Microplastics’ Occurrence in Edible Fish Species (Mullus barbatus and M. surmuletus) from an Italian Marine Protected Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Microplastic Analyses
2.3. Quality Assurance and Control
2.4. Polymer Identification
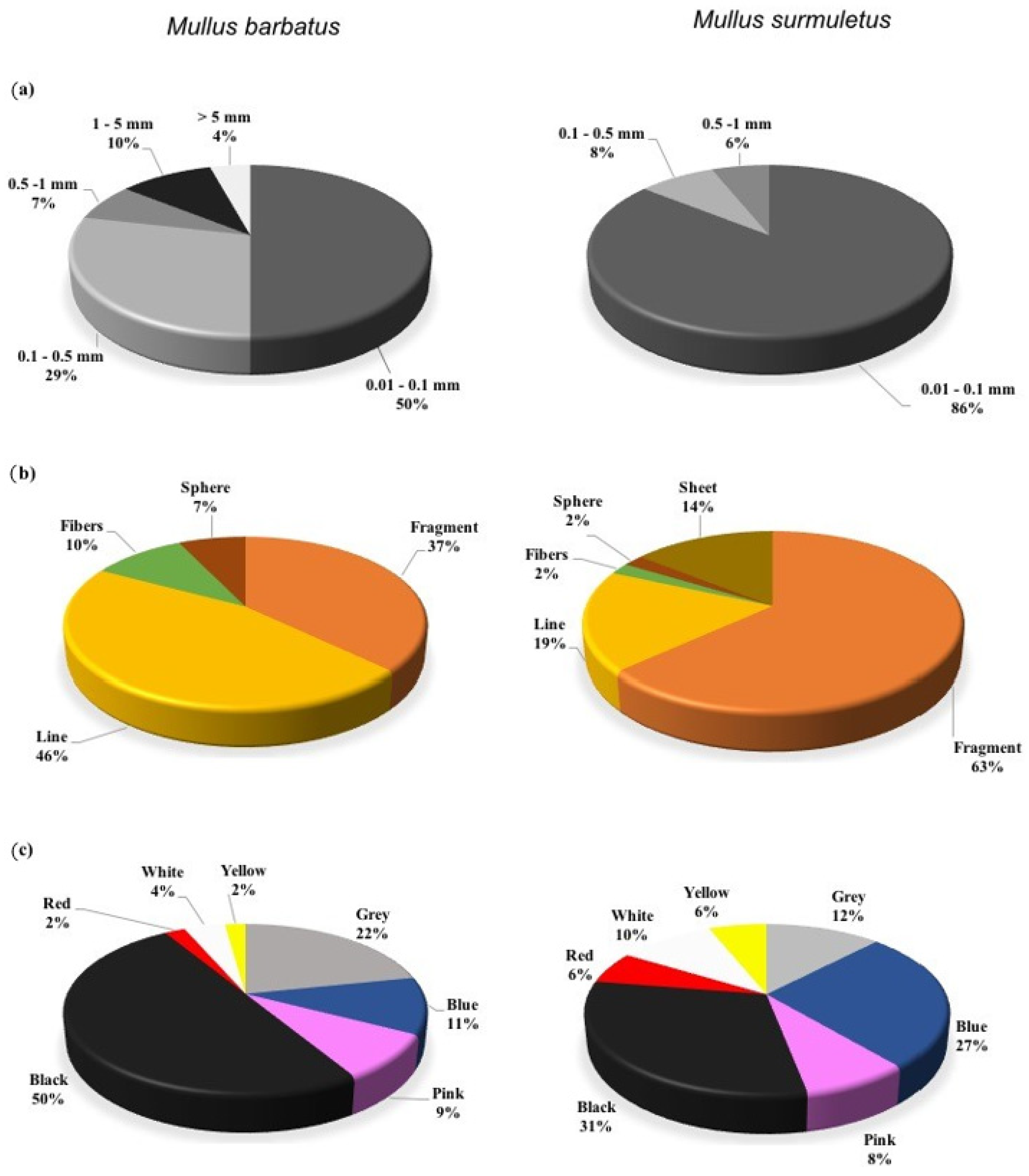
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, M.; Lebreton, L.C.M.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, G.W.; Lubchenco, J.; Carr, M.H. Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl. 1998, 8, 79–92. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Blašković, A.; Fastelli, P.; Čižmek, H.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the Croatian marine protected area of the natural park of TelašČica bay (Adriatic Sea). Mar. Pollut. Bull. 2017, 114, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Abessa, D.M.S.; Albuquerque, H.C.; Morais, L.G.; Araújo, G.S.; Fonseca, T.G.; Cruz, A.C.F.; Campos, B.G.; Camargo, J.B.D.A.; Gusso-Choueri, P.K.; Perina, F.C.; et al. Pollution status of marine protected areas worldwide and the consequent toxic effects are unknown. Environ. Pollut. 2018, 243, 1450–1459. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Morley, S.A.; Bell, J.; Brewin, P.; Brigden, K.; Collins, M.; Glass, T.; Goodall-Copestake, W.P.; Henry, L.; Laptikhovsky, V.; et al. Marine plastics threaten giant Atlantic Marine Protected Areas. Curr. Biol. 2018, 28, R1121–R1142. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Bergmann, M.; Gutow, L.; Klages, M. (Eds.) Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319165097. [Google Scholar]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Budziak, A.; Van Franeker, J.; Galgani, F.; Hanke, G.; Maes, T.; Matiddi, M.; Nilsson, P.; Oosterbaan, L.; Priestland, E.; et al. Harm Caused by Marine Litter; MSFD GES TG Marine Litter–Thematic Report; JRC Technical Report; EUR 28317 EN; European Union: Luxembourg, 2016; ISBN 1517-8382. