Overview of Roles of Novel Components in the Regulation of DNA Damage Repair in BRCA1-Deficient Cancers: An Update
Abstract
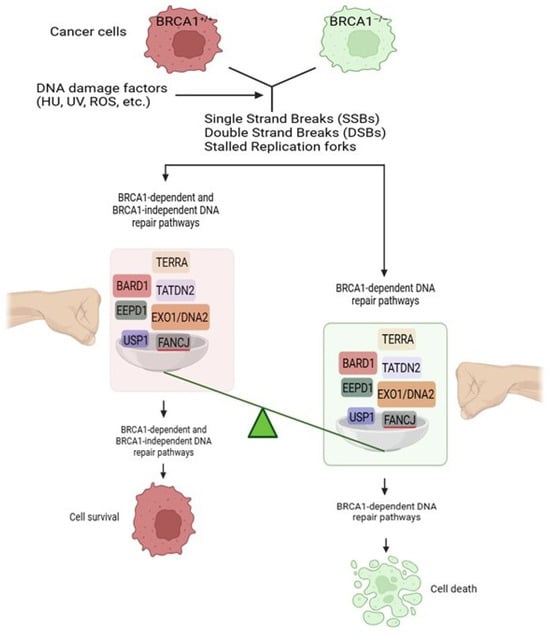
:1. Introduction
2. Novel DNA Repair Components Required for Survival of BRCA1-Deficient, but Not BRCA1-Proficient, Cancers
2.1. Role of TATDN2 in BRCA1-Deficient Cancers
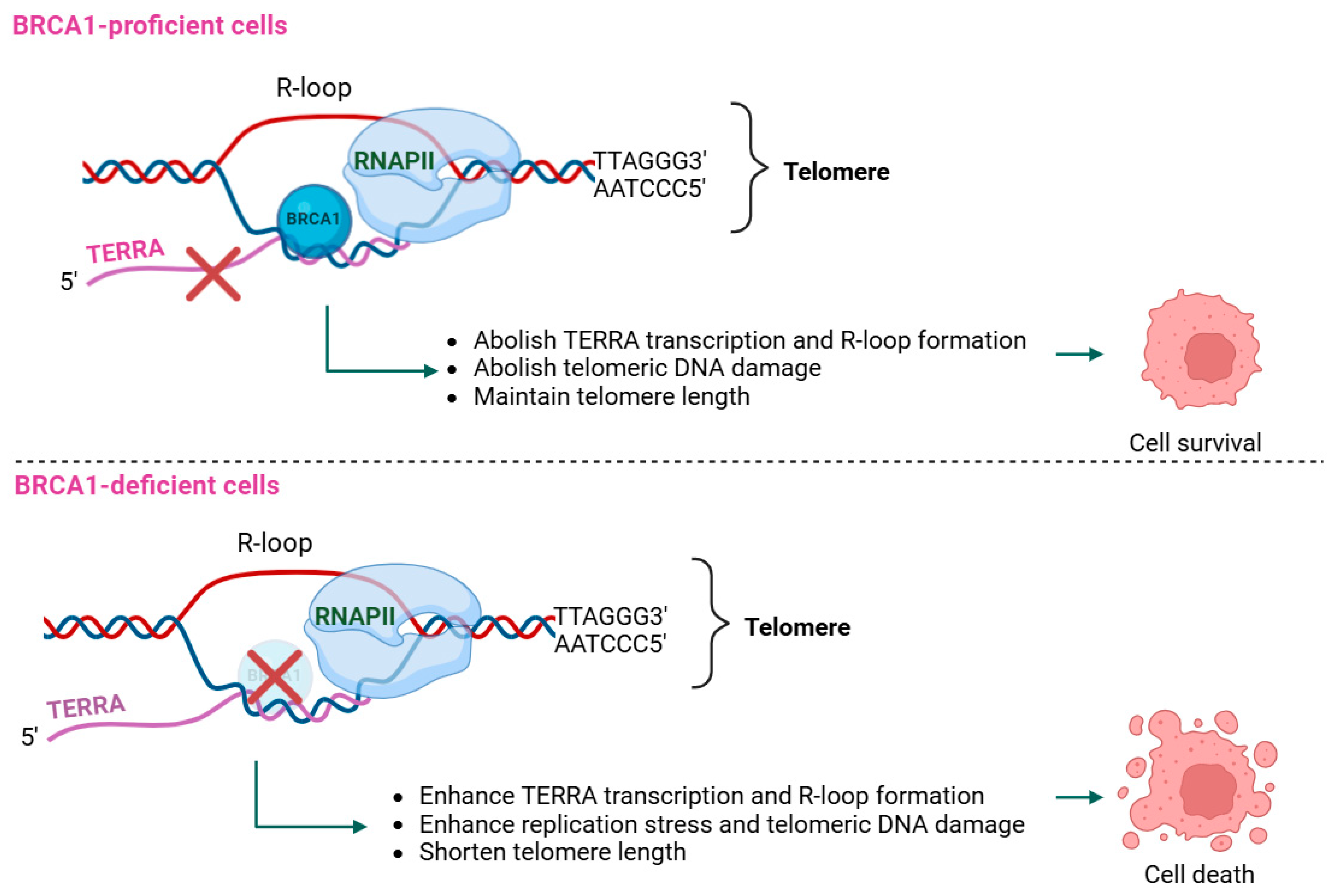
2.2. Role of TERRA in BRCA1-Deficient Cancers
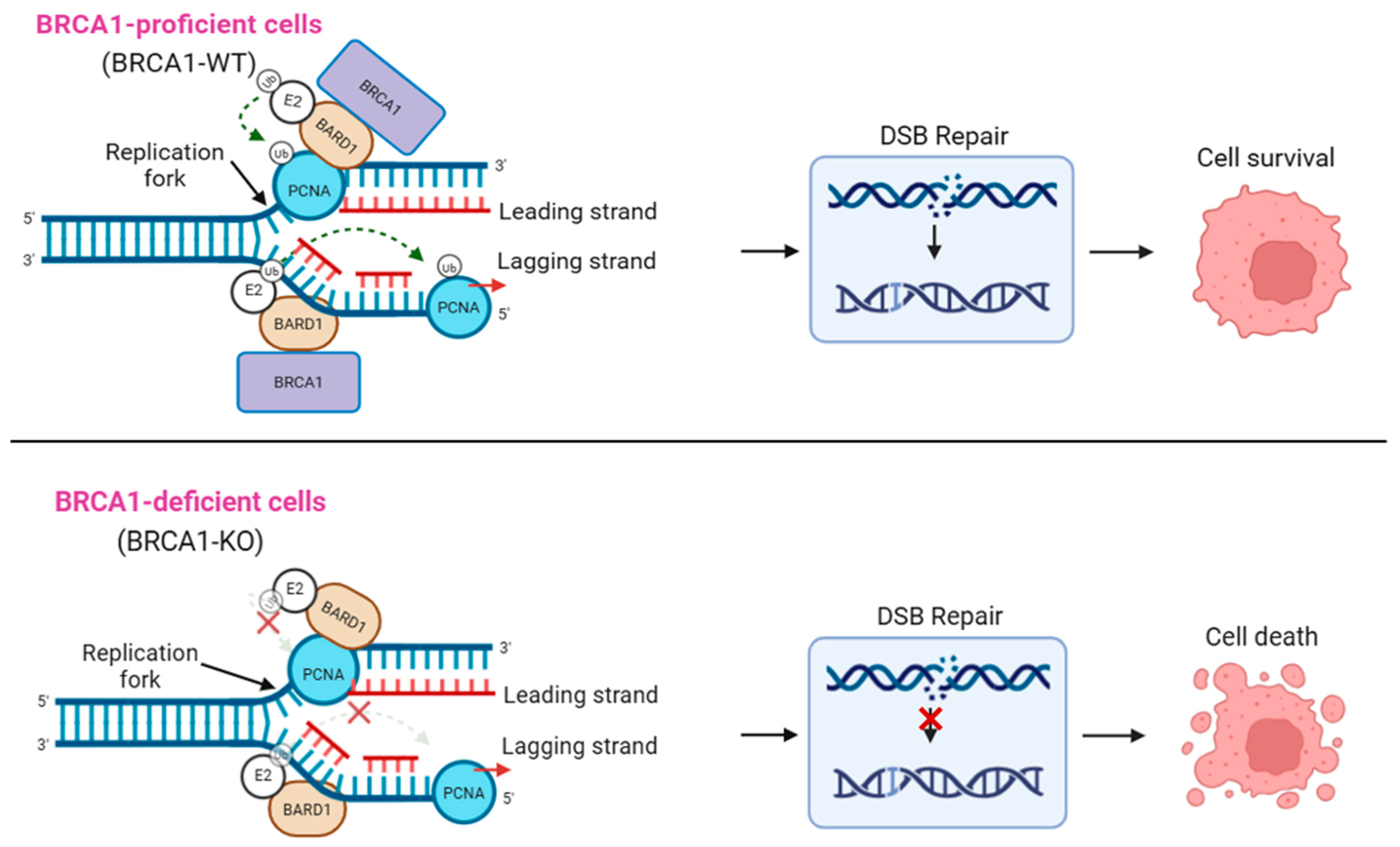
2.3. Role of BARD1 in BRCA1-Deficient Cancers
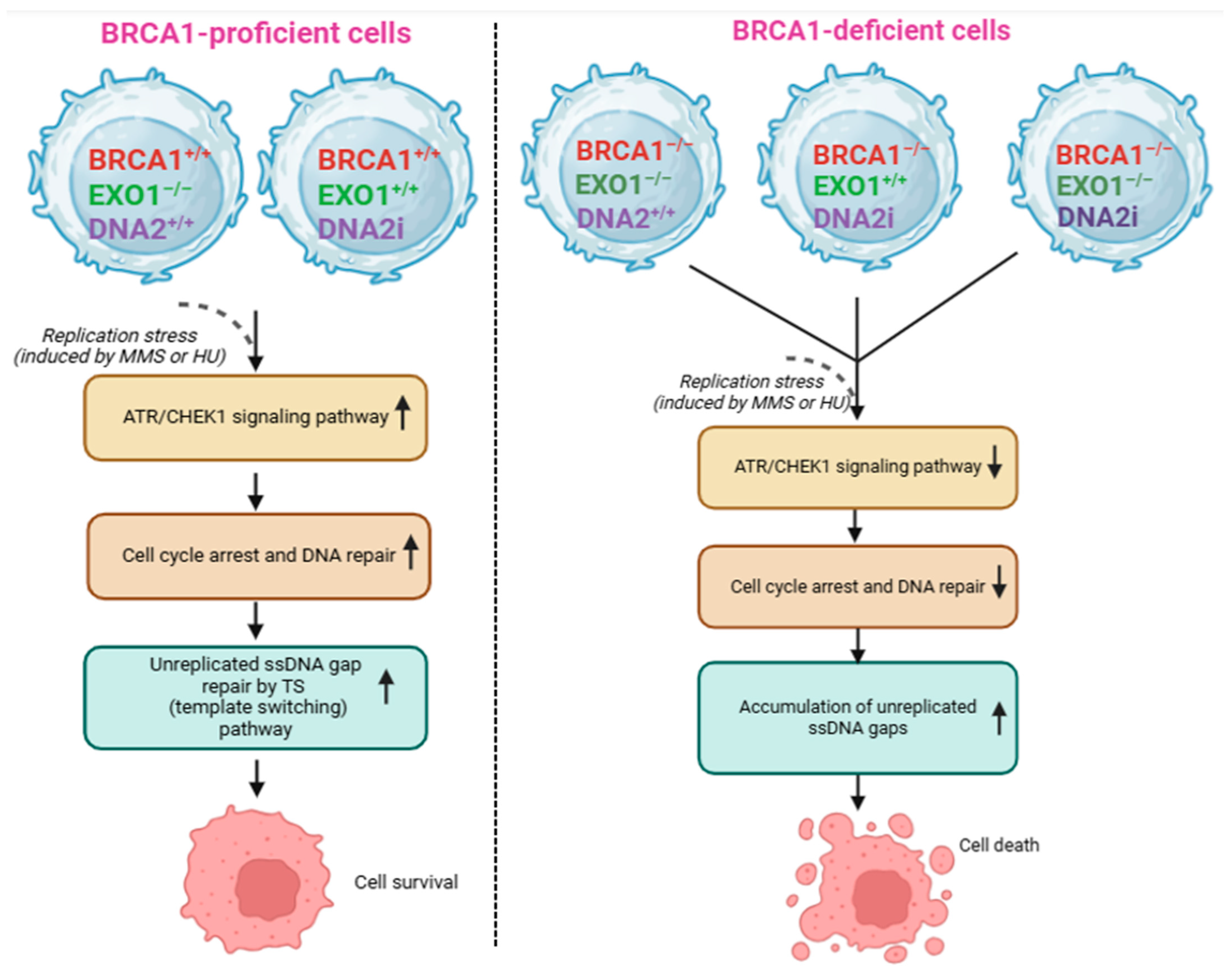
2.4. Role of EXO1 in BRCA1-Deficient Cancers
3. Other Potential Components
3.1. Role of EEPD1 in BRCA1-Deficient Cancers
3.2. Role of FANCJ in BRCA1-Deficient Cancers
3.3. Role of USP1 in BRCA1-Deficient Cancers
4. Discussion
| Components | DNA repair mechanisms in BRCA1-deficient cancer cells | Types of cancer with BRCA1 deficiency |
| TATDN2 | Bound to R-loops and degraded the RNA strand but not the DNA of multiple forms of R-loops in vitro in Mg2+-dependent manner. Silencing of TATDN2 triggers poor replication fork progression in the presence of increased cellular R-loops, thereby leading to DNA damage and chromosomal abnormalities [55]. | (1) Breast cancer cells (MDA-MB-436 and HCC1937) (2) Ovarian cancer cells (UWB1.289) |
| TERRA | Upregulated, leading to accumulated R-loops at telomeres, which causes replication stress, telomeric DNA damage, and detrimental effects on telomere stability in response to BRCA1 deficiency [70]. | Not identified. |
| EXO1 | Processing dsDNA ends by trimming DNA in a 5′-3′ direction, playing key roles in SSA and alt-NHEJ pathways in RAD52-dependent manner. Knockdown of EXO1 significantly impairs cancer cell survival [86]. | (1) Breast cancer cell lines: MDA-MB-231; MDA-MB-436. (2) Colorectal adenocarcinoma cell line: DLD-1. |
| EEPD1 | Required for death of BRCA1-deficient breast cancer cells depleted of RAD52. Co-depletion of EEPD1 and RAD52 accelerates restart of stalled replication forks, and abolishes chromosome aberrations and mitotic catastrophe, showing that BRCA1-deficient breast cancer cells depleted of EEPD1 switch to be dependent upon alt-NHEJ pathway for survival [107]. | Breast cancer cell lines: MDA-MB-436; SUM149PT and MCF7. |
| FANCJ | Serving as a vital driver of PARP1-induced ssDNA gaps and sensitivity during DNA replication in BRCA1-deficient breast and ovarian cancer cells. Depletion of FANCJ leads to deactivate PARP1 through modifying the replisome composition and/or DNA secondary structures, thereby contributing synthetic lethality in BRCA1-deficient cells [112]. | Not identified |
| USP1 | Overexpressed in BRCA1-deficient cancer cells, and regulating PCNA deubiquitylation. Depletion of USP remarkedly reduces cancer cell survival [113]. | Ovarian cancer cell line: UWB1.289 |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRCA1 | Breast cancer type 1 susceptibility protein |
| BRCA2 | Breast cancer type 2 susceptibility protein |
| HR | Homologous recombination |
| NHEJ | Non-homologous end joining |
| cNHEJ | Classical non-homologous end joining |
| alt-NHEJ | Alternative non-homologous end joining |
| SSA | Single-strand annealing |
| BER | Base excision repair |
| TNBCs | Triple-negative breast cancers |
| DDR | DNA damage repair |
| DSBs | DNA double-strand breaks |
| ssDNA | Single-stranded DNA |
| ERs | Estrogen receptors |
| PRs | Progesterone receptors |
| HERs | Epidermal growth factor receptor 2 |
| PARP1 | Poly ADP-ribose polymerase 1 |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit complex |
| TATDN2 | Twin-arginine translocation (TatD) DNase domain containing 2 |
| EEPD1 | Exonuclease/Endonuclease/Phosphatase domain-1 |
| TERRA | Telomeric Repeat-containing RNA |
| FANCJ | Fanconi anemia complementation group J. |
