1. Introduction
DNA interstrand cross-links (ICLs), which form when the opposing strands of duplex DNA become covalently linked, are among the most deleterious forms of DNA damage [
1]. As ICLs mark an absolute block to essential cellular processes such as DNA replication and transcription [
2], if left unrepaired, these lesions can cause chromosomal aberrations and cell death. Exogenous ICL-inducing agents (e.g., cyclophosphamide, mitomycin C) are highly toxic, especially to rapidly dividing cells, and have been widely used in cancer treatment [
3]. The cellular resistance to these ICL-inducing agents has prompted research on the formation, recognition, and repair of ICLs in vivo [
4]. ICLs are removed through a variety of repair pathways, including replication-coupled [
5], replication-independent [
6], and ICL traverse pathways [
7] as select examples. These cross-links are of particular importance to the pathology of Fanconi anemia, a rare genetic disease caused by mutations in a cluster of genes associated with replication-coupled ICL repair [
8]. Fanconi anemia is characterized by bone marrow failure, developmental abnormalities, and a predisposition to cancer, and is often fatal. The search for ICL-inducing agents has led to the identification of new classes of ICLs induced by alcohol [
9], acetaldehyde [
10], unsaturated aldehydes [
11], abasic sites [
12], nucleotidyl radical [
13], and colibactin [
14]. The goal of the present study is to discover potential ICLs that can be induced by light and/or oxidative stress.
Under oxidative stress, cellular levels of reactive oxygen species (ROS) are elevated to give rise to 7,8-dihydro-8-oxoguanine (oxoG) and 7,8-dihydro-8-oxoadenine (oxoA) as major lesions [
15]. Due to lower redox potentials of oxoG (0.74 V) and oxoA (0.92 V) compared to those of undamaged bases [e.g., guanine (1.29 V), adenine (1.42 V)], these C8-oxidized purines can undergo further oxidation via the long-range electron transfer mechanism [
16]. The major oxidative adenine lesion oxoA has been shown to generate oxoA ICLs under in vitro oxidative conditions [
17], where the proposed mechanism involves the oxidation-mediated formation of an iminopurine followed by the nucleophilic attack of the N2 of guanine on the C2 of the iminopurine (
Figure 1A). In our initial report of oxoA-mediated cross-link formation, we have shown that oxoA ICLs are produced at modest yields (~20%) in the presence of reactive halogen species, sodium hypochlorite (NaOCl) [
17]. While these conditions are relevant to some biological processes like inflammation, it would be of broad interest if these ICLs could form in the presence of ROS, including hydroxyl radical (OH
•), peroxides (ROOR), singlet oxygen (
1O
2), and superoxide anion (
•O
2−). These ROS are byproducts of normal oxygen metabolism but are often generated at elevated levels during oxidative stress in response to metabolic stress [
18], inflammation [
19], or upon irradiation by UV light exposure [
20]. We contemplated that ROS generated by irradiation may trigger further oxidation of oxoA into the reactive iminoquinone electrophile, which can react with a neighboring nucleophile to form oxoA-mediated cross-links.
Human skin cells contain a wide range of chromophores (e.g., riboflavin, flavins, heme, melanin, NADPH), which can absorb UVA photons to generate ROS via photosensitization [
21]. Riboflavin, which is present at high levels in skin tissue (7.9 μmol per kg) and eye fluid (4.5 μmol per kg) [
22,
23], is the main component of flavin adenine dinucleotide and flavin mononucleotide in flavin oxidoreductase proteins and is involved in the electron transfer process. Riboflavin absorbs light at maximum wavelengths of 356 and 445 nm, generating a relatively long-lived triplet excited state of riboflavin, which in turn reacts with ground-state triplet oxygen to produce singlet oxygen (
Figure 1C) [
23]. The inclusion of riboflavin has been shown to greatly increase UVA-induced oxidative damage to DNA [
24].
We herein report the effect of light and riboflavin on the formation of ICLs mediated by oxoA lesion. The production of oxoA-mediated cross-links is significantly influenced by the wavelength of light and the presence of riboflavin. While UVA or visible rays alone did not trigger oxoA ICL formation, the inclusion of riboflavin in the reaction readily generated oxoA ICLs, underscoring the critical role of the cellular photosensitizer in the cross-linking reaction.
2. Methods
Oligonucleotides for UV irradiation reactions: PAGE-purified oxoA-containing oligodeoxynucleotide (5′-GGCGCGC[oxoA]CGCGC-3′) (100 µM, Midland Co., Midland, TX, USA) was mixed with 1 molar equivalent of its complementary strand (5′-GCGCGCTGCGCGCC-3′, 100 µM) in an annealing buffer composed of 10 mM sodium phosphate (pH 7.0) and 100 mM NaCl. The solution was then heated at 90 °C for 5 min and allowed to slowly cool to room temperature. The total volume of each reaction was 40 µL containing 8 µL of the 50 µM duplex DNA (0.4 nmol), varying concentrations of riboflavin (0–250 µM), and annealing buffer. A negative control used for the following experiments was duplex DNA containing the oxoA-modified base but left untreated. Duplex DNA containing oxoA treated with 5 molar equivalents of N-bromosuccinimide (NBS) was used as a positive control due to its known induction of oxoA-G ICL [
17].
