Evaluating AlphaFold 3 Folding of the Intrinsically Disordered Human DNA Topoisomerase IIα C-Terminal Domain
Abstract
1. Introduction
2. Materials and Methods
3. Results
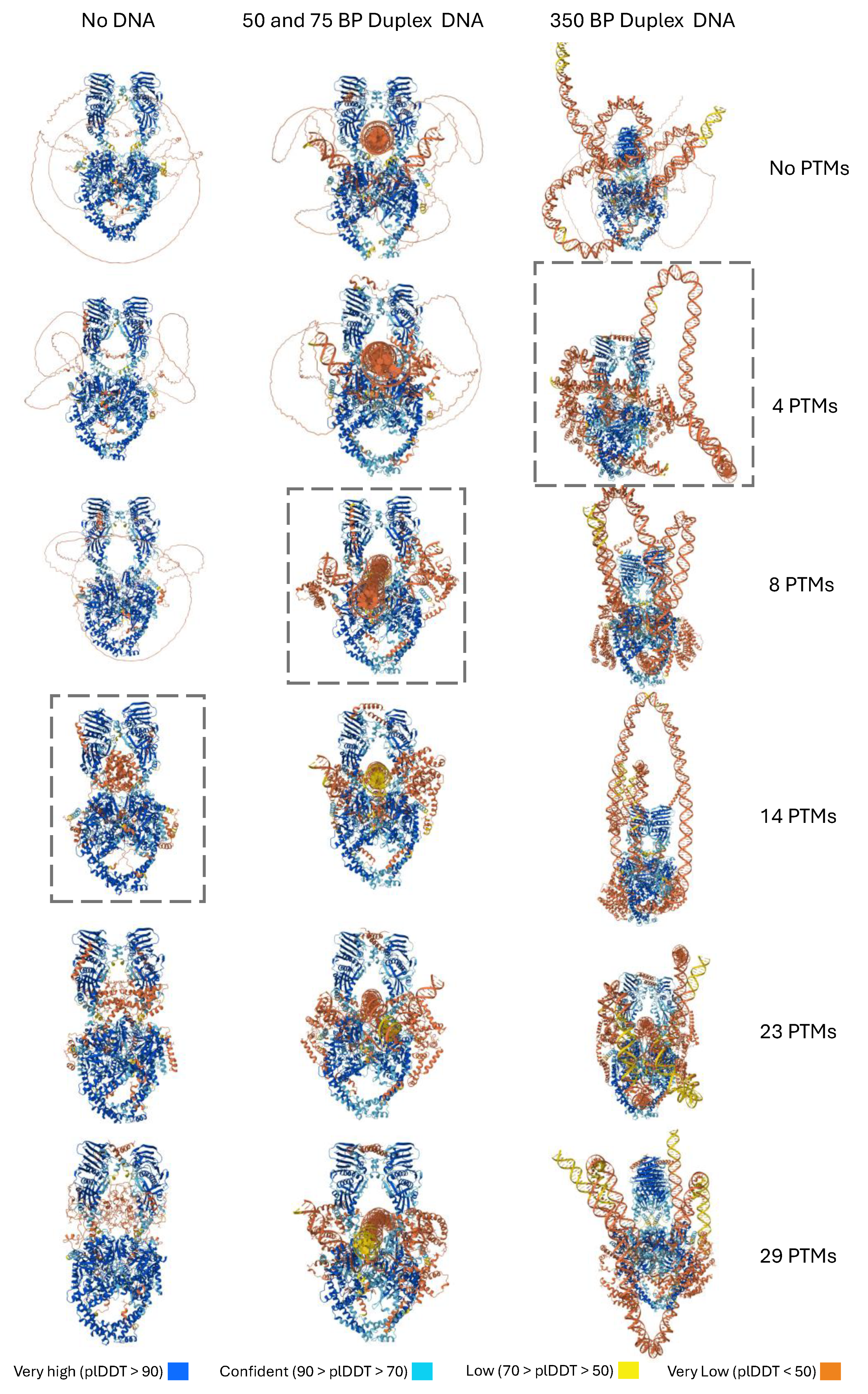
3.1. Post-Translational Modifications Are Sufficient to Induce Predicted Folding
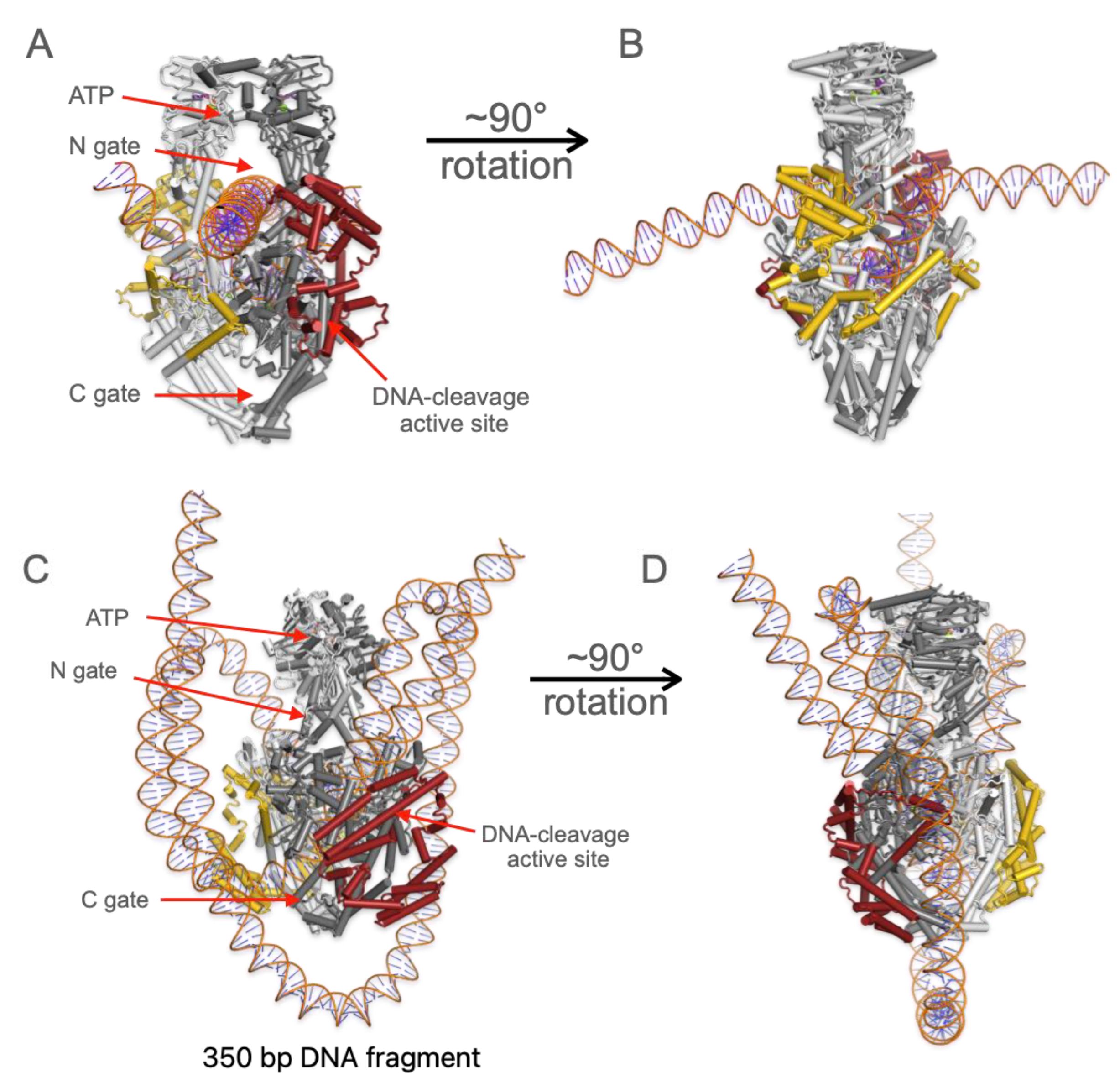
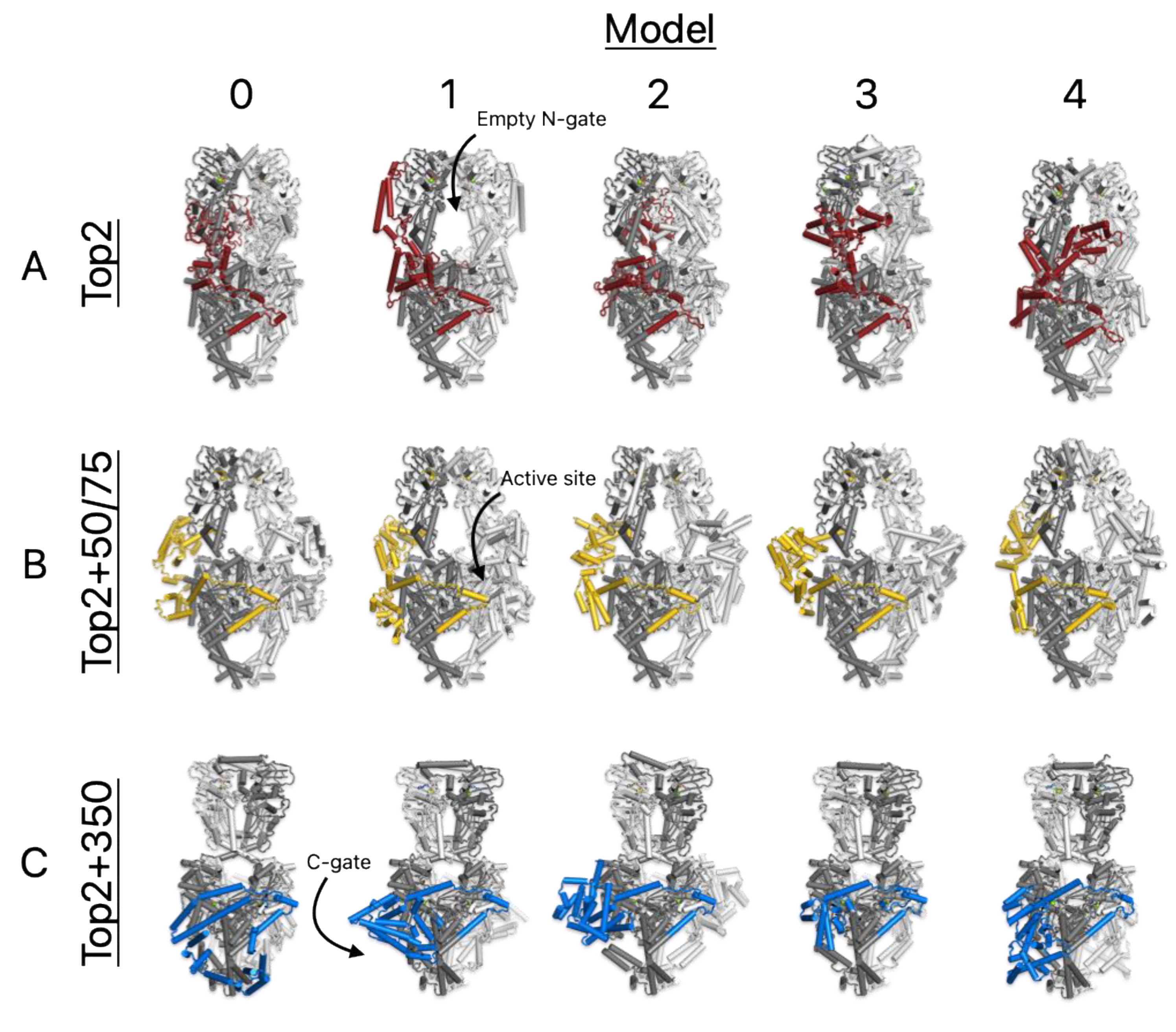
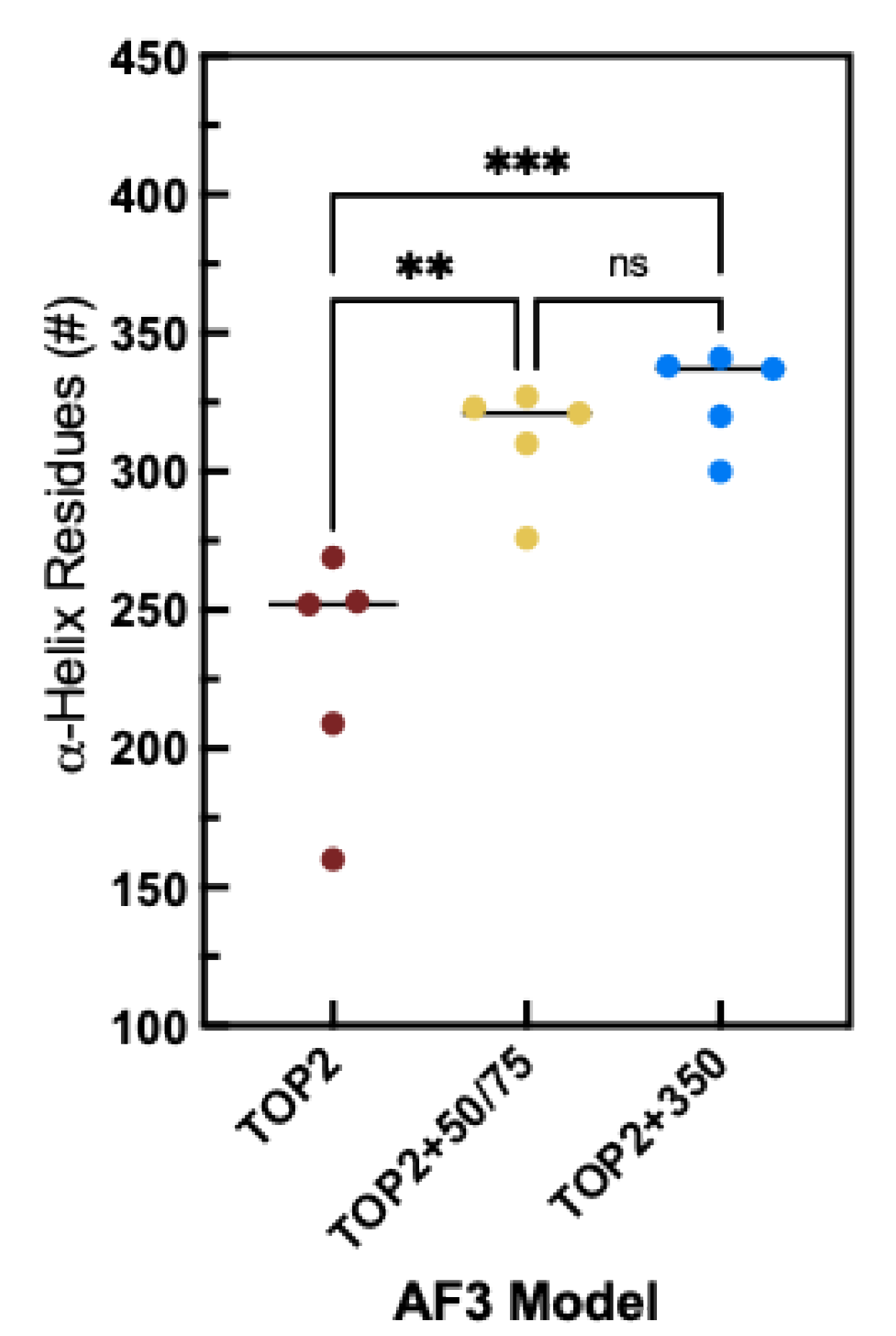
3.2. DNA Substrates Alter Folding and Interact with the CTD
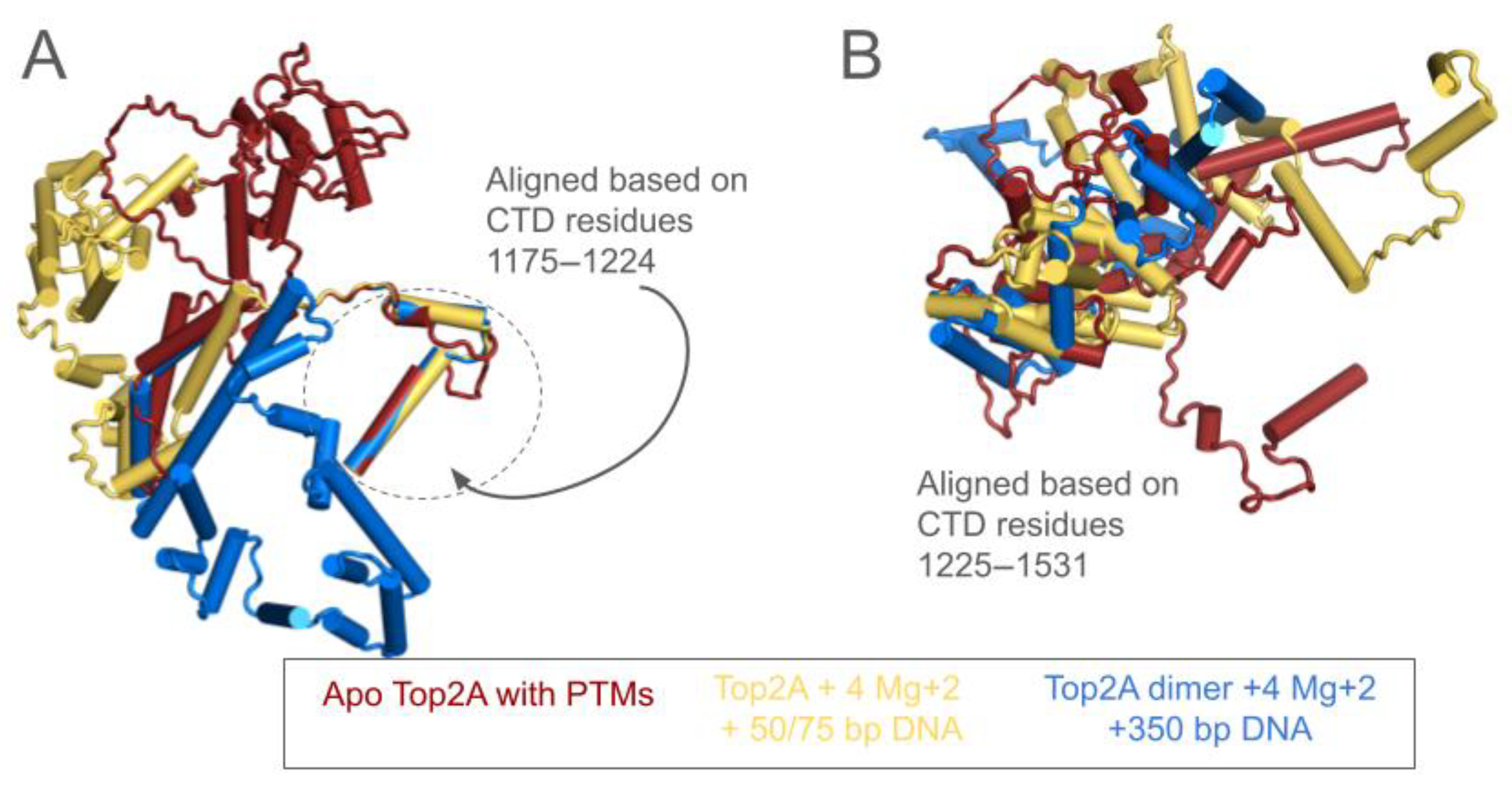
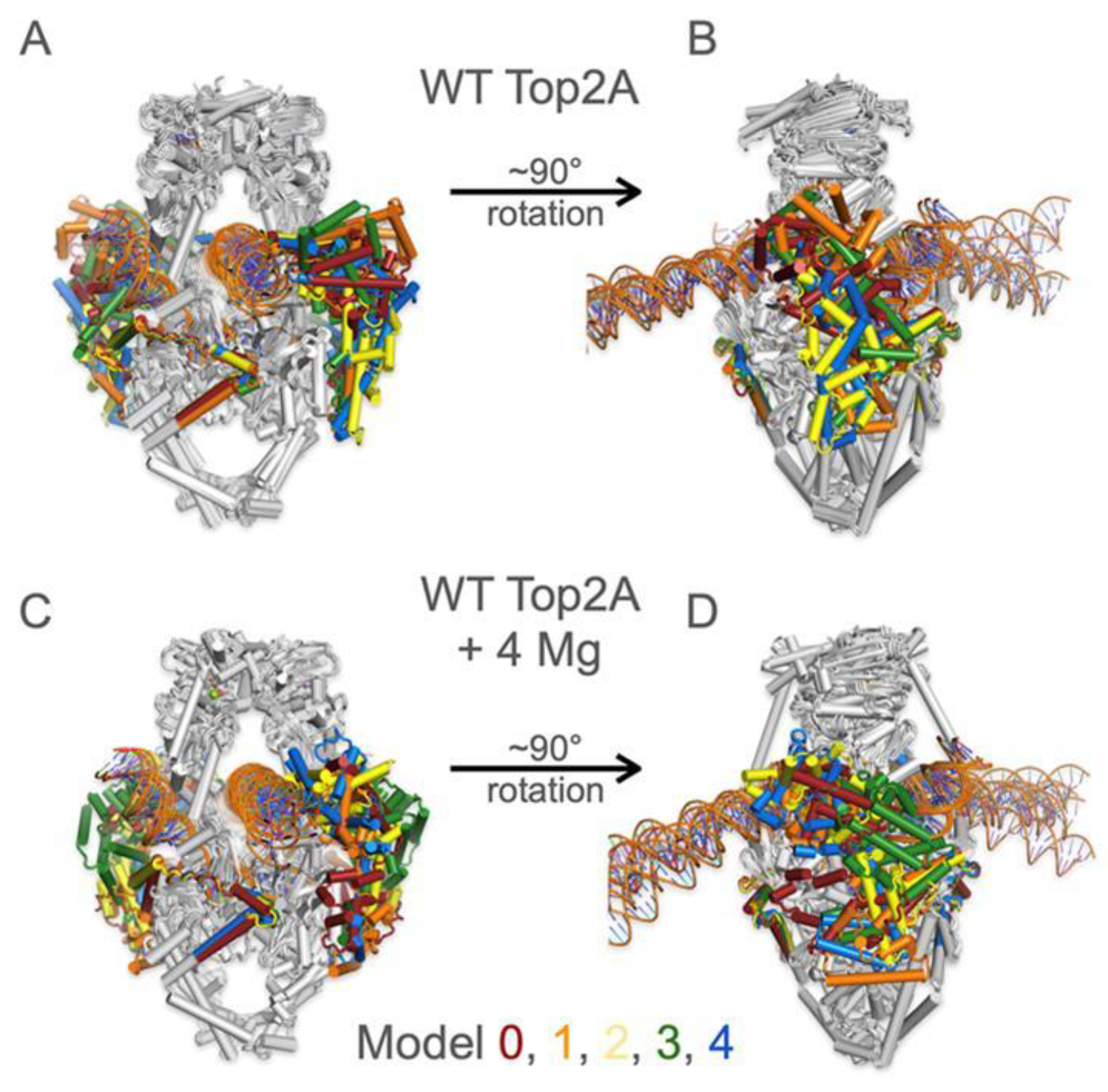
3.3. The CTD Fold Predictions Do Appear to Move Based upon the Substrate
3.4. AlphaFold Model Predictions for CTD in the Presence of Metal Ions
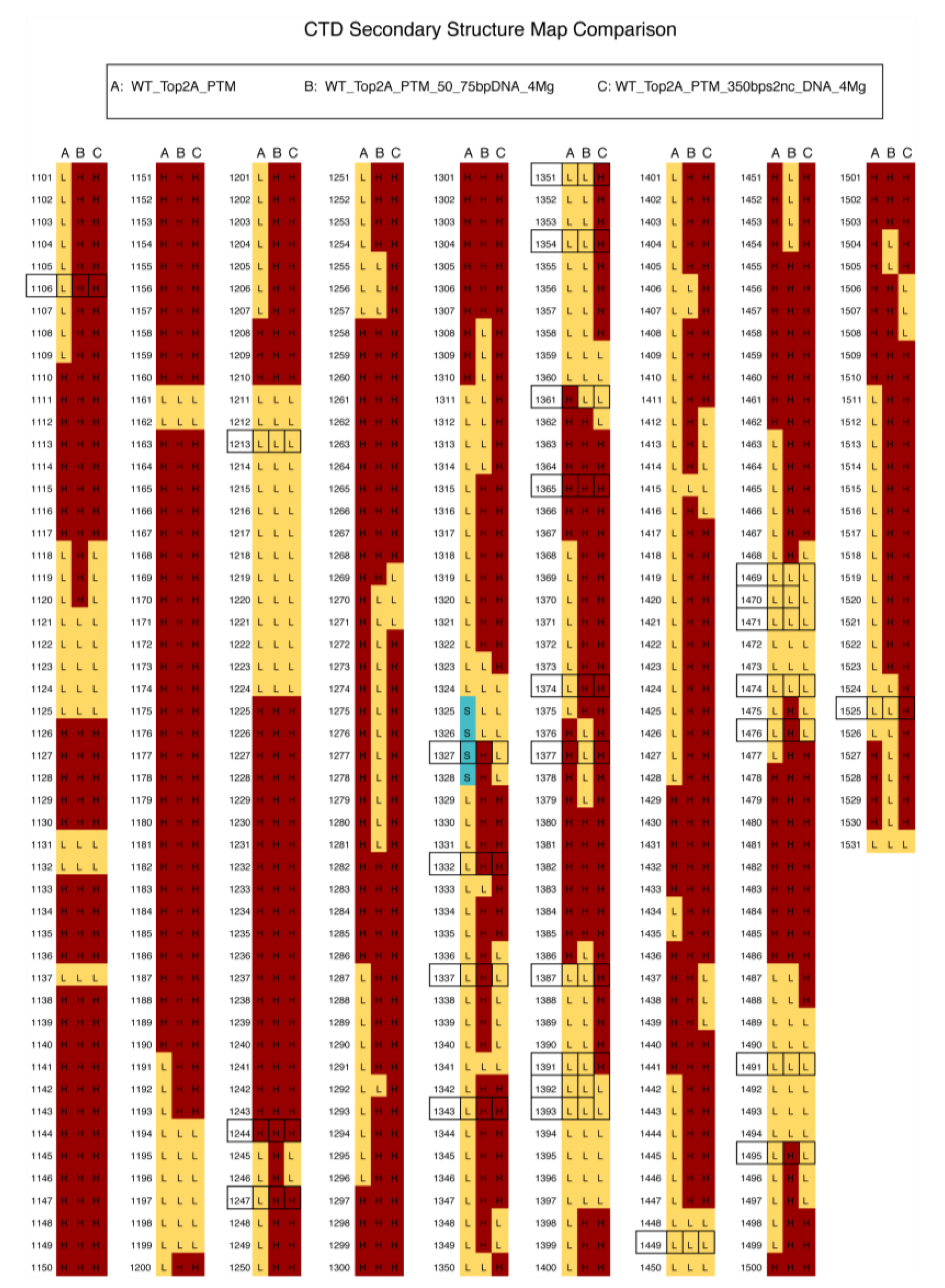
3.5. Folding of the CTD Decreases as PTMs Are Removed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF3 | AlphaFold 3 |
| CTD | C-terminal domain |
| IDR | Intrinsically disordered region |
| NLS | Nuclear Localization Sequence |
| plDDT | Predicted Local Distance Difference Test (also pLDDT) |
| PTM | Post-translational modification |
| TOP2A | Topoisomerase IIα |
| TOP2B | Topoisomerase IIβ |
| WT | Wild type |
Appendix A. DNA Sequences Used in Modeling and Quality Metrics from Structures
| Sequence | |
|---|---|
| 50 base pair forward strand | TTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGAAAAAGAGTTGGT |
| 75 base pair forward strand | TCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAG AATCAGGGGATAACGCAGG |