| ATR | Ataxia telangiectasia and Rad3-related. |
| IRE1 | RNA-degrading inositol-requiring enzyme type 1 |
| BARD1 | BRCA1-associated RING domain protein 1 |
| PCNA | Proliferating cell nuclear antigen |
| CtIP | CtBP (C-terminal binding protein) interacting protein |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| APE1 | AP-endonuclease 1 |
| DNA interstrand crosslinks | ICL |
| PROTACS | Proteolysis-targeting chimeras |
References
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef]
- Yu, W.; Lescale, C.; Babin, L.; Bedora-Faure, M.; Lenden-Hasse, H.; Baron, L.; Demangel, C.; Yelamos, J.; Brunet, E.; Deriano, L. Repair of G1 induced DNA double-strand breaks in S-G2/M by alternative NHEJ. Nat. Commun. 2020, 11, 5239. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, N.R.; Watanabe, G.; Lieber, M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10512–10523. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Majumdar, R.; Moore, G.M.; Narang, H.; Buechelmaier, E.S.; Bazil, M.J.; Ravindran, P.T.; Leeman, J.E.; Li, Y.; Jalan, M.; et al. Measuring nonhomologous end-joining, homologous recombination and alternative end-joining simultaneously at an endogenous locus in any transfectable human cell. Nucleic Acids Res. 2021, 49, e74. [Google Scholar] [CrossRef]
- Hanscom, T.; McVey, M. Regulation of Error-Prone DNA Double-Strand Break Repair and Its Impact on Genome Evolution. Cells 2020, 9, 1657. [Google Scholar] [CrossRef] [PubMed]
- Dueva, R.; Iliakis, G. Alternative pathways of non-homologous end joining (NHEJ) in genomic instability and cancer. Transl. Cancer Res. 2013, 2, 163–177. [Google Scholar]
- Bennardo, N.; Cheng, A.; Huang, N.; Stark, J.M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008, 4, e1000110. [Google Scholar] [CrossRef]
- Sfeir, A.; Symington, L.S. Microhomology-Mediated End Joining: A Back-up Survival Mechanism or Dedicated Pathway? Trends Biochem. Sci. 2015, 40, 701–714. [Google Scholar] [CrossRef]
- Paul, A.; Paul, S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front. Biosci. (Landmark Ed.) 2014, 19, 605–618. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hirasawa, A. Homologous Recombination Deficiencies and Hereditary Tumors. Int. J. Mol. Sci. 2021, 23, 348. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Ao, D. Targeting DNA repair pathway in cancer: Mechanisms and clinical application. MedComm 2021, 2, 654–691. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Jaiswal, A.S.; Sharma, N.; Williamson, E.A.; Tran, M.T.; Arris, D.; Yang, M.; Hromas, R. Cellular Responses to Widespread DNA Replication Stress. Int. J. Mol. Sci. 2023, 24, 16903. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.; McVey, M. Error-Prone Repair of DNA Double-Strand Breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Torgovnick, A.; Schumacher, B. DNA repair mechanisms in cancer development and therapy. Front. Genet. 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Sishc, B.J.; Davis, A.J. The Role of the Core Non-Homologous End Joining Factors in Carcinogenesis and Cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Knudsen, K.E. DNA Damage Response in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2019, 9, a030486. [Google Scholar] [CrossRef]
- Deng, C.X. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006, 34, 1416–1426. [Google Scholar] [CrossRef]
- Wu, W.; Koike, A.; Takeshita, T.; Ohta, T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div. 2008, 3, 1. [Google Scholar] [CrossRef]
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.; Boehning, D. Structure-Function Of The Tumor Suppressor BRCA1. Comput. Struct. Biotechnol. J. 2012, 1, e201204005. [Google Scholar] [CrossRef]