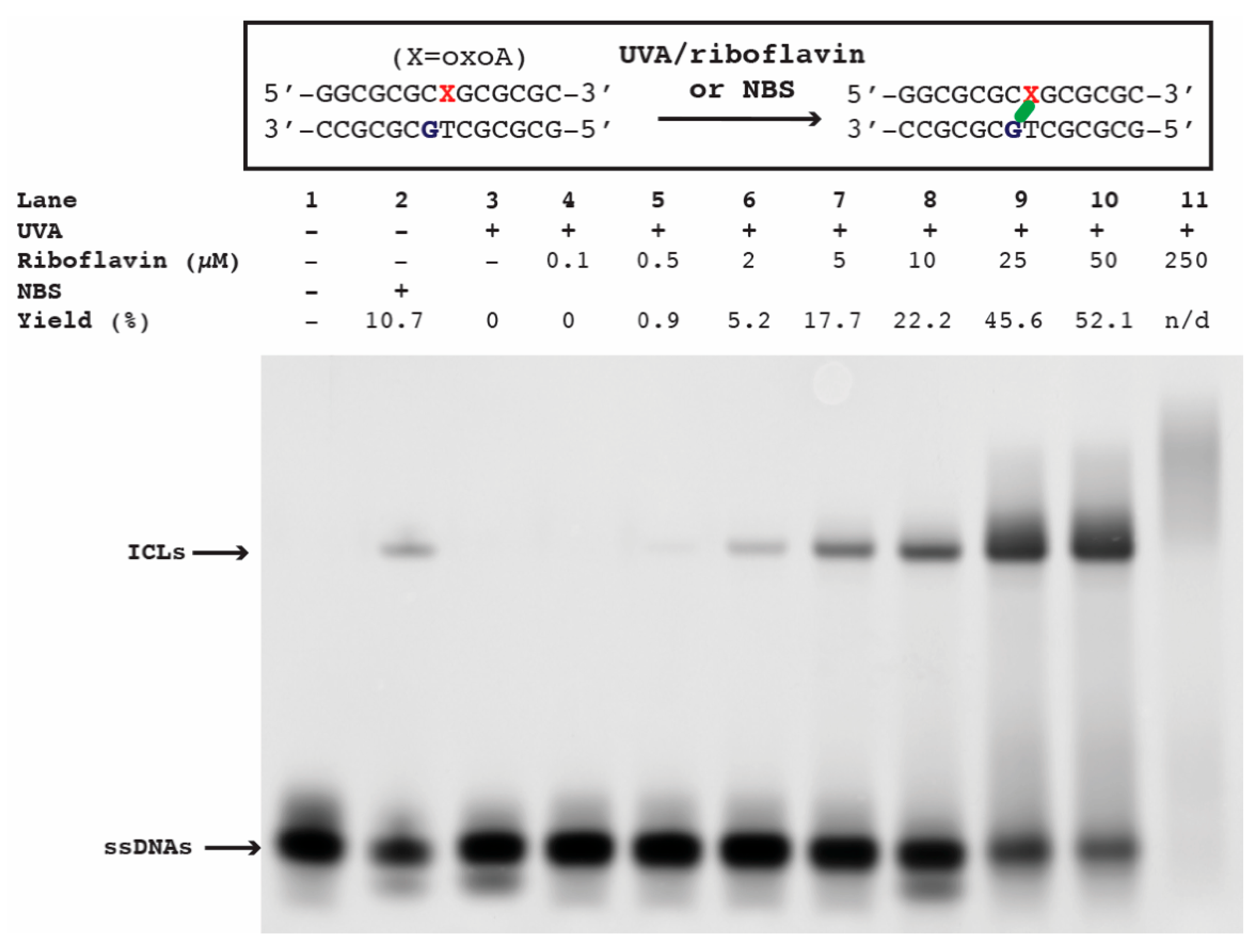
Production of oxoA-mediated ICLs in the presence of UVA and riboflavin: OxoA-containing DNA (0.4 nmol), varying concentrations of riboflavin (0, 0.5, 2, 5, 10, 25, 50, and 250 µM), and annealing buffer were mixed to achieve the desired final DNA concentration (10 µM), totaling 40 µL per reaction. All reactions were performed in PCR tubes that were placed approximately 1 cm in distance to the light source emitting UVA (365 nm, Spectroline, Model ENF-280C (Spectronics, Melville, NY, USA); photon flux: 2.9 × 1018; photon flux density: 2.3 × 1017 photons/cm2/s; quantum yield: 1.5%) for 75 min. Reactions were then stopped by adding an equal volume of 2× reaction quenching buffer (98% formamide, 1 mM EDTA, 1 mg/mL bromophenol blue). Approximately 50 pmol of DNA from each quenched reaction was added to a 20% denaturing gel and run for ~3 h at 300 V, followed by SYBR-gold staining for 1 h and imaging on a Typhoon 9500 for band visualization. Using the image processing program ImageJ (version 1.54p), the integrated density of all present PAGE bands was measured per lane and normalized relative to an average of five background levels measured per gel. The percent yield of the ICL bands was calculated by dividing their integrated density by the total integrated density measured per lane and multiplying by one hundred. All percent yields were calculated with their respective negative control set to absolute zero and averaged from triplicate reactions.
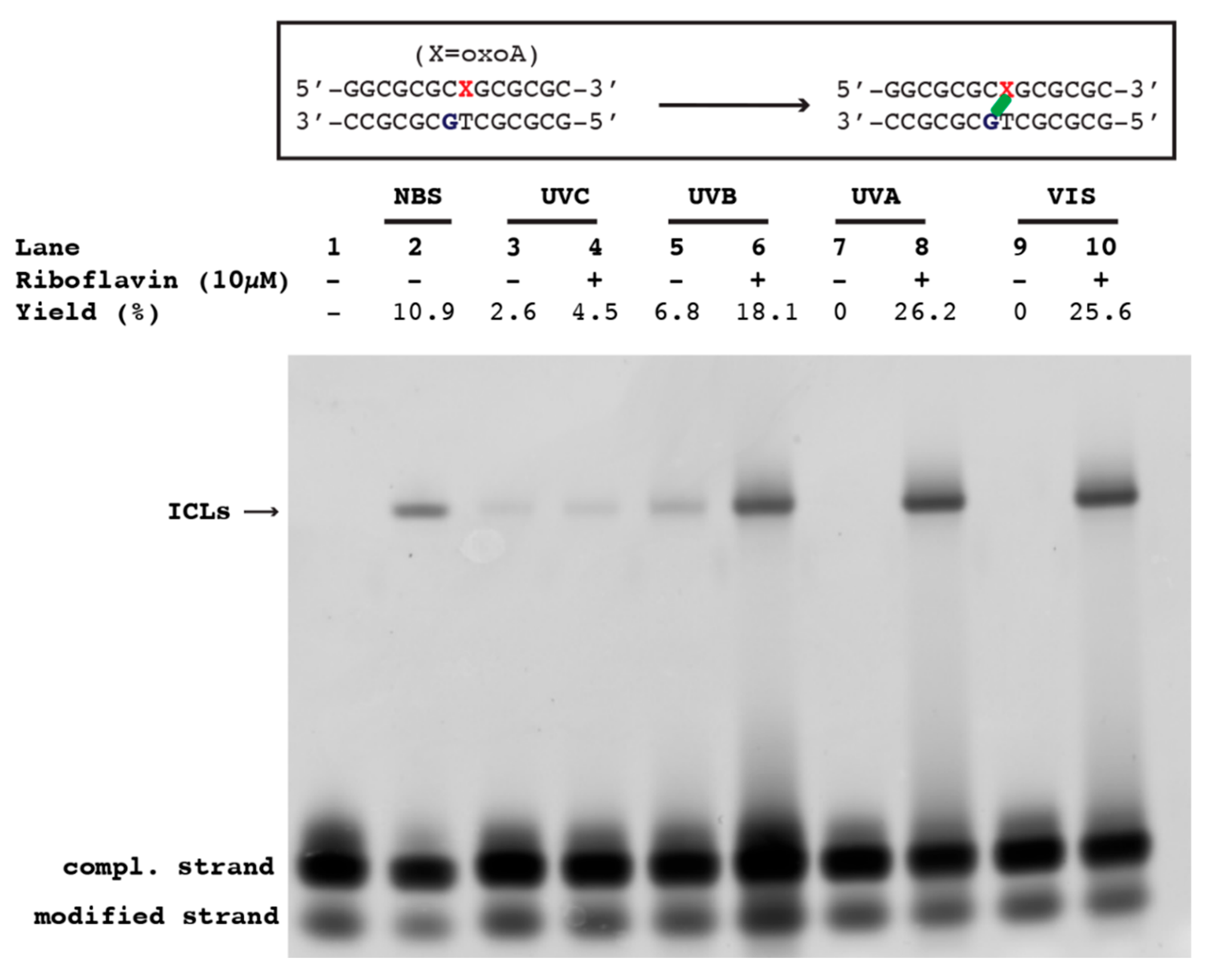
Production of oxoA-mediated ICLs with a varying wavelength of light: In the presence or absence of riboflavin (10 µM), oxoA-containing duplex DNA was irradiated with UVC (254 nm), UVB (302 nm), UVA (365 nm), or visible light for 75 min using a UV lamp (254 nm and 365 nm: Spectroline, Model ENF-280C; 302 nm: Analytikjena, UVM-57 Handheld UV lamp). All reactions were performed in PCR tubes that were placed approximately 1 cm in distance from the light source. After irradiation, the reactions were immediately quenched by adding 2× quenching buffer (98% formamide, 1 mM EDTA, 1 mg/mL bromophenol blue). The resulting solution containing approximately 50 pmol of the reacted DNA was run on a 20% urea PAGE gel, stained by SYBR-gold, and visualized by Typhoon 9500 imager. Untreated oxoA-containing duplex DNA and NBS-treated oxoA-containing duplex DNA were used as a negative control and a positive control, respectively. Percent yields of ICL bands were measured as previously stated, with measured integrated densities normalized relative to an average of background levels per gel, as well as respective negative controls set to absolute zero and averaged using ImageJ for quantification.