| 350 base pair forward strand | CGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATC GTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACT GGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAG TTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGGACAGTAT TTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGT TGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGT TTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCT CAAGAAGATCCTTTGATCTTTTCTACGGGGT |
| Structure | ipTM * | pTM ** |
|---|---|---|
| TOP2A only | 0.59 | 0.61 |
| TOP2A with PTM | 0.57 | 0.59 |
| TOP2A with PTM and 50/75 bp duplexes | 0.58 | 0.62 |
| TOP2A with PTM and 350 bp duplex | 0.55 | 0.59 |
| Number of PTMs | PTM Sites |
|---|---|
| 4 | S1469, S1470, S1471, S1474 |
| 8 | S1213, S1247, S1377, S1469, S1470, S1471, S1474, S1476 |
| 14 | S1106, S1213, S1247, S1332, S1337, S1354, S1374, S1377, S1469, S1470, S1471, S1474, S1476, S1525 |
| 23 | S1106, S1213, S1244, S1247, S1332, S1337, S1343, S1351, S1354, S1374, S1377, S1387, S1391, S1392, S1393, S1449, S1469, S1470, S1471, S1474, S1476, S1495, S1525 |
| 29 | S390, S709, S1106, S1213, S1244, S1247, S1332, S1337, S1343, S1351, S1354, S1361, S1365, S1374, S1377, S1387, S1391, S1392, S1393, S1449, S1469, S1470, S1471, S1474, S1476, S1491, S1495, S1525 |
References
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Katuwawala, A.; Oldfield, C.J.; Hu, G.; Wu, Z.; Uversky, V.N.; Kurgan, L. Intrinsic Disorder in Human RNA-Binding Proteins. J. Mol. Biol. 2021, 433, 167229. [Google Scholar] [CrossRef]
- Musselman, C.A.; Kutateladze, T.G. Characterization of functional disordered regions within chromatin-associated proteins. iScience 2021, 24, 102070. [Google Scholar] [CrossRef]