- Leung, C.C.; Glover, J.N. BRCT domains: Easy as one, two, three. Cell Cycle 2011, 10, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Tarsounas, M.; Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.M.; Fan, S.; Ma, Y. BRCA1 regulation of transcription. Cancer Lett. 2006, 236, 175–185. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Pan, H.; He, Z.; Ling, L.; Ding, Q.; Chen, L.; Zha, X.; Zhou, W.; Liu, X.; Wang, S. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: Results from ten studies. Cancer Epidemiol. 2014, 38, 1–8. [Google Scholar] [CrossRef]
- Shao, F.; Sun, H.; Deng, C.X. Potential therapeutic targets of triple-negative breast cancer based on its intrinsic subtype. Oncotarget 2017, 8, 73329–73344. [Google Scholar] [CrossRef]
- Peshkin, B.N.; Alabek, M.L.; Isaacs, C. BRCA1/2 mutations and triple negative breast cancers. Breast Dis. 2010, 32, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Burga, L.N.; Hu, H.; Juvekar, A.; Tung, N.M.; Troyan, S.L.; Hofstatter, E.W.; Wulf, G.M. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res. 2011, 13, R30. [Google Scholar] [CrossRef]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with 1 and 2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef]
- Castro, E.; Eeles, R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J. Androl. 2012, 14, 409–414. [Google Scholar] [CrossRef]
- Narod, S.A.; Metcalfe, K.; Finch, A.; Chan, A.-W.; Armel, S.R.; Aeilts, A.; Eisen, A.; Karlan, B.; Bordeleau, L.; Tung, N.; et al. The risk of skin cancer in women who carry BRCA1 or BRCA2 mutations. Hered. Cancer Clin. Pract. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Devico Marciano, N.; Kroening, G.; Dayyani, F.; Zell, J.A.; Lee, F.C.; Cho, M.; Valerin, J.G. BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms. Cancers 2022, 14, 2453. [Google Scholar] [CrossRef]
- Patel, A.G.; Sarkaria, J.N.; Kaufmann, S.H. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Liang, S.; Blundell, T.L. Human DNA-dependent protein kinase activation mechanism. Nat. Struct. Mol. Biol. 2023, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Couto, C.A.; Wang, H.Y.; Green, J.C.; Kiely, R.; Siddaway, R.; Borer, C.; Pears, C.J.; Lakin, N.D. PARP regulates nonhomologous end joining through retention of Ku at double-strand breaks. J. Cell Biol. 2011, 194, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Dale Rein, I.; Solberg Landsverk, K.; Micci, F.; Patzke, S.; Stokke, T. Replication-induced DNA damage after PARP inhibition causes G2 delay, and cell line-dependent apoptosis, necrosis and multinucleation. Cell Cycle 2015, 14, 3248–3260. [Google Scholar] [CrossRef]
- Wang, S.S.Y.; Jie, Y.E.; Cheng, S.W.; Ling, G.L.; Ming, H.V.Y. PARP Inhibitors in Breast and Ovarian Cancer. Cancers 2023, 15, 2357. [Google Scholar] [CrossRef]
- Valabrega, G.; Scotto, G.; Tuninetti, V.; Pani, A.; Scaglione, F. Differences in PARP Inhibitors for the Treatment of Ovarian Cancer: Mechanisms of Action, Pharmacology, Safety, and Efficacy. Int. J. Mol. Sci. 2021, 22, 4203. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP inhibitors: Enhancing efficacy through rational combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef]
- Dilmac, S.; Ozpolat, B. Mechanisms of PARP-Inhibitor-Resistance in BRCA-Mutated Breast Cancer and New Therapeutic Approaches. Cancers 2023, 15, 3642. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Swisher, E.M.; Taniguchi, T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011, 102, 663–669. [Google Scholar] [CrossRef]
- Giudice, E.; Gentile, M.; Salutari, V.; Ricci, C.; Musacchio, L.; Carbone, M.V.; Ghizzoni, V.; Camarda, F.; Tronconi, F.; Nero, C.; et al. PARP Inhibitors Resistance: Mechanisms and Perspectives. Cancers 2022, 14, 1420. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Lheureux, S.; Moore, K.N. PARP Inhibitors for Ovarian Cancer: Current Indications, Future Combinations, and Novel Assets in Development to Target DNA Damage Repair. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e116–e131. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int. J. Environ. Res. Public Health 2022, 19, 8577. [Google Scholar] [CrossRef]