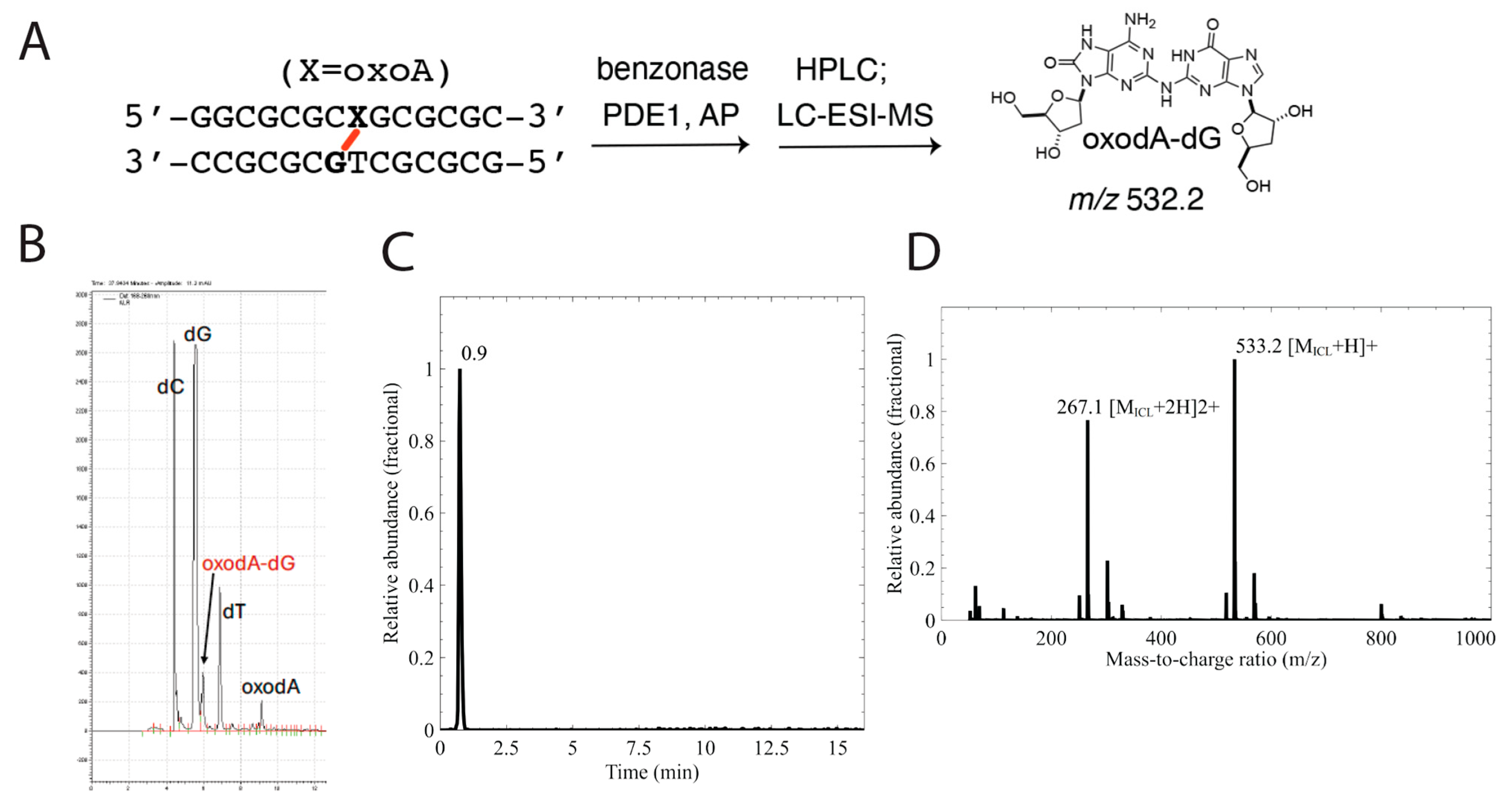
Characterization of the oxoA cross-links by LC-MS analysis: We performed mass spectrometric analyses to characterize the chemical structure of the oxoA cross-links in duplex DNA. The cross-linked duplex DNAs (20 nmol) were digested with an enzyme cocktail containing MgCl2 (20 mM), NaCl (100 mM), Tris-HCl pH 7.9 (20 mM), alkaline phosphatase (20 U), benzonase (25 U), phosphodiesterase I (0.03 U), and erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (0.05 mM). The enzyme cocktail was incubated for ~18 h at 37 °C in a Thermomixer C at 400 rpm for a fully digested cross-linked dinucleoside along with constituent nucleosides. The fully digested DNA was extracted via phenol-chloroform and enriched through HPLC using a C18 column (250 × 4.6 mm, Hypersil GoldTM, Thermo Fisher Scientific (Waltham, MA, USA)) and a detector wavelength of 254 nm with a simultaneous linear increase to 30% of acetonitrile (MeCN) and decrease to 70% of 0.1 M TEAA, pH 7, 5% vol MeCN at 1 mL/min for 30 min followed by a linear increase to 100% MeCN and a decrease to 0% of 0.1 M TEAA, pH 7, 5% MeCN at 1 mL/min for 8 min for full elution. The enriched mixtures were subjected to LC-ESI-MS analysis to identify residues present in the cross-links. The LC-ESI-MS analysis was performed using a SeQuant Zic-HILIC 5 μm column (150 × 4.6 mm). The flow rate was set to 0.5 mL/min, and the column temperature was maintained at 30 °C. The mobile phase gradient consisted of 10 mM ammonium acetate and acetonitrile. The injection volume was 10 μL, with the sample dissolved in 50 μL of water. The spray voltage was set to 3.5 |kV|, with a capillary temperature of 320 °C. The S-lens RF level was maintained at 70.0, and the auxiliary gas heater temperature was set to 250 °C.
3. Results
To evaluate whether singlet oxygen can induce oxoA-mediated ICLs, we first screened ICL formation under the influence of UVA and riboflavin, which are known to efficiently produce singlet oxygen. The DNA duplex containing a site-specific oxoA modification (
Figure 2) was annealed at a concentration of 10 µM in buffer containing 100 mM NaCl and 10 mM sodium phosphate (pH 7) before the addition of one molar equivalent (10 µM) riboflavin. This oxoA-containing oligonucleotide could generate ICLs involving the oxoA nucleobase and the adjacent guanine on the complementary strand based on our previous work [