- Biesaga, M.; Frigolé-Vivas, M.; Salvatella, X. Intrinsically disordered proteins and biomolecular condensates as drug targets. Curr. Opin. Chem. Biol. 2021, 62, 90–100. [Google Scholar] [CrossRef]
- Fuxreiter, M. Classifying the Binding Modes of Disordered Proteins. Int. J. Mol. Sci. 2020, 21, 8615. [Google Scholar] [CrossRef]
- Chillemi, G.; Kehrloesser, S.; Bernassola, F.; Desideri, A.; Dotsch, V.; Levine, A.J.; Melino, G. Structural Evolution and Dynamics of the p53 Proteins. Cold Spring Harb. Perspect. Med. 2017, 7, a028308. [Google Scholar] [CrossRef]
- Kriwacki, R.W.; Hengst, L.; Tennant, L.; Reed, S.I.; Wright, P.E. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: Conformational disorder mediates binding diversity. Proc. Natl. Acad. Sci. USA 1996, 93, 11504–11509. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Lotz, C.; Drillien, R.; Haas, L.; Bedez, C.; Lamour, V. Structural basis for allosteric regulation of Human Topoisomerase IIalpha. Nat. Commun. 2021, 12, 2962. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Ecsédi, P.; Érfalvy, D.; Kovács, Z.J.; Katran, V.; Pálinkás, J.; Cervenak, M.; Pancsa, R.; Harami, G.M.; Smeller, L.; Kovács, M. Selective engineering of condensation properties of single-stranded DNA binding (SSB) protein via its intrinsically disordered linker region. Nucleic Acids Res. 2025, 53, gkaf481. [Google Scholar] [CrossRef]
- Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, J.H.; Carcamo, C.C.; Parker, M.W.; Berger, J.M. DNA-Stimulated Liquid-Liquid phase separation by eukaryotic topoisomerase ii modulates catalytic function. eLife 2022, 11, e81786. [Google Scholar] [CrossRef]