- Veneris, J.T.; Matulonis, U.A.; Liu, J.F.; Konstantinopoulos, P.A. Choosing wisely: Selecting PARP inhibitor combinations to promote anti-tumor immune responses beyond BRCA mutations. Gynecol. Oncol. 2020, 156, 488–497. [Google Scholar] [CrossRef]
- Yazinski, S.A.; Comaills, V.; Buisson, R.; Genois, M.M.; Nguyen, H.D.; Ho, C.K.; Todorova Kwan, T.; Morris, R.; Lauffer, S.; Nussenzweig, A.; et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev. 2017, 31, 318–332. [Google Scholar] [CrossRef]
- Nolan, E.; Savas, P.; Policheni, A.N.; Darcy, P.K.; Vaillant, F.; Mintoff, C.P.; Dushyanthen, S.; Mansour, M.; Pang, J.B.; Fox, S.B.; et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci. Transl. Med. 2017, 9, eaal4922. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, C.L.; Hsiao, Y.Y.; Duh, Y.; Yuan, H.S. Structure and function of TatD exonuclease in DNA repair. Nucleic Acids Res. 2014, 42, 10776–10785. [Google Scholar] [CrossRef]
- Petrů, M.; Wideman, J.; Moore, K.; Alcock, F.; Palmer, T.; Doležal, P. Evolution of mitochondrial TAT translocases illustrates the loss of bacterial protein transport machines in mitochondria. BMC Biol. 2018, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Kudva, R.; Denks, K.; Kuhn, P.; Vogt, A.; Müller, M.; Koch, H.G. Protein translocation across the inner membrane of Gram-negative bacteria: The Sec and Tat dependent protein transport pathways. Res. Microbiol. 2013, 164, 505–534. [Google Scholar] [CrossRef]
- Dorival, J.; Eichman, B.F. Human and bacterial TatD enzymes exhibit apurinic/apyrimidinic (AP) endonuclease activity. Nucleic Acids Res. 2023, 51, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Jamsen, J.; Wu, Z.; Wang, Y.; Chen, J.; Zheng, L.; Shen, B. The DNase domain-containing protein TATDN1 plays an important role in chromosomal segregation and cell cycle progression during zebrafish eye development. Cell Cycle 2012, 11, 4626–4632. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.S.; Dutta, A.; Srinivasan, G.; Yuan, Y.; Zhou, D.; Shaheen, M.; Sadideen, D.T.; Kirby, A.; Williamson, E.A.; Gupta, Y.K.; et al. TATDN2 resolution of R-loops is required for survival of BRCA1-mutant cancer cells. Nucleic Acids Res. 2023, 51, 12224–12241. [Google Scholar] [CrossRef]
- Crossley, M.P.; Bocek, M.; Cimprich, K.A. R-Loops as Cellular Regulators and Genomic Threats. Mol. Cell 2019, 73, 398–411. [Google Scholar] [CrossRef]
- García-Muse, T.; Aguilera, A. R Loops: From Physiological to Pathological Roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef]
- Chang, E.Y.; Stirling, P.C. Replication Fork Protection Factors Controlling R-Loop Bypass and Suppression. Genes 2017, 8, 33. [Google Scholar] [CrossRef]
- Bettin, N.; Oss Pegorar, C.; Cusanelli, E. The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells 2019, 8, 246. [Google Scholar] [CrossRef]
- Porro, A.; Feuerhahn, S.; Reichenbach, P.; Lingner, J. Molecular Dissection of Telomeric Repeat-Containing RNA Biogenesis Unveils the Presence of Distinct and Multiple Regulatory Pathways. Mol. Cell. Biol. 2010, 30, 4808–4817. [Google Scholar] [CrossRef]
- Redon, S.; Reichenbach, P.; Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010, 38, 5797–5806. [Google Scholar] [CrossRef] [PubMed]
- Chebly, A.; Ropio, J.; Baldasseroni, L.; Prochazkova-Carlotti, M.; Idrissi, Y.; Ferrer, J.; Farra, C.; Beylot-Barry, M.; Merlio, J.P.; Chevret, E. Telomeric Repeat-Containing RNA (TERRA): A Review of the Literature and First Assessment in Cutaneous T-Cell Lymphomas. Genes 2022, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Romero, C.A.; Chartrand, P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef]