17]. The irradiation of oxoA-containing duplex with UVA alone did not give rise to ICLs, while the inclusion of riboflavin triggered the production of ICLs in a riboflavin concentration-dependent manner (
Figure 2). ICLs began to form at 0.5 μM riboflavin, and the ICL yield reached ~50% at 25–50 μM riboflavin. The UVA/riboflavin-mediated ICLs and the NBS-mediated ICLs migrated similarly, suggesting that the UVA/riboflavin induced oxoA-G ICLs.
To assess the effect of the wavelength of light on the formation of oxoA ICLs in the presence/absence of riboflavin, we irradiated the oxoA-containing duplex DNA with varying sources of light, including short-wave UV (λ = 254 nm, UVC), medium-wave UV (λ = 302 nm, UVB), long-wave UV (λ = 360 nm, UVA), and visible rays. Interestingly, in the presence of riboflavin, all light exposure groups produced a slow-migrating band in the region of the gel where oxoA-G ICLs were expected to appear. The reaction induced by UVC/riboflavin revealed a slow-migrating band in 4.5 ± 0.8% yield (
Figure 3, lane 4). Here, the cross-link band migrated at the same rate as the band generated by the NBS-treated control (
Figure 3, lane 2), which has been shown to generate oxoA-G ICLs. Another control reaction involving UVC rays in the absence of riboflavin also revealed the same slow-migrating band in 2.6 ± 0.6% yield (
Figure 3, lane 3). This indicates that the presence of riboflavin does not significantly promote UVC-mediated formation of oxoA-G ICLs. These results mark a rare formation of UVC-induced ICLs. The lane containing the oxoA-modified duplex exposed to UVB in the presence of riboflavin possessed a slow-migrating cross-link band at a yield of 18.1 ± 0.9% (
Figure 3, lane 6). UVB exposure alone resulted in a decrease in ICL yield (6.8 ± 1.6%), indicating UVB and UVB/riboflavin are more efficient at generating oxoA-G ICLs than UVC and UVC/riboflavin, respectively. UVA irradiation in the presence of riboflavin resulted in the high-yielding oxoA cross-link with a yield of 26.2 ± 2.0% (
Figure 3, lane 8), while UVA alone did not generate an ICL band (lane 7). Finally, exposure of the oxoA-modified duplex to visible light in the presence of riboflavin revealed a cross-link band upon PAGE analysis at a yield of 25.6 ± 2.1% (
Figure 3, lane 10), while the control reaction did not produce a cross-link band.
