; Chen, J.; Tan, Q.; Tian, Y. Occurrence, Removal and Potential Threats Associated with Microplastics in Drinking Water Sources. J. Environ. Chem. Eng. 2020, 8, 104527. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal Efficiency of Micro- and Nanoplastics (180 Nm–125 Μm) during Drinking Water Treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Deshani Igalavithana, A.; Gedara, M.; Mahagamage, Y.L.; Gajanayake, P.; Abeynayaka, A.; Jagath, P.; Gamaralalage, D.; Ohgaki, M.; Takenaka, M.; Fukai, T.; et al. Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures. Microplastics 2022, 1, 102–120. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Trivalent Chromium Removal from Wastewater Using Low Cost Activated Carbon Derived from Agricultural Waste Material and Activated Carbon Fabric Cloth. J. Hazard. Mater. 2006, 135, 280–295. [Google Scholar] [CrossRef]
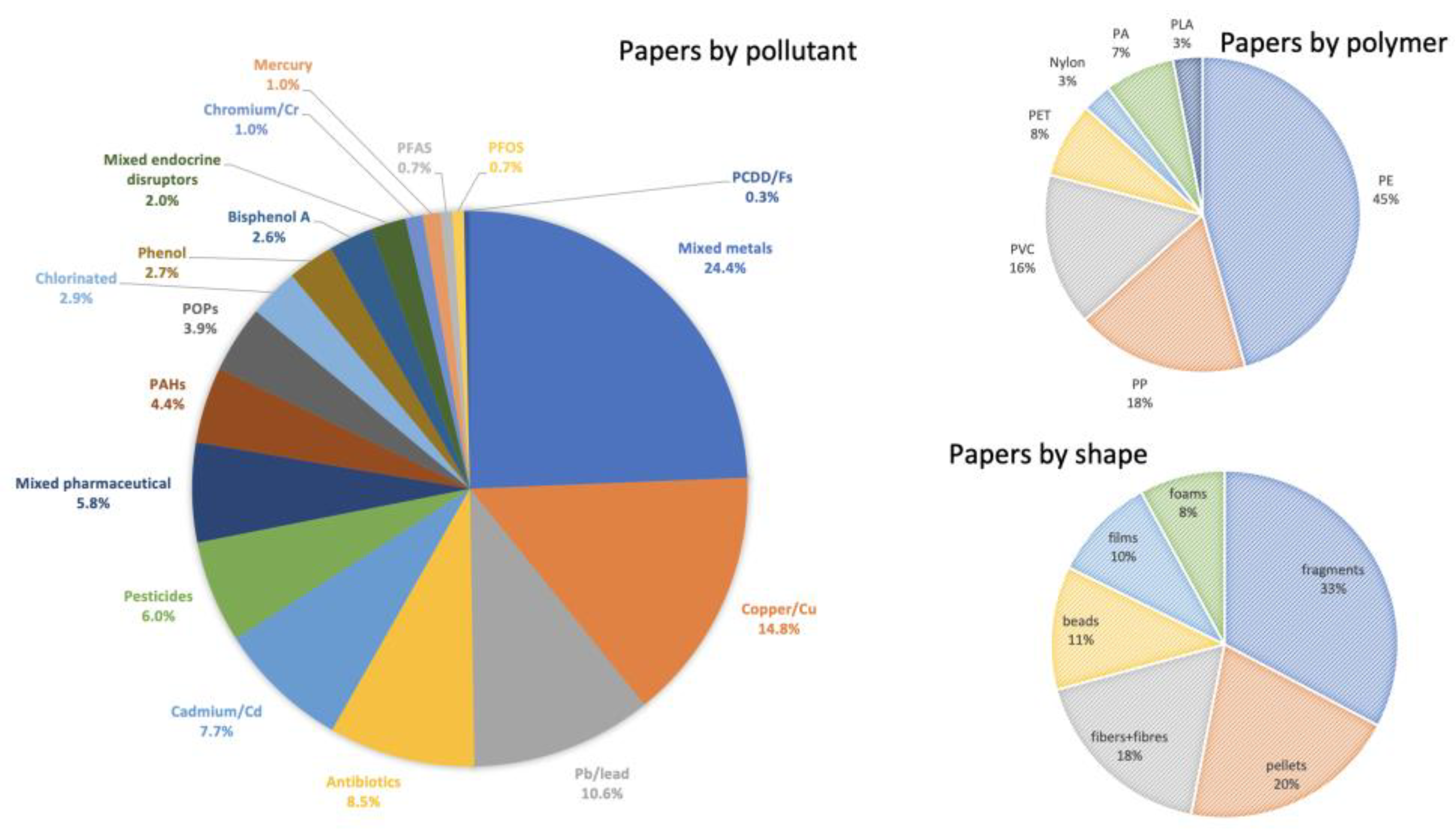
| Reference | Pollutant Studied | Polymer | Main Findings |
|---|---|---|---|
| Abaroa et al. [82] | POPs | PE and PP from beaches | Increase in adsorption with aging |
| Abbasi et al. [93] | Naphthalene and phenanthrene | PET | MPs adsorb 97% of the naphthalene and 27% of the phenanthrene present |
| Bakir et al. [23] | Phenanthrene and DDT | Different types | Interaction between phenanthrene and DDT |
| Bakir et al. [24] | PFOA and DEHP | Different types | Interaction between PFOA and DEHP. The desorption in the intestinal tract is much faster than in seawater |
| Bao et al. [92] | Phenanthrene and its hydroxy-derivatives | PVC | Pseudo-second-order model for the adsorption process |
| Bao et al. [92] | Phenanthrene and its hydroxy-derivatives | PVC | Presence of the -OH group inhibits the fixation of the compound on the surface |
| Černá et al. [29] | PAHs | PU | No significant differences with aging |
| Černá et al. [29] | PAHs | Biodegradable and conventional PU | Much faster adsorption in the biodegradable |
| Chen et al. [31] | POPs related to dioxins | Different types | Aging of MPs increases the presence of compounds related to dioxins |
| Chen et al. [31] | PCDD/Fs | PS, PE, PVC, PP | PS has much more ability to adsorb dioxins |
| Chua et al. [15] | PBDEs | Different types | PBDEs are assimilated by living organisms, especially the more brominated congeners. Particles are eliminated. |
| Cormier et al. [32] | PFOA | Different types | Rapid desorption under conditions similar to those existing in the stomach |
| Ding et al. [35] | HOCs | PS | Aging increases adsorption capacity |
| Gao et al. [41] | Oil (similar to PAHs) | PE | Pseudo-second-order adsorption model with internal diffusion being a rate-controlling step |
| Gui et al. [43] | PAHs, pesticides, benzene-derivatives | PE and chlorinated PE | PAHs and chlorobenzene adsorb faster on chlorinated PE, hydrophobicity being a key point |
| Hanslik et al. [44] | Benzo(k)fluoranthene | PE and PMMA | The potential of MPs as vectors is limited |
| Kleinteich et al. [16] | PAHs | PE | Bacteria show decreased bioavailability when MPs are present |
| Li et al. [78] | PAHs and HOCs | 3 types of PE | Desorption dominated by external diffusion |
| Li et al. [105] | PAHs | PE | Aging and etching surface area of the plastics produces increase in the adsorption of PAHs on polymers |
| Li et al. [49] | N-O-S PAHs | PVC, PS | Presence of extractable organic matter prevents the adsorption of contaminants |
| Lin et al. [50] | PAHs and PCBs | PS | Diffusion in the pores controls the reaction rate |