- Lotz, C.; Lamour, V. The interplay between DNA topoisomerase 2α post-translational modifications and drug resistance. Cancer Drug Resist. 2020, 3, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.G.; Menzie, R.A.; Rhoades, J.H.; Fief, C.A.; Deweese, J.E. Reviewing the Modification, Interactions, and Regulation of the C-terminal Domain of Topoisomerase IIα as a Prospect for Future Therapeutic Targeting. EC Pharmacol. Toxicol. 2020, 8, 27–43. [Google Scholar]
- Bedez, C.; Lotz, C.; Batisse, C.; Broeck, A.V.; Stote, R.H.; Howard, E.; Pradeau-Aubreton, K.; Ruff, M.; Lamour, V. Post-translational modifications in DNA topoisomerase 2alpha highlight the role of a eukaryote-specific residue in the ATPase domain. Sci. Rep. 2018, 8, 9272. [Google Scholar] [CrossRef]
- Kozuki, T.; Chikamori, K.; Surleac, M.D.; Micluta, M.A.; Petrescu, A.J.; Norris, E.J.; Elson, P.; Hoeltge, G.A.; Grabowski, D.R.; Porter, A.C.G.; et al. Roles of the C-terminal domains of topoisomerase IIalpha and topoisomerase IIbeta in regulation of the decatenation checkpoint. Nucleic Acids Res. 2017, 45, 5995–6010. [Google Scholar] [CrossRef]
- Clarke, D.J.; Azuma, Y. Non-Catalytic Roles of the Topoisomerase IIalpha C-Terminal Domain. Int. J. Mol. Sci. 2017, 18, 2438. [Google Scholar] [CrossRef]
- Lane, A.B.; Gimenez-Abian, J.F.; Clarke, D.J. A novel chromatin tether domain controls topoisomerase IIalpha dynamics and mitotic chromosome formation. J. Cell Biol. 2013, 203, 471–486. [Google Scholar] [CrossRef]
- Gilroy, K.L.; Austin, C.A. The impact of the C-terminal domain on the interaction of human DNA topoisomerase II alpha and beta with DNA. PLoS ONE 2011, 6, e14693. [Google Scholar] [CrossRef]