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; Mcgonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.R.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Bio. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Fossi, M.C.; Pedà, C.; Compa, M.; Tsangaris, C.; Alomar, C.; Claro, F.; Ioakeimidis, C.; Galgani, F.; Hema, T.; Deudero, S.; et al. Bioindicators for monitoring marine litter ingestion and its impacts on Mediterranean biodiversity. Environ. Pollut. 2018, 237, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T.T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Anastasopoulou, A.; Fortibuoni, T.; Ronchi, F.Z. Marine Litter Assessment in the Adriatic & Ionian Seas; ISPRA: Ispra, Italy, 2017; ISBN 9789606793257.
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Giani, D.; Baini, M.; Galli, M.; Casini, S.; Fossi, M.C. Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 129–137. [Google Scholar] [CrossRef]
- Wright, S.L.; Levermore, J.M.; Kelly, F.J. Raman Spectral Imaging for the Detection of Inhalable Microplastics in Ambient Particulate Matter Samples. Environ. Sci. Technol. 2019, 53, 8947–8956. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Nigamatzyanova, L.; Fakhrullina, G.; Fakhrullin, R. Label-free identification of microplastics in human cells: Dark-field microscopy and deep learning study. Anal. Bioanal. Chem. 2022, 414, 1297–1312. [Google Scholar] [CrossRef]