- Chu, H.P.; Cifuentes-Rojas, C.; Kesner, B.; Aeby, E.; Lee, H.G.; Wei, C.; Oh, H.J.; Boukhali, M.; Haas, W.; Lee, J.T. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell 2017, 170, 86–101.e116. [Google Scholar] [CrossRef] [PubMed]
- Rivosecchi, J.; Jurikova, K.; Cusanelli, E. Telomere-specific regulation of TERRA and its impact on telomere stability. Semin. Cell Dev. Biol. 2024, 157, 3–23. [Google Scholar] [CrossRef]
- MacKenzie, D., Jr.; Watters, A.K.; To, J.T.; Young, M.W.; Muratori, J.; Wilkoff, M.H.; Abraham, R.G.; Plummer, M.M.; Zhang, D. ALT Positivity in Human Cancers: Prevalence and Clinical Insights. Cancers 2021, 13, 2384. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y. R-Loops at Chromosome Ends: From Formation, Regulation, and Cellular Consequence. Cancers 2023, 15, 2178. [Google Scholar] [CrossRef]
- Al-Turki, T.M.; Maranon, D.G.; Nelson, C.B.; Lewis, A.M.; Luxton, J.J.; Taylor, L.E.; Altina, N.; Wu, F.; Du, H.; Kim, J.; et al. Telomeric RNA (TERRA) increases in response to spaceflight and high-altitude climbing. Commun. Biol. 2024, 7, 698. [Google Scholar] [CrossRef]
- Cusanelli, E.; Chartrand, P. Telomeric repeat-containing RNA TERRA: A noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 2015, 6, 143. [Google Scholar] [CrossRef]
- Vohhodina, J.; Goehring, L.J.; Liu, B.; Kong, Q.; Botchkarev Jr, V.V.; Huynh, M.; Liu, Z.; Abderazzaq, F.O.; Clark, A.P.; Ficarro, S.B.; et al. BRCA1 binds TERRA RNA and suppresses R-Loop-based telomeric DNA damage. Nat. Commun. 2021, 12, 3542. [Google Scholar] [CrossRef]
- Flynn, R.L.; Cox, K.E.; Jeitany, M.; Wakimoto, H.; Bryll, A.R.; Ganem, N.J.; Bersani, F.; Pineda, J.R.; Suvà, M.L.; Benes, C.H.; et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015, 347, 273–277. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Z.; Stong, N.; Plasschaert, R.; Moczan, A.; Chen, H.S.; Hu, S.; Wikramasinghe, P.; Davuluri, R.V.; Bartolomei, M.S.; et al. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J. 2012, 31, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Lorusso, D.; Italiano, A.; Kaye, S.B.; Aracil, M.; Tanović, A.; D’Incalci, M. Trabectedin as a chemotherapy option for patients with BRCA deficiency. Cancer Treat. Rev. 2016, 50, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Llop-Guevara, A.; Garber, J.E.; Arun, B.K.; Pérez Fidalgo, J.A.; Lluch, A.; Telli, M.L.; Fernández, C.; Kahatt, C.; Galmarini, C.M.; et al. Multicenter Phase II Study of Lurbinectedin in BRCA-Mutated and Unselected Metastatic Advanced Breast Cancer and Biomarker Assessment Substudy. J. Clin. Oncol. 2018, 36, 3134–3143. [Google Scholar] [CrossRef]
- Shakya, R.; Szabolcs, M.; McCarthy, E.; Ospina, E.; Basso, K.; Nandula, S.; Murty, V.; Baer, R.; Ludwig, T. The basal-like mammary carcinomas induced by Brca1 or Bard1 inactivation implicate the BRCA1/BARD1 heterodimer in tumor suppression. Proc. Natl. Acad. Sci. USA 2008, 105, 7040–7045. [Google Scholar] [CrossRef]
- Edwards, R.A.; Lee, M.S.; Tsutakawa, S.E.; Williams, R.S.; Nazeer, I.; Kleiman, F.E.; Tainer, J.A.; Glover, J.N. The BARD1 C-terminal domain structure and interactions with polyadenylation factor CstF-50. Biochemistry 2008, 47, 11446–11456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Steinfeld, J.B.; Liang, F.; Chen, X.; Maranon, D.G.; Jian Ma, C.; Kwon, Y.; Rao, T.; Wang, W.; Sheng, C.; et al. BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature 2017, 550, 360–365. [Google Scholar] [CrossRef]
- Mallery, D.L.; Vandenberg, C.J.; Hiom, K. Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. EMBO J. 2002, 21, 6755–6762. [Google Scholar] [CrossRef]