- Luo, K.; Yuan, J.; Chen, J.; Lou, Z. Topoisomerase IIalpha controls the decatenation checkpoint. Nat. Cell Biol. 2009, 11, 204–210. [Google Scholar] [CrossRef]
- Meczes, E.L.; Gilroy, K.L.; West, K.L.; Austin, C.A. The impact of the human DNA topoisomerase II C-terminal domain on activity. PLoS ONE 2008, 3, e1754. [Google Scholar] [CrossRef]
- McClendon, A.K.; Gentry, A.C.; Dickey, J.S.; Brinch, M.; Bendsen, S.; Andersen, A.H.; Osheroff, N. Bimodal recognition of DNA geometry by human topoisomerase II alpha: Preferential relaxation of positively supercoiled DNA requires elements in the C-terminal domain. Biochemistry 2008, 47, 13169–13178. [Google Scholar] [CrossRef]
- Linka, R.M.; Porter, A.C.; Volkov, A.; Mielke, C.; Boege, F.; Christensen, M.O. C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 2007, 35, 3810–3822. [Google Scholar] [CrossRef]
- McClendon, A.K.; Osheroff, N. The geometry of DNA supercoils modulates topoisomerase-mediated DNA cleavage and enzyme response to anticancer drugs. Biochemistry 2006, 45, 3040–3050. [Google Scholar] [CrossRef]
- McClendon, A.K.; Dickey, J.S.; Osheroff, N. Ability of viral topoisomerase II to discern the handedness of supercoiled DNA: Bimodal recognition of DNA geometry by type II enzymes. Biochemistry 2006, 45, 11674–11680. [Google Scholar] [CrossRef][Green Version]
- Dickey, J.S.; Osheroff, N. Impact of the C-terminal domain of topoisomerase IIα on the DNA cleavage activity of the human enzyme. Biochemistry 2005, 44, 11546–11554. [Google Scholar] [CrossRef] [PubMed]