- Nigamatzyanova, L.; Fakhrullin, R. Dark-field hyperspectral microscopy for label-free microplastics and nanoplastics detection and identification in vivo: A Caenorhabditis elegans study. Environ. Pollut. 2021, 271, 116337. [Google Scholar] [CrossRef]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Becker, J.; Ehlers, S.M.; Fritz, M.; Fischer, C.B.; Koop, J.H.E.; Winkelmann, C.; Imhof, W. Quantitative analysis of PET microplastics in environmental model samples using quantitative 1H-NMR spectroscopy: Validation of an optimized and consistent sample clean-up method. Anal. Bioanal. Chem. 2019, 411, 7409–7418. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scholz-Böttcher, B.M. Microplastics analysis in environmental samples-recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal. Methods 2019, 11, 2489–2497. [Google Scholar] [CrossRef]
- De Benedetto, G.E.; Fico, D.; Margapoti, E.; Pennetta, A.; Cassiano, A.; Minerva, B. The study of the mural painting in the 12th century monastery of Santa Maria delle Cerrate (Puglia-Italy): Characterization of materials and techniques used. J. Raman Spectrosc. 2013, 44, 899–904. [Google Scholar] [CrossRef]
- Laurentie, M.; Amara, R.; Boricaud, B.; Hermabessiere, L.; Kazour, M.; Paul-Pont, I.; Cassone, A.-L.; Himber, C.; Soudant, P.; Duflos, G.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.R.D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci.-Proc. Impacts 2013, 15, 1949. [Google Scholar] [CrossRef] [Green Version]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Zhou, X.X.; Hao, L.T.; Wang, H.Y.Z.; Li, Y.J.; Liu, J.F. Cloud-Point Extraction Combined with Thermal Degradation for Nanoplastic Analysis Using Pyrolysis Gas Chromatography-Mass Spectrometry. Anal. Chem. 2019, 91, 1785–1790. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Kovač Viršek, M.; Bojanić Varezić, D.; Digka, N.; Fortibuoni, T.; Koren, Š.; Mandić, M.; Mytilineou, C.; Pešić, A.; Ronchi, F.; et al. Assessment on marine litter ingested by fish in the Adriatic and NE Ionian Sea macro-region (Mediterranean). Mar. Pollut. Bull. 2018, 133, 841–851. [Google Scholar] [CrossRef]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
| Species | TL (cm) | FW (g) | ||||
|---|---|---|---|---|---|---|
| Min | Max | Mean (SE) | Min | Max | Mean (SE) | |
| M. surmuletus | 14.5 | 20.5 | 18 ± 0.5 | 31 | 135 | 79 ± 0.6 |
| M. barbatus | 15 | 18 | 16.7 ± 0.3 | 56 | 82 | 70 ± 3 |
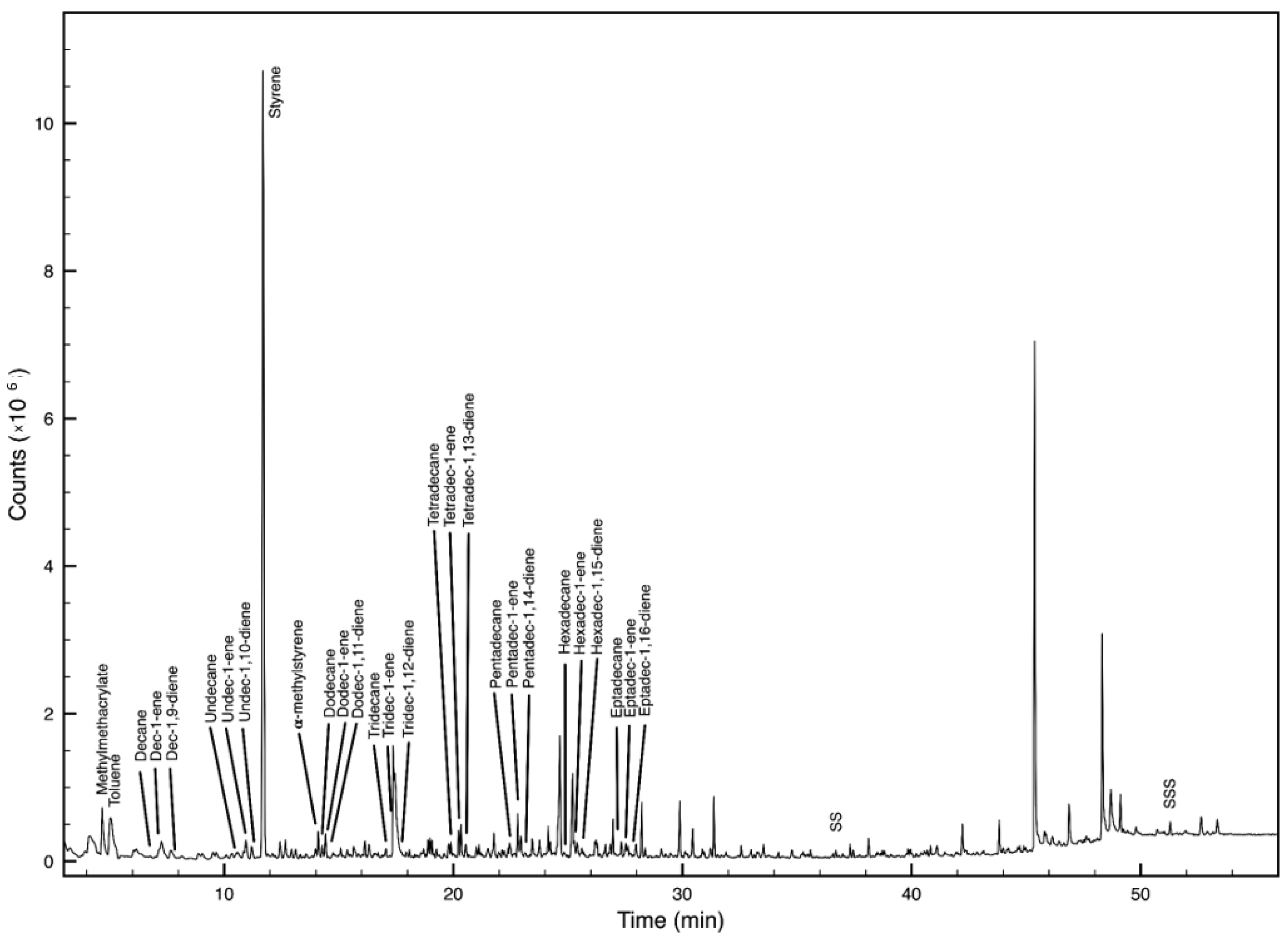
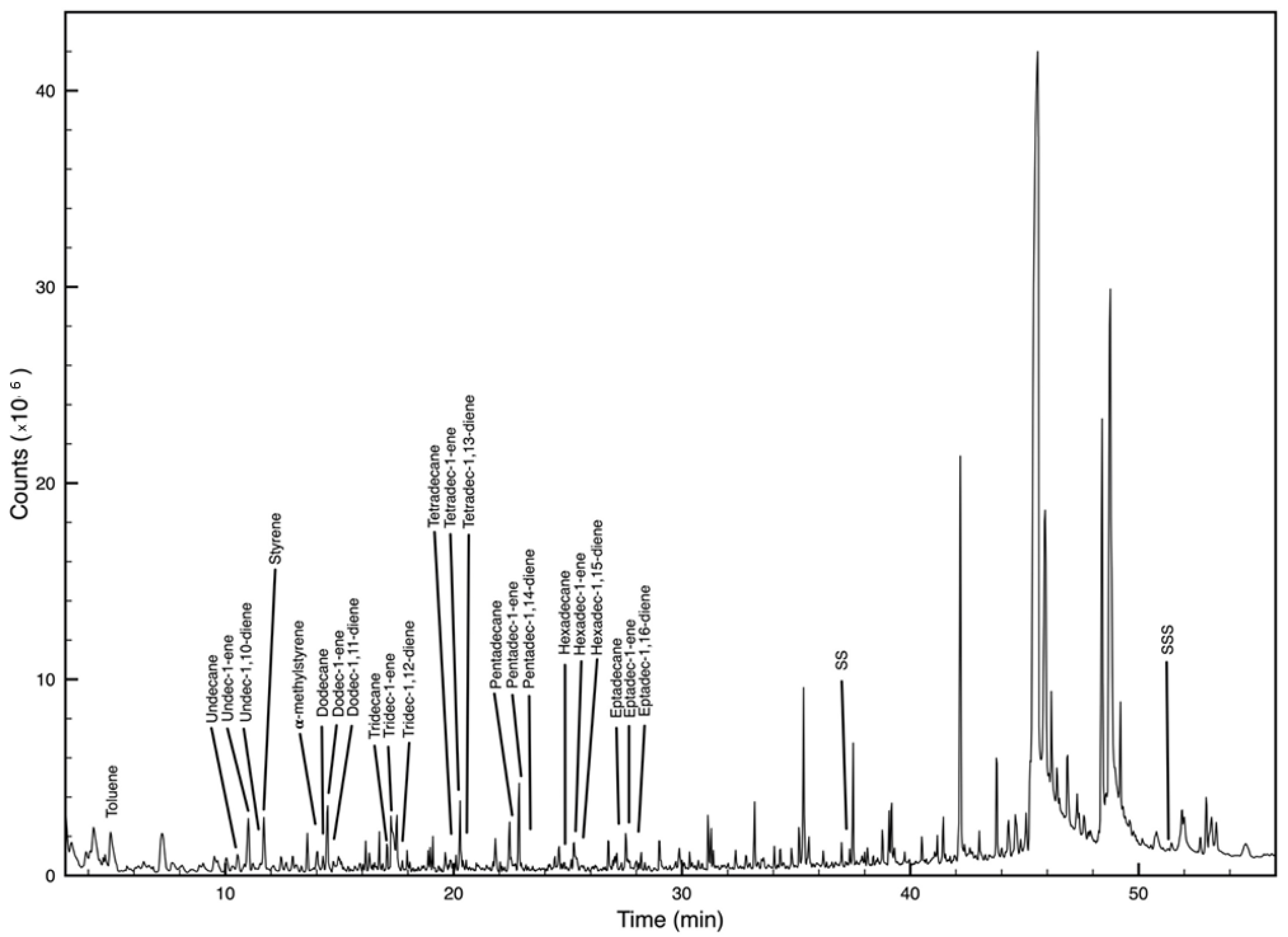
| Polymer | Main Decomposition Products | M (m/z) | Indicator Ions (m/z) | RT * |
|---|---|---|---|---|
| PE | alkanes (ex. CH3(CH2)8CH3) | 142 | 85, 71 | 6.8 |
| α-alkenes (ex. CH2=CH(CH2)7CH3) | 140 | 97, 83 | 7.3 | |
| α,ω-alkadienes (ex. CH2=CH(CH2)6CH=CH2) | 138 | 95, 81 | 8 | |
| PP | 2,4-dimethylhept-1-ene | 126 | 126, 70 | 3.5 |
| 2,4,6-trimethyl-1-nonene (meso form) | 168 | 111, 112, 83, 69 | 10.1 | |
| 2,4,6-trimethyl-1-nonene (racemic form) | 168 | 112, 111, 83, 69 | 10.3 | |
| 2,4,6,8-tetramethyl-1-undecene (isotactic) | 210 | 111, 83, 69 | 17.3 | |
| 2,4,6,8-tetramethyl-1-undecene (heterotactic) | 210 | 111, 83, 69 | 17.6 | |
| 2,4,6,8-tetramethyl-1-undecene (syndiotactic) | 210 | 111, 83, 69 | 17.9 | |
| PS | styrene | 104 | 104, 78 | 11.7 |
| α-methylstyrene | 118 | 117, 118, 103 | 14.1 | |
| C=C(Ph)-C-C-Ph (dimer) | 208 | 208, 91 | 36.7 | |
| C=C(Ph)-C-C(Ph)-C-C-Ph (trimer) | 312 | 312, 194, 91 | 51.2 | |
| PVC | Chlorobenzene | 112 | 112, 114, 77 | 10.3 |
| Styrene | 104 | 104, 78 | 11.7 | |
| Indene | 116 | 116, 115 | 18.1 | |
| Naphthalene | 128 | 128 | 24.2 | |
| 1-methylnaphthalene | 142 | 142, 141, 115 | 26.7 | |