- Densham, R.M.; Garvin, A.J.; Stone, H.R.; Strachan, J.; Baldock, R.A.; Daza-Martin, M.; Fletcher, A.; Blair-Reid, S.; Beesley, J.; Johal, B.; et al. Human BRCA1–BARD1 ubiquitin ligase activity counteracts chromatin barriers to DNA resection. Nat. Struct. Mol. Biol. 2016, 23, 647–655. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, C.-F.; Li, S.; Chen, Y.; Wang, C.-C.; Xiao, J.; Chen, P.-L.; Sharp, Z.D.; Lee, W.-H. Association of BRCA1 with the hRad50-hMre11-p95 Complex and the DNA Damage Response. Science 1999, 285, 747–750. [Google Scholar] [CrossRef]
- Chen, L.; Nievera, C.J.; Lee, A.Y.-L.; Wu, X. Cell Cycle-dependent Complex Formation of BRCA1·CtIP·MRN Is Important for DNA Double-strand Break Repair. J. Biol. Chem. 2008, 283, 7713–7720. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Adam, S.; Wang, Y.; Sasanuma, H.; Callén, E.; Murga, M.; Day, A.; Kruhlak, M.J.; Wong, N.; Munro, M.; et al. BRCA1 Haploinsufficiency Is Masked by RNF168-Mediated Chromatin Ubiquitylation. Mol. Cell 2019, 73, 1267–1281.e1267. [Google Scholar] [CrossRef]
- Sherker, A.; Chaudhary, N.; Adam, S.; Heijink, A.M.; Noordermeer, S.M.; Fradet-Turcotte, A.; Durocher, D. Two redundant ubiquitin-dependent pathways of BRCA1 localization to DNA damage sites. EMBO Rep. 2021, 22, e53679. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lloret, D.; García-Rodríguez, N.; Soto-Hidalgo, E.; González-Vinceiro, L.; Espejo-Serrano, C.; Giebel, L.; Mateos-Martín, M.L.; de Ru, A.H.; van Veelen, P.A.; Huertas, P.; et al. BRCA1/BARD1 ubiquitinates PCNA in unperturbed conditions to promote continuous DNA synthesis. Nat. Commun. 2024, 15, 4292. [Google Scholar] [CrossRef]
- van de Kooij, B.; Schreuder, A.; Pavani, R.S.; Garzero, V.; Van Hoeck, A.; San Martin Alonso, M.; Koerse, D.; Wendel, T.J.; Callen, E.; Boom, J.; et al. EXO1-mediated DNA repair by single-strand annealing is essential for BRCA1-deficient cells. bioRxiv, 2023. [Google Scholar] [CrossRef]
- García-Rodríguez, N.; Domínguez-García, I.; Domínguez-Pérez, M.D.C.; Huertas, P. EXO1 and DNA2-mediated ssDNA gap expansion is essential for ATR activation and to maintain viability in BRCA1-deficient cells. Nucleic Acids Res. 2024, 52, 6376–6391. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.M.; Ceppi, I.; Anand, R.; Geiger, R.; Cejka, P. The internal region of CtIP negatively regulates DNA end resection. Nucleic Acids Res. 2020, 48, 5485–5498. [Google Scholar] [CrossRef]
- Patterson-Fortin, J.; D’Andrea, A.D. Exploiting the Microhomology-Mediated End-Joining Pathway in Cancer Therapy. Cancer Res. 2020, 80, 4593–4600. [Google Scholar] [CrossRef]
- Zhao, F.; Kim, W.; Kloeber, J.A.; Lou, Z. DNA end resection and its role in DNA replication and DSB repair choice in mammalian cells. Exp. Mol. Med. 2020, 52, 1705–1714. [Google Scholar] [CrossRef]
- Halder, S.; Sanchez, A.; Ranjha, L.; Reginato, G.; Ceppi, I.; Acharya, A.; Anand, R.; Cejka, P. Double-stranded DNA binding function of RAD51 in DNA protection and its regulation by BRCA2. Mol. Cell 2022, 82, 3553–3565.e3555. [Google Scholar] [CrossRef]
- Bunting, S.F.; Callén, E.; Kozak, M.L.; Kim, J.M.; Wong, N.; López-Contreras, A.J.; Ludwig, T.; Baer, R.; Faryabi, R.B.; Malhowski, A.; et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol. Cell 2012, 46, 125–135. [Google Scholar] [CrossRef]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Greene, E.C. DNA Repair Pathway Choices in CRISPR-Cas9-Mediated Genome Editing. Trends Genet. 2021, 37, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Onyango, D.O.; Stark, J.M. Regulation of Single-Strand Annealing and its Role in Genome Maintenance. Trends Genet. 2016, 32, 566–575. [Google Scholar] [CrossRef]