Taken together, these results show that in the absence of riboflavin, both UVC and UVB rays, but not UVA and visible rays, induce oxoA-G ICLs. In the presence of riboflavin, UVA and visible rays dramatically induce oxoA-G ICL formation, highlighting the impact of the naturally occurring photosensitizer riboflavin on oxoA-mediated ICL formation. The effect of riboflavin on UVC- and UVB-induced oxoA-G ICL formation is less pronounced than that on UVA- and visible ray-induced ICL formation. This differential effect is consistent with the fact that the absorption maxima of riboflavin (365 and 445 nm) are within UVA and visible rays. These ICLs represent rare examples of light-induced ICLs mediated by oxidative DNA lesions and differ from those produced by photoactivation of exogenous alkylating agents such as furan and binitroimidazole [
25,
26]. We conclude that UVC and UVB, as well as UVA/riboflavin and visible rays/riboflavin, can facilitate ICL formation. While it is clear that singlet oxygen, which can be generated by UVA/riboflavin and visible rays/riboflavin, is an efficient ICL-inducing agent, these results also highlight that other types of ROS or the absorption of UVC or UVB alone can promote ICL formation.
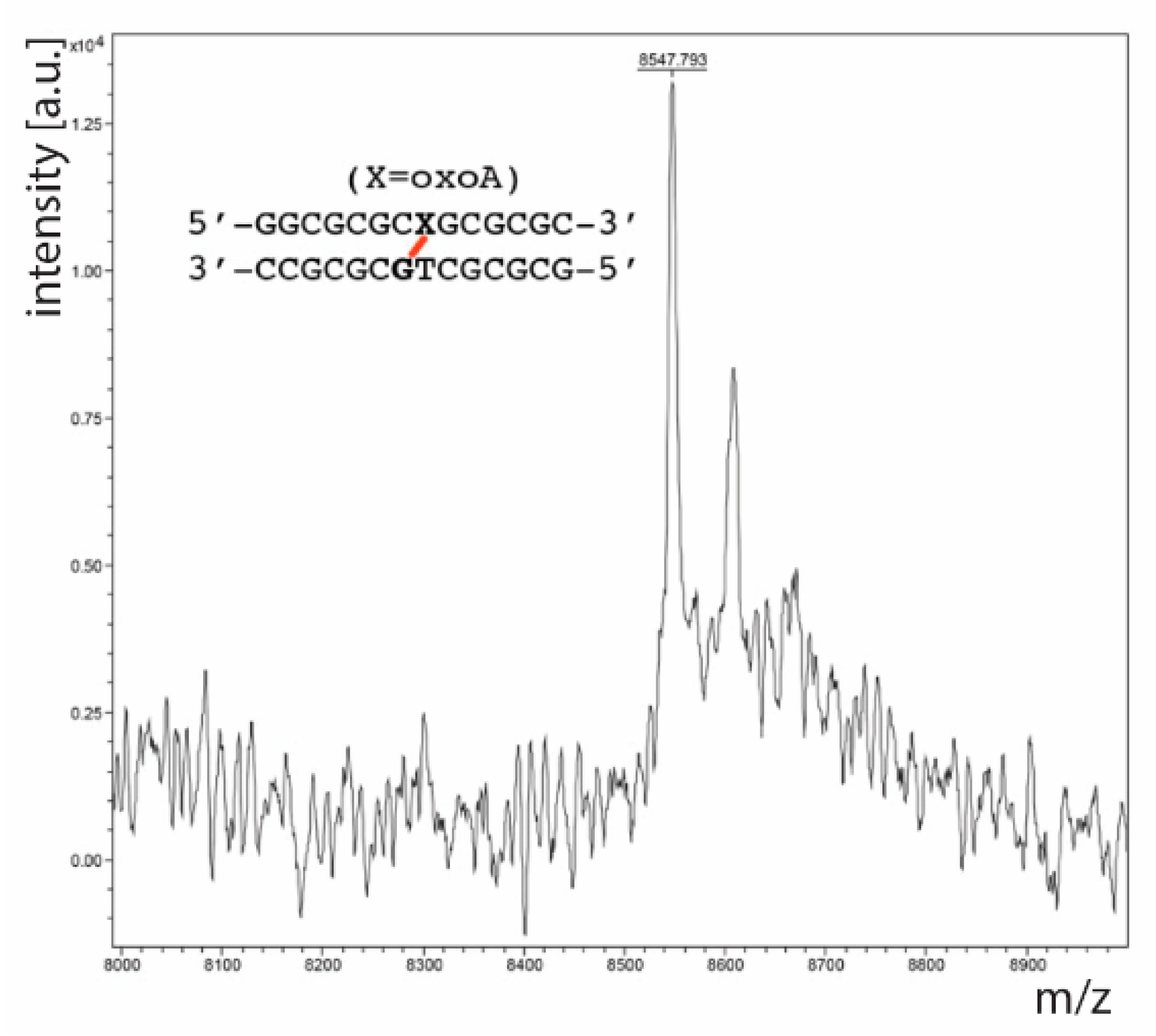
To confirm that the slow-migrating band observed upon denaturing PAGE analysis was an oxoA-G cross-link, we performed a series of mass spectrometric analyses. First, we performed MALDI-TOF-MS analysis on a PAGE-purified oxoA-modified duplex DNA exposed to UVA light in the presence of riboflavin. This MALDI-TOF-MS analysis revealed an
m/
z of 8547.8 (calculated
m/
z = 8546.6,
Figure 4), which suggests that the slow-migrating band in question is a cross-linked duplex containing oxoA. The results also suggest that oxoA is preferentially oxidized over normal bases in the presence of light-induced ROS. Next, we treated the same cross-linked oxoA-modified duplex DNA with a cocktail of enzymes to yield a mixture containing individual nucleosides along with the putative oxoA-G cross-linked dinucleoside. This nucleoside mixture was purified through HPLC, and the fractions believed to contain cross-linked dinucleoside were analyzed via LC-ESI-MS to identify the exact mass of the product. This analysis produced a single peak on the selected-ion chromatogram (SIC) trace, which was revealed to have an
m/
z consistent with an oxoA-G cross-linked dinucleoside as shown in
Figure 5 (
m/
z = 533.2 [M+H]
+). Specifically, these results are consistent with those observed in our previous report regarding oxoA cross-linking and suggest that this cross-linking reaction occurs between the C2 position of oxoA and the exocyclic N2-amino group of an opposing guanine residue. Together, these results confirm that the product observed upon incubation of oxoA-modified duplex DNA with riboflavin and exposure to various light sources is a DNA ICL that involves oxoA and guanine residues on opposing strands of the duplex.