- Shaiu, W.L.; Hu, T.; Hsieh, T.S. The hydrophilic, protease-sensitive terminal domains of eucaryotic DNA topoisomerases have essential intracellular functions. Pac. Symp. Biocomput. 1999, 4, 578–589. [Google Scholar] [CrossRef]
- Mirski, S.E.; Gerlach, J.H.; Cole, S.P. Sequence determinants of nuclear localization in the alpha and beta isoforms of human topoisomerase II. Exp. Cell Res. 1999, 251, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Mirski, S.E.; Gerlach, J.H.; Cummings, H.J.; Zirngibl, R.; Greer, P.A.; Cole, S.P. Bipartite nuclear localization signals in the C terminus of human topoisomerase IIa. Exp. Cell Res. 1997, 237, 452–455. [Google Scholar] [CrossRef]
- Adachi, N.; Miyaike, M.; Kato, S.; Kanamaru, R.; Koyama, H.; Kikuchi, A. Cellular distribution of mammalian DNA topoisomerase II is determined by its catalytically dispensible C-terminal domain. Nucleic Acids Res. 1997, 25, 3135–3142. [Google Scholar] [CrossRef]
- Endsley, C.E.; Moore, K.A.; Townsley, T.D.; Durston, K.K.; Deweese, J.E. Bioinformatic Analysis of Topoisomerase IIalpha Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues. Int. J. Mol. Sci. 2024, 25, 5674. [Google Scholar] [CrossRef] [PubMed]
- Musselman, J.R.; England, D.C.; Fielding, L.A.; Durham, C.T.; Baxter, E.; Jiang, X.; Lisic, E.C.; Deweese, J.E. Topoisomerase IIα C-terminal Domain Mutations and Catalytic Function. bioRxiv 2023. [Google Scholar] [CrossRef]
- Townsley, T.D.; Wilson, J.T.; Akers, H.; Bryant, T.; Cordova, S.; Wallace, T.L.; Durston, K.K.; Deweese, J.E. PSICalc: A novel approach to identifying and ranking critical non-proximal interdependencies within the overall protein structure. Bioinform. Adv. 2022, 2, vbac058. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A.C.; Hawaz, M.G.; Hoang, K.G.; Trac, J.; Keck, J.M.; Ayes, C.; Deweese, J.E. Exploration of the Role of the C-Terminal Domain of Human DNA Topoisomerase IIalpha in Catalytic Activity. ACS Omega 2021, 6, 25892–25903. [Google Scholar] [CrossRef]
- Chang, J.W.; O’Brian, A.K.; Thomas, A.J.; Hardin, M.R.; Latham, B.D.; Ngabonziza, D.; Simpson, L.G.; Wade, B.D.; Kuhnhenrich, L.; Thompson, N.M.; et al. Mutagenesis of Intrinsically Disordered Domain Impacts Topoisomerase IIalpha Catalytic Activity. Int. J. Mol. Sci. 2025, 26, 3604. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Murphy, M.B.; Mercer, S.L.; Deweese, J.E. Inhibitors and Poisons of Mammalian Type II Topoisomerases. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 11, pp. 203–240. [Google Scholar]
- Deweese, J.E.; Osheroff, N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2009, 37, 738–749. [Google Scholar] [CrossRef]
- Pendleton, M.; Lindsey, R.H., Jr.; Felix, C.A.; Grimwade, D.; Osheroff, N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014, 1310, 98–110. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Cowell, I.G.; Casement, J.W.; Austin, C.A. To Break or Not to Break: The Role of TOP2B in Transcription. Int. J. Mol. Sci. 2023, 24, 14806. [Google Scholar] [CrossRef]
- Wu, M.; Beck, C.; Lee, J.H.; Fulbright, R.M.; Jeong, J.; Inman, J.T.; Woodhouse, M.V.; Berger, J.M.; Wang, M.D. Human Topoisomerase IIα Promotes Chromatin Condensation Via a Phase Transition. bioRxiv 2024. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2024, 53, D609–D617. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 16 December 2024).