- van de Kooij, B.; Schreuder, A.; Pavani, R.; Garzero, V.; Uruci, S.; Wendel, T.J.; van Hoeck, A.; San Martin Alonso, M.; Everts, M.; Koerse, D.; et al. EXO1 protects BRCA1-deficient cells against toxic DNA lesions. Mol. Cell 2024, 84, 659–674.e657. [Google Scholar] [CrossRef]
- Jaiswal, A.S.; Kim, H.S.; Schärer, O.D.; Sharma, N.; Williamson, E.A.; Srinivasan, G.; Phillips, L.; Kong, K.; Arya, S.; Misra, A.; et al. EEPD1 promotes repair of oxidatively-stressed replication forks. NAR Cancer 2023, 5, zcac044. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lee, S.H.; Williamson, E.A.; Reinert, B.L.; Cho, J.H.; Xia, F.; Jaiswal, A.S.; Srinivasan, G.; Patel, B.; Brantley, A.; et al. EEPD1 Rescues Stressed Replication Forks and Maintains Genome Stability by Promoting End Resection and Homologous Recombination Repair. PLoS Genet. 2015, 11, e1005675. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Fleming, A.M.; Zhu, J.; Ding, Y.; Burrows, C.J. 8-Oxo-7,8-dihydroguanine in the Context of a Gene Promoter G-Quadruplex Is an On–Off Switch for Transcription. ACS Chem. Biol. 2017, 12, 2417–2426. [Google Scholar] [CrossRef]
- Sugden, K.D.; Martin, B.D. Guanine and 7,8-dihydro-8-oxo-guanine-specific oxidation in DNA by chromium(V). Environ. Health Perspect. 2002, 110 (Suppl. S5), 725–728. [Google Scholar] [CrossRef]
- Andrs, M.; Stoy, H.; Boleslavska, B.; Chappidi, N.; Kanagaraj, R.; Nascakova, Z.; Menon, S.; Rao, S.; Oravetzova, A.; Dobrovolna, J.; et al. Excessive reactive oxygen species induce transcription-dependent replication stress. Nat. Commun. 2023, 14, 1791. [Google Scholar] [CrossRef]
- Yoshizawa-Sugata, N.; Masai, H. Human Tim/Timeless-interacting protein, Tipin, is required for efficient progression of S phase and DNA replication checkpoint. J. Biol. Chem. 2007, 282, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.S.; Cortez, D. New insights into abasic site repair and tolerance. DNA Repair 2020, 90, 102866. [Google Scholar] [CrossRef]
- Rajan, A.; Varghese, G.R.; Yadev, I.; Anandan, J.; Latha, N.R.; Patra, D.; Krishnan, N.; Kuppusamy, K.; Warrier, A.V.; Bhushan, S.; et al. Modulation of BRCA1 mediated DNA damage repair by deregulated ER-α signaling in breast cancers. Am. J. Cancer Res. 2022, 12, 17–47. [Google Scholar]
- Kim, H.S.; Nickoloff, J.A.; Wu, Y.; Williamson, E.A.; Sidhu, G.S.; Reinert, B.L.; Jaiswal, A.S.; Srinivasan, G.; Patel, B.; Kong, K.; et al. Endonuclease EEPD1 Is a Gatekeeper for Repair of Stressed Replication Forks. J. Biol. Chem. 2017, 292, 2795–2804. [Google Scholar] [CrossRef]
- Hromas, R.; Kim, H.S.; Sidhu, G.; Williamson, E.; Jaiswal, A.; Totterdale, T.A.; Nole, J.; Lee, S.H.; Nickoloff, J.A.; Kong, K.Y. The endonuclease EEPD1 mediates synthetic lethality in RAD52-depleted BRCA1 mutant breast cancer cells. Breast Cancer Res. 2017, 19, 122. [Google Scholar] [CrossRef]
- Awate, S.; Sommers, J.A.; Datta, A.; Nayak, S.; Bellani, M.A.; Yang, O.; Dunn, C.A.; Nicolae, C.M.; Moldovan, G.L.; Seidman, M.M.; et al. FANCJ compensates for RAP80 deficiency and suppresses genomic instability induced by interstrand cross-links. Nucleic Acids Res. 2020, 48, 9161–9180. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.W.; Tripathi, K.; Dorsman, J.C.; Palle, K. FANCJ protein is important for the stability of FANCD2/FANCI proteins and protects them from proteasome and caspase-3 dependent degradation. Oncotarget 2015, 6, 28816–28832. [Google Scholar] [CrossRef]
- Bogliolo, M.; Surrallés, J. The Fanconi Anemia/BRCA Pathway: FANCD2 at the Crossroad between Repair and Checkpoint Responses to DNA Damage. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2006. [Google Scholar]
- Fang, C.B.; Wu, H.T.; Zhang, M.L.; Liu, J.; Zhang, G.J. Fanconi Anemia Pathway: Mechanisms of Breast Cancer Predisposition Development and Potential Therapeutic Targets. Front. Cell Dev. Biol. 2020, 8, 160. [Google Scholar] [CrossRef]