4. Discussion
DNA ICLs are among the most cytotoxic and genotoxic lesions; as few as 20 unrepaired ICLs can be sufficient to kill mammalian cells [
1]. The formation of oxoA ICLs in the presence of light and riboflavin provides new insights into the lesions induced by solar radiation. The ubiquitous genotoxic agent solar UV radiation has been shown to generate a wide range of DNA lesions, including cyclobutane pyrimidine dimers (CPDs) [
27], 6–4 photoproducts (6–4 PPs) [
28], oxidized bases [
16,
29,
30], strand breaks [
31,
32], and DNA protein cross-links (DPCs) [
33]. In addition, UVC rays (220–280 nm) have been suggested to induce DNA ICLs in vitro [
34,
35,
36,
37], although their sequence context and chemical structures are unknown. UVA radiation (315–400 nm) is known to directly or indirectly induce various types of DNA lesions (e.g., thymine-thymine CPDs, oxoG, strand breaks), as well as mutations in skin cells [
27,
29,
38,
39]. In the presence of the physiologic dose of UVA/B rays, the FA/BRCA pathway is activated by FANCD2 monoubiquitination [
40], suggesting UVA/B-generated ROS may give rise to ICLs [
41]. The ICL formation by UVA/B rays, however, has not been reported so far. Our observation of the light-induced oxoA ICL formation represents a rare example of ICLs induced by UVA/B and visible light.
Solar rays produce various ROS, which can give rise to oxidative DNA lesions. The formation of UVA- and visible ray-induced oxoA ICLs is likely to involve singlet oxygen, which is known to mainly contribute to the UVA-mediated oxidative damage to cellular DNA via the type II photosensitization mechanism [
42,
43]. In the case of undamaged DNA, singlet oxygen can primarily react with guanine nucleobase to generate oxoG, the main biomarker for oxidative stress [
44]. However, as the redox potential of oxoA is less than that of guanine, oxoA may be preferentially modified by singlet oxygen to produce ICLs. In the absence of a photosensitizer, the ability of UVA and visible rays to produce singlet oxygen is significantly reduced [
43,
45,
46], which is consistent with the lack of the oxoA ICL formation upon radiation with UVA and visible rays. Singlet oxygen is proposed to be the major source of ROS in UVC-induced oxidative damage [
47]. The higher yields of oxoA ICLs in the presence of UVA/riboflavin and visible ray/riboflavin (~25%) compared to UVC/riboflavin (~5%) may be attributed to riboflavin’s absorption maximum wavelengths (λ
max) that are within UVA (356 nm) and visible ray (445 nm).
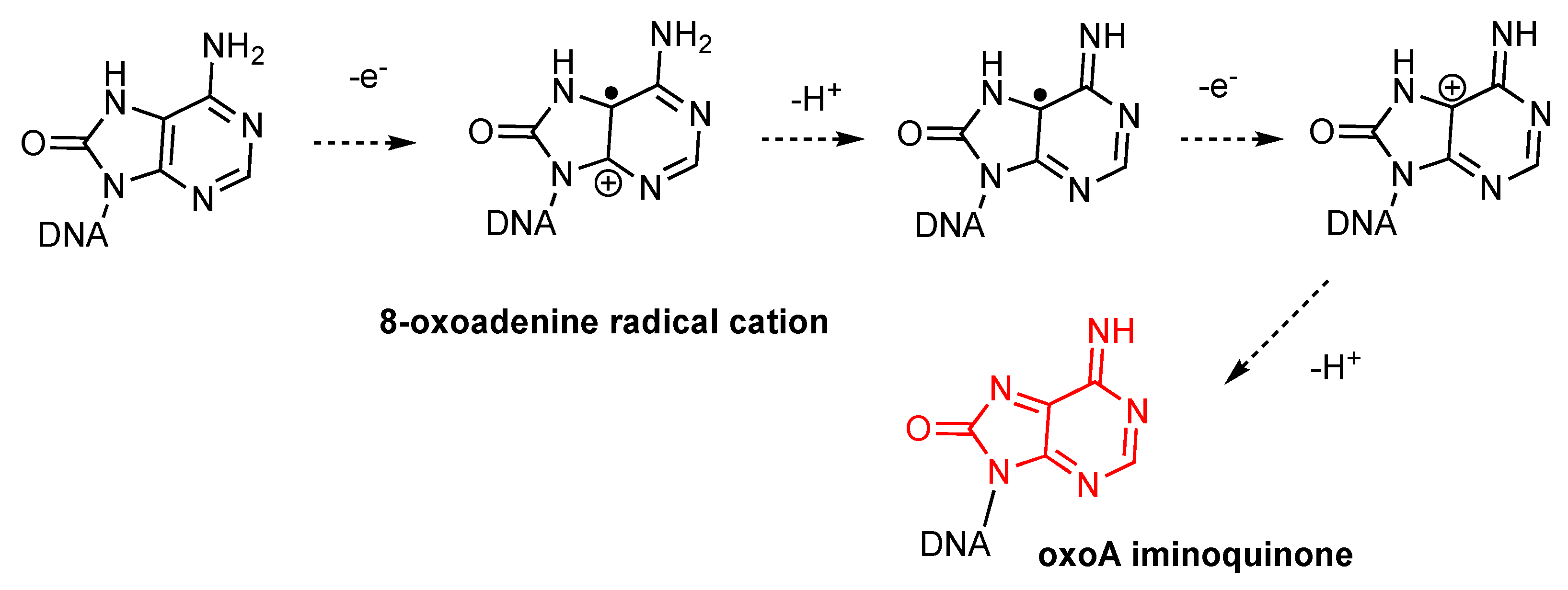
The mechanism of photosensitized oxoA iminoquinone formation in the presence of riboflavin likely involves overall two-electron oxidation and deprotonation (
Figure 6). While singlet oxygenation of deoxyguanosine can produce endoperoxide intermediates [
48,
49], riboflavin-mediated photosensitization induces 8-oxoguanine formation through a mechanism that does not involve reactive oxygen species [
50]. In this process, one electron oxidation of guanine gives rise to a guanine radical cation, which reacts with a water molecule to form 8-oxoguanine. Similarly, the photosensitization of 8-oxoadenine would generate an 8-oxoadenine radical cation, which could undergo further modification to produce the highly reactive oxoA iminoquinone intermediate.