- Deweese, J.E.; Burgin, A.B.; Osheroff, N. Using 3′-bridging phosphorothiolates to isolate the forward DNA cleavage reaction of human topoisomerase IIα. Biochemistry 2008, 47, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Mamun, Y.; Aguado, A.; Preza, A.; Kadel, A.; Mogallur, A.; Gonzalez, B.; De La Rosa, J.; Diaz, D.; Evdokimova, P.; Karki, U.; et al. Substrate binding of human and bacterial type IA topoisomerase: An experimentation with AlphaFold 3.0. Comput. Struct. Biotechnol. J. 2025, 27, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M., Jr.; Sun, X.; Anusha; Batchelder-Schwab, E.J.; Li, J.; Siraj, N.; Jampana, R.; Zhang, Y.; Bai, Y.; Mao, C. AlphaFold 3 modeling of DNA nanomotifs: Is it reliable? Nanoscale Horiz. 2025, 10, 1428–1435. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, S.C.; Chen, K.L.; Huang, Y.C.; Liao, C.Y.; Lin, G.J.; Lee, H.; Chen, P.Y. Analysing protein complexes in plant science: Insights and limitation with AlphaFold 3. Bot. Stud. 2025, 66, 14. [Google Scholar] [CrossRef]
- Wendorff, T.J.; Schmidt, B.H.; Heslop, P.; Austin, C.A.; Berger, J.M. The structure of DNA-bound human topoisomerase IIα: Conformational mechanisms for coordinating inter-subunit interactions with DNA cleavage. J. Mol. Biol. 2012, 424, 109–124. [Google Scholar] [CrossRef]
- Schmidt, B.H.; Osheroff, N.; Berger, J.M. Structure of a topoisomerase II-DNA-nucleotide complex reveals a new control mechanism for ATPase activity. Nat. Struct. Mol. Biol. 2012, 19, 1147–1154. [Google Scholar] [CrossRef]
- Schmidt, B.H.; Burgin, A.B.; Deweese, J.E.; Osheroff, N.; Berger, J.M. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature 2010, 465, 641–644. [Google Scholar] [CrossRef]
- Ruff, K.M.; Pappu, R.V. AlphaFold and Implications for Intrinsically Disordered Proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef] [PubMed]
- Pajkos, M.; Clerc, I.; Zanon, C.; Bernadó, P.; Cortés, J. AFflecto: A web server to generate conformational ensembles of flexible proteins from AlphaFold models. J. Mol. Biol. 2025, 437, 169003. [Google Scholar] [CrossRef] [PubMed]
- Özmen, Z.A.; Çaylı, F.N.; Uversky, V.N.; Woo, J.A.; Kang, D.E.; Coskuner-Weber, O. Effects of pathological mutations on the CHCHD2 monomer structure: A study by AlphaFold3 linked to the generation of conformational ensembles. Comput. Biol. Med. 2025, 196, 110810. [Google Scholar] [CrossRef] [PubMed]
- Majila, K.; Ullanat, V.; Viswanath, S. A deep learning method for predicting interactions for intrinsically disordered regions of proteins. bioRxiv 2025. [Google Scholar] [CrossRef]
- Oughtred, R.; Stark, C.; Breitkreutz, B.J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef]
- Cho, W.K.; Spille, J.H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartey, C.M.; Deweese, J.E. Evaluating AlphaFold 3 Folding of the Intrinsically Disordered Human DNA Topoisomerase IIα C-Terminal Domain. DNA 2025, 5, 46. https://doi.org/10.3390/dna5040046
Nartey CM, Deweese JE. Evaluating AlphaFold 3 Folding of the Intrinsically Disordered Human DNA Topoisomerase IIα C-Terminal Domain. DNA. 2025; 5(4):46. https://doi.org/10.3390/dna5040046
Chicago/Turabian StyleNartey, Charisse M., and Joseph E. Deweese. 2025. "Evaluating AlphaFold 3 Folding of the Intrinsically Disordered Human DNA Topoisomerase IIα C-Terminal Domain" DNA 5, no. 4: 46. https://doi.org/10.3390/dna5040046
APA StyleNartey, C. M., & Deweese, J. E. (2025). Evaluating AlphaFold 3 Folding of the Intrinsically Disordered Human DNA Topoisomerase IIα C-Terminal Domain. DNA, 5(4), 46. https://doi.org/10.3390/dna5040046