| Liu et al. [52] | Phenanthrene | Different types | Lower presence of pollutants if MPs are present |
| Liu et al. [54] | Ciprofloxacin | PE, PVC, and PET | Chlorination of polymer decreases adsorption in PE, but not with PVC and PET |
| Liu et al. [53] | PAHs | PS | Pre-extraction of hydrophobic fraction decreases adsorption |
| Liu et al. [51] | Bisphenol A | PU, PA | Hydrogen bonds in MPs improve adsorption capacity |
| Llorca et al. [56] | PCBs | Different types | Low-chlorinated compounds show higher adsorption in all polymers. PET and PS have higher affinity for PCBs than PE |
| Lončarsk et al. [57] | PAHs | PLA | Second-order modeling indicates chemisorption mechanism |
| Lončarsk et al. [57] | PAHs | PLA | Very low and insignificant adsorption |
| Luo et al. [59] | PAHs and HOCs | Different types | Presence of oxygenated groups reduces adsorption of organic compounds |
| Munoz et al. [62] | drugs | PS, PET, PP, PE | Natural organic matter blocks active sites reducing adsorption |
| Sharma et al. [21] | PAHs | Different types | Adsorption capacity is between 46 and 236 μg/g, in 45 min |
| Sorensen et al. [17] | Fluorene and phenanthrene | PE, PS | MP-adsorbed PAHs do not accumulate in crustaceans. Salinity is a very important point in the process |
| Yang et al. [90] | Pyrene and its substituted derivatives | PS | Rate is second order with respect to the amount of contaminant |
| Yang et al. [90] | Pyrene and its substituted derivatives | Different types | CH3- substituted pollutant increases adsorption, NH2-, OH-, and COOH- decrease |
| Yu et al. [91] | Naphthalene | PS, modified with -COOH | No differences when introducing functional groups |
| Yu et al. [91] | Naphthalene and NH2-, OH-, and COOH- derivatives | PS | The presence of these groups causes adsorption to occur faster |
| Zhang et al. [18] | PAHs | Agricultural MPs | Aging decreases the affinity of MPs with organic pollutant, by increasing oxygenated surface |
| Zhang et al. [18] | PAHs | PU, polyurea, and urea-formaldehyde | Negative charges on the surface of polymers allows PAHs to be easily adsorbed |
| Zhang et al. [18] | Phenanthrene | PU, polyurea, urea-formaldehyde | Increase in the salinity produces an increase in the adsorption capacity |
| Zhang et al. [106] | Nitro-anthracene | PS, PP, PE | Presence of salts decrease adsorption rate |
| Zhao et al. [98] | Phenanthrene, pyrene and derivatives | Different types | Pseudo-second-order model for the adsorption process |
| Zhao et al. [98] | Phenanthrene and pyrene and polar derivatives | PBS, PCL, PU, PS | Adsorption in polar polymers is much faster than in conventional ones |
| Zhu et al. [100] | Phenanthrene | Different types | Presence of MPs delays the leaching of phenanthrene in soil |
| Zhu et al. [100] | Phenanthrene | PS, PVC, PE | Adsorption capacity: PS > PE > PVC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, J.A. Adsorption of PAHs and PCDD/Fs in Microplastics: A Review. Microplastics 2022, 1, 346-358. https://doi.org/10.3390/microplastics1030026
Conesa JA. Adsorption of PAHs and PCDD/Fs in Microplastics: A Review. Microplastics. 2022; 1(3):346-358. https://doi.org/10.3390/microplastics1030026
Chicago/Turabian StyleConesa, Juan A. 2022. "Adsorption of PAHs and PCDD/Fs in Microplastics: A Review" Microplastics 1, no. 3: 346-358. https://doi.org/10.3390/microplastics1030026
APA StyleConesa, J. A. (2022). Adsorption of PAHs and PCDD/Fs in Microplastics: A Review. Microplastics, 1(3), 346-358. https://doi.org/10.3390/microplastics1030026