While our study provides novel insights into oxoA ICL formation induced by UV light in the presence of riboflavin, several limitations should be noted. First, the experiments were conducted in vitro, and thus, the physiological relevance of the observed oxoA ICL formation remains uncertain. Second, the study does not examine the biological consequences of these lesions in cellular systems, including their cytotoxicity, mutagenic potential, activation of DNA damage responses, or engagement with repair pathways. Lastly, the lack of comparison with other UV-induced DNA lesions limits our ability to assess the relative significance of oxoA ICLs within the broader context of solar radiation-induced DNA damage. These limitations underscore the need for further investigation to determine the biological relevance and mechanistic details of oxoA ICL formation under physiologically relevant conditions.
5. Conclusions
The results presented here demonstrate that a common product of oxidative damage to genomic DNA, oxoA, has the potential to form DNA interstrand cross-links upon irradiation by solar rays. UVC and UVB can trigger further modification of oxoA lesion into oxoA ICLs, while UVA and visible rays dramatically induce oxoA-mediated ICL formation in the presence of a naturally occurring photosensitizer. The light-induced cross-linking reaction would involve overall two-electron oxidation and deprotonation of oxoA into an iminoquinone electrophile, followed by the nucleophilic attack of opposite guanine on the iminoquinone to form the oxoA(C2)-G(N2) covalent bond. The oxidative conditions studied in this report are more general than those previously investigated, which provides further evidence that these oxoA-induced ICLs may form in a cellular environment and are of broad interest to the DNA damage and repair field. However, the physiological relevance of oxoA ICLs under solar exposure remains speculative. Future research will aim to validate the formation of light-induced oxoA ICLs in vivo and to investigate their potential impact on cellular processes, including recognition, repair, cytotoxicity, mutagenicity, and the activation of DNA damage responses.
Author Contributions
Conceptualization, N.R., A.L.R. and S.L.; methodology, N.R.; software, N.R.; validation, N.R. and S.L.; investigation, N.R., A.L.R. and S.L.; resources, S.L.; data curation, N.R.; writing—original draft preparation, N.R., A.L.R. and S.L.; writing—review and editing, N.R., A.L.R. and S.L.; visualization, N.R.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Cancer Prevention and Research Institute of Texas RP230334.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All supporting data are included within the main article. Original data, including gel images (
Figure 2 and
Figure 3), MALDI-TOF MS (
Figure 4), and ESI-MS (
Figure 5), will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lawley, P.D.; Phillips, D.H. DNA adducts from chemotherapeutic agents. Mutat. Res.-Fundam. Mol. Mech. Mutag. 1996, 355, 13–40. [Google Scholar] [CrossRef]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and Repair of Interstrand Cross-Links in DNA. Chem. Rev. 2006, 106, 277–301. [Google Scholar] [CrossRef]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Guainazzi, A.; Schärer, O.D. Using synthetic DNA interstrand crosslinks to elucidate repair pathways and identify new therapeutic targets for cancer chemotherapy. Cell. Mol. Life Sci. 2010, 67, 3683–3697. [Google Scholar] [CrossRef] [PubMed]
- Räschle, M.; Knipscheer, P.; Enoiu, M.; Angelov, T.; Sun, J.; Griffith, J.D.; Ellenberger, T.E.; Schärer, O.D.; Walter, J.C. Mechanism of Replication-Coupled DNA Interstrand Crosslink Repair. Cell 2009, 137, 972. [Google Scholar] [CrossRef]
- Williams, H.L.; Gottesman, M.E.; Gautier, J. Replication-Independent Repair of DNA Interstrand Crosslinks. Mol. Cell 2012, 47, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, S.; Bellani, M.A.; Thazhathveetil, A.K.; Ling, C.; de Winter, J.P.; Wang, Y.; Wang, W.; Seidman, M.M. The DNA Translocase FANCM/MHF Promotes Replication Traverse of DNA Interstrand Crosslinks. Mol. Cell 2013, 52, 434–446. [Google Scholar] [CrossRef]
- Knipscheer, P.; Räschle, M.; Smogorzewska, A.; Enoiu, M.; Ho The, V.; Schärer Orlando, D.; Elledge Stephen, J.; Walter Johannes, C. The Fanconi Anemia Pathway Promotes Replication-Dependent DNA Interstrand Cross-Link Repair. Science 2009, 326, 1698–1701. [Google Scholar] [CrossRef]
- Hodskinson, M.R.; Bolner, A.; Sato, K.; Kamimae-Lanning, A.N.; Rooijers, K.; Witte, M.; Mahesh, M.; Silhan, J.; Petek, M.; Williams, D.M.; et al. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms. Nature 2020, 579, 603–608. [Google Scholar] [CrossRef]
- Liu, X.; Lao, Y.; Yang, I.Y.; Hecht, S.S.; Moriya, M. Replication-coupled repair of crotonaldehyde/acetaldehyde-induced guanine-guanine interstrand cross-links and their mutagenicity. Biochemistry 2006, 45, 12898–12905. [Google Scholar] [CrossRef]
- Stone, M.P.; Cho, Y.-J.; Huang, H.; Kim, H.-Y.; Kozekov, I.D.; Kozekova, A.; Wang, H.; Minko, I.G.; Lloyd, R.S.; Harris, T.M.; et al. Interstrand DNA Cross-Links Induced by α,β-Unsaturated Aldehydes Derived from Lipid Peroxidation and Environmental Sources. Acc. Chem. Res. 2008, 41, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chowdhury, G.; Gates, K.S. Interstrand Cross-Links Generated by Abasic Sites in Duplex DNA. J. Am. Chem. Soc. 2007, 129, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S.; Ding, H.; Greenberg, M.M. Oxygen independent DNA interstrand cross-link formation by a nucleotide radical. J. Am. Chem. Soc. 2006, 128, 485–491. [Google Scholar] [CrossRef]
- Wilson Matthew, R.; Jiang, Y.; Villalta Peter, W.; Stornetta, A.; Boudreau Paul, D.; Carrá, A.; Brennan Caitlin, A.; Chun, E.; Ngo, L.; Samson Leona, D.; et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 2019, 363, eaar7785. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.-L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res.-Fundam. Mol. Mech. Mutag. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Hall, D.B.; Holmlin, R.E.; Barton, J.K. Oxidative DNA damage through long-range electron transfer. Nature 1996, 382, 731–735. [Google Scholar] [CrossRef]