- Cong, K.; MacGilvary, N.; Lee, S.; MacLeod, S.G.; Calvo, J.; Peng, M.; Nedergaard Kousholt, A.; Day, T.A.; Cantor, S.B. FANCJ promotes PARP1 activity during DNA replication that is essential in BRCA1 deficient cells. Nat. Commun. 2024, 15, 2599. [Google Scholar] [CrossRef]
- Lim, K.S.; Li, H.; Roberts, E.A.; Gaudiano, E.F.; Clairmont, C.; Sambel, L.A.; Ponnienselvan, K.; Liu, J.C.; Yang, C.; Kozono, D.; et al. USP1 Is Required for Replication Fork Protection in BRCA1-Deficient Tumors. Mol. Cell 2018, 72, 925–941.e924. [Google Scholar] [CrossRef] [PubMed]
- García-Santisteban, I.; Peters, G.J.; Giovannetti, E.; Rodríguez, J.A. USP1 deubiquitinase: Cellular functions, regulatory mechanisms and emerging potential as target in cancer therapy. Mol. Cancer 2013, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Dharadhar, S.; Clerici, M.; van Dijk, W.J.; Fish, A.; Sixma, T.K. A conserved two-step binding for the UAF1 regulator to the USP12 deubiquitinating enzyme. J. Struct. Biol. 2016, 196, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Yang, K.; Dejsuphong, D.; Hirota, K.; Takeda, S.; D’Andrea, A.D. The USP1/UAF1 complex promotes double-strand break repair through homologous recombination. Mol. Cell Biol. 2011, 31, 2462–2469. [Google Scholar] [CrossRef]
- Edmunds, C.E.; Simpson, L.J.; Sale, J.E. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell 2008, 30, 519–529. [Google Scholar] [CrossRef]
- Ghosal, G.; Chen, J. DNA damage tolerance: A double-edged sword guarding the genome. Transl. Cancer Res. 2013, 2, 107–129. [Google Scholar]
- Nicolae, C.M.; Aho, E.R.; Vlahos, A.H.S.; Choe, K.N.; De, S.; Karras, G.I.; Moldovan, G.-L. The ADP-ribosyltransferase PARP10/ARTD10 Interacts with Proliferating Cell Nuclear Antigen (PCNA) and Is Required for DNA Damage Tolerance. J. Biol. Chem. 2014, 289, 13627–13637. [Google Scholar] [CrossRef]
- Venkadakrishnan, J.; Lahane, G.; Dhar, A.; Xiao, W.; Bhat, K.M.; Pandita, T.K.; Bhat, A. Implications of Translesion DNA Synthesis Polymerases on Genomic Stability and Human Health. Mol. Cell Biol. 2023, 43, 401–425. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Rosenthal, A.S.; Liang, Q.; Chen, J.; Villamil, M.A.; Kerns, E.H.; Simeonov, A.; Jadhav, A.; Zhuang, Z.; Maloney, D.J. Discovery of ML323 as a Novel Inhibitor of the USP1/UAF1 Deubiquitinase Complex. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Tung, N.M.; Garber, J.E. BRCA1/2 testing: Therapeutic implications for breast cancer management. Br. J. Cancer 2018, 119, 141–152. [Google Scholar] [CrossRef]
- Arun, B.; Couch, F.J.; Abraham, J.; Tung, N.; Fasching, P.A. BRCA-mutated breast cancer: The unmet need, challenges and therapeutic benefits of genetic testing. Br. J. Cancer 2024, 131, 1400–1414. [Google Scholar] [CrossRef]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; Genereviews Is a Registered Trademark of the University of Washington; Copyright © 1993–2024; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.; Arris, D.; Tran, M.T. Overview of Roles of Novel Components in the Regulation of DNA Damage Repair in BRCA1-Deficient Cancers: An Update. DNA 2025, 5, 17. https://doi.org/10.3390/dna5020017
Nguyen N, Arris D, Tran MT. Overview of Roles of Novel Components in the Regulation of DNA Damage Repair in BRCA1-Deficient Cancers: An Update. DNA. 2025; 5(2):17. https://doi.org/10.3390/dna5020017
Chicago/Turabian StyleNguyen, Nhat, Dominic Arris, and Manh Tien Tran. 2025. "Overview of Roles of Novel Components in the Regulation of DNA Damage Repair in BRCA1-Deficient Cancers: An Update" DNA 5, no. 2: 17. https://doi.org/10.3390/dna5020017
APA StyleNguyen, N., Arris, D., & Tran, M. T. (2025). Overview of Roles of Novel Components in the Regulation of DNA Damage Repair in BRCA1-Deficient Cancers: An Update. DNA, 5(2), 17. https://doi.org/10.3390/dna5020017