| 2-methylnaphthalene | 142 | 142, 141, 115 | 27.4 | |
| PET | Acetophenone | 105 | 120, 105, 77, 51 | 22.1 |
| Vinyl benzoate | 148 | 148, 105, 77, 52 | 22.2 | |
| Benzoic acid | 122 | 122, 105, 77 | 32.6 | |
| Divinyl terephthalate | 218 | 175, 104 | 34.7 | |
| PMMA | Methyl methacrylate | 100 | 100, 69, 41, 85 | 4.2 |
| Nylon | ε-caprolactam | 113 | 113, 85, 83 | 29.9 |
| Polymer Type | Composition (%) |
|---|---|
| Polyethylene (PE) | 80 |
| Polystyrene (PS) | 33.4 |
| Polymethylmethacrylate (PMMA) | 6.7 |
| Polyvinylchloride (PVC) | 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felline, S.; Piccardo, M.; De Benedetto, G.E.; Malitesta, C.; Terlizzi, A. Microplastics’ Occurrence in Edible Fish Species (Mullus barbatus and M. surmuletus) from an Italian Marine Protected Area. Microplastics 2022, 1, 291-302. https://doi.org/10.3390/microplastics1020021
Felline S, Piccardo M, De Benedetto GE, Malitesta C, Terlizzi A. Microplastics’ Occurrence in Edible Fish Species (Mullus barbatus and M. surmuletus) from an Italian Marine Protected Area. Microplastics. 2022; 1(2):291-302. https://doi.org/10.3390/microplastics1020021
Chicago/Turabian StyleFelline, Serena, Manuela Piccardo, Giuseppe Egidio De Benedetto, Cosimino Malitesta, and Antonio Terlizzi. 2022. "Microplastics’ Occurrence in Edible Fish Species (Mullus barbatus and M. surmuletus) from an Italian Marine Protected Area" Microplastics 1, no. 2: 291-302. https://doi.org/10.3390/microplastics1020021
APA StyleFelline, S., Piccardo, M., De Benedetto, G. E., Malitesta, C., & Terlizzi, A. (2022). Microplastics’ Occurrence in Edible Fish Species (Mullus barbatus and M. surmuletus) from an Italian Marine Protected Area. Microplastics, 1(2), 291-302. https://doi.org/10.3390/microplastics1020021