- Rozelle, A.L.; Cheun, Y.; Vilas, C.K.; Koag, M.-C.; Lee, S. DNA interstrand cross-links induced by the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Nat. Commun. 2021, 12, 1897. [Google Scholar] [CrossRef]
- Wellen, K.E.; Thompson, C.B. Cellular Metabolic Stress: Considering How Cells Respond to Nutrient Excess. Mol. Cell 2010, 40, 323–332. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2013, 20, 1126–1167. [Google Scholar] [CrossRef]
- Ahmad, S.I. (Ed.) Ultraviolet Light in Human Health, Diseases and Environment; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 996, pp. 15–23. [Google Scholar] [CrossRef]
- Huhner, J.; Ingles-Prieto, A.; Neususs, C.; Lammerhofer, M.; Janovjak, H. Quantification of riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in mammalian model cells by CE with LED-induced fluorescence detection. Electrophoresis 2015, 36, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Besaratinia, A.; Kim, S.I.; Bates, S.E.; Pfeifer, G.P. Riboflavin activated by ultraviolet A1 irradiation induces oxidative DNA damage-mediated mutations inhibited by vitamin C. Proc. Natl. Acad. Sci. USA 2007, 104, 5953–5958. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, W.; Kuang, Y.; Sun, H.; Wang, Z.; Peng, X. UV-Induced DNA Interstrand Cross-Linking and Direct Strand Breaks from a New Type of Binitroimidazole Analogue. Chem. Res. Toxicol. 2015, 28, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Veliz Montes, C.; Memczak, H.; Gyssels, E.; Torres, T.; Madder, A.; Schneider, R.J. Photoinduced Cross-Linking of Short Furan-Modified DNA on Surfaces. Langmuir 2017, 33, 1197–1201. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005, 571, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.A.; Doetsch, P.W.; Haseltine, W.A. Structural determination of the ultraviolet light-induced thymine-cytosine pyrimidine-pyrimidone (6–4) photoproduct. Nucleic Acids Res. 1985, 13, 5317–5325. [Google Scholar] [CrossRef]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res 2012, 40, 10263–10273. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Wang, T.C.; Smith, K.C. Postreplication repair in ultraviolet-irradiated human fibroblasts: Formation and repair of DNA double-strand breaks. Carcinogenesis 1986, 7, 389–392. [Google Scholar] [CrossRef]
- Limoli, C.L.; Giedzinski, E.; Bonner, W.M.; Cleaver, J.E. UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, gamma -H2AX formation, and Mre11 relocalization. Proc. Natl. Acad. Sci. USA 2002, 99, 233–238. [Google Scholar] [CrossRef]
- Stutzer, A.; Welp, L.M.; Raabe, M.; Sachsenberg, T.; Kappert, C.; Wulf, A.; Lau, A.M.; David, S.S.; Chernev, A.; Kramer, K.; et al. Analysis of protein-DNA interactions in chromatin by UV induced cross-linking and mass spectrometry. Nat. Commun. 2020, 11, 5250. [Google Scholar] [CrossRef]
- Marmur, J.; Grossman, L. Ultraviolet light induced linking of deoxyribonucleic acid strands and its reversal by photoreactivating enzyme. Proc. Natl. Acad. Sci. USA 1961, 47, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Nejedly, K.; Kittner, R.; Kypr, J. Genomic DNA regions whose complementary strands are prone to UV light-induced crosslinking. Arch. Biochem. Biophys. 2001, 388, 216–224. [Google Scholar] [CrossRef]
- Love, J.D.; Nguyen, H.T.; Or, A.; Attri, A.K.; Minton, K.W. UV-induced interstrand cross-linking of d(GT)n.d(CA)n is facilitated by a structural transition. J. Biol. Chem. 1986, 261, 10051–10057. [Google Scholar] [CrossRef]
- Cuesta-Lopez, S.; Menoni, H.; Angelov, D.; Peyrard, M. Guanine radical chemistry reveals the effect of thermal fluctuations in gene promoter regions. Nucleic Acids Res. 2011, 39, 5276–5283. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Whiteside, J.R.; McMillan, T.J.; Allinson, S.L. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int. J. Radiat. Biol. 2009, 85, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Dunn, J.; Potter, M.; Rees, A.; Runger, T.M. Activation of the Fanconi anemia/BRCA pathway and recombination repair in the cellular response to solar ultraviolet light. Cancer Res. 2006, 66, 11140–11147. [Google Scholar] [CrossRef]
- Lopez-Martinez, D.; Liang, C.C.; Cohn, M.A. Cellular response to DNA interstrand crosslinks: The Fanconi anemia pathway. Cell. Mol. Life Sci. 2016, 73, 3097–3114. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to the guanine moiety of DNA: Mechanistic aspects and formation in cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L.; Di Mascio, P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem. Photobiol. Sci. 2009, 8, 903–911. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J. Investig. Dermatol. 2011, 131, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli-Neto, O.; Ferreira, A.S.; Martins, W.K.; Pavani, C.; Severino, D.; Faiao-Flores, F.; Maria-Engler, S.S.; Aliprandini, E.; Martinez, G.R.; Di Mascio, P.; et al. Melanin photosensitization and the effect of visible light on epithelial cells. PLoS ONE 2014, 9, e113266. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cai, Q.; Rahn, R.; Zhang, X. Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free Radic. Biol. Med. 1997, 23, 148–154. [Google Scholar] [CrossRef]
- Ravanat, J.L.; Di Mascio, P.; Martinez, G.R.; Medeiros, M.H.; Cadet, J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2000, 275, 40601–40604. [Google Scholar] [CrossRef]
- Sheu, C.; Foote, C.S. Endoperoxide formation in a guanosine derivative. J. Am. Chem. Soc. 1993, 115, 10446–10447. [Google Scholar] [CrossRef]
- Kasai, H.; Yamaizumi, Z.; Berger, M.; Cadet, J. Photosensitized formation of 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-hydroxy-2′-deoxyguanosine) in DNA by riboflavin: A nonsinglet oxygen-mediated reaction. J. Am. Chem. Soc. 1992, 114, 9692–9694. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